Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9889
Peer-review started: April 19, 2021
First decision: June 23, 2021
Revised: June 26, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: November 16, 2021
Processing time: 204 Days and 11.3 Hours
Treatment of synchronous multiple primary malignancies is quite often very challenging. Herein, we report on a rare case of synchronous multiple primary malignancies in the esophagus, stomach, and jejunum.
A 50-year-old man who was a heavy drinker and smoker with a poor diet, and had a family history of cancer sought treatment due to dysphagia lasting for 4 mo. He was finally diagnosed with lower esophageal squamous cell carcinoma (pT3N2M0, G2, stage IIIB), gastric angular adenocarcinoma (pT3N2M0, G2-G3, stage IIIA) with greater omental lymph node metastasis, and jejunal stromal tumor (high risk). The high-risk jejunal stromal tumor was found during surgery. In spite of radical resection and adjuvant chemotherapy, lymph node metastasis occurred 21 mo later. The patient responded poorly to additional chemotherapy and refused further examination and therapy. He died of widespread metastases 33 mo after surgery.
This case indicates a poor prognosis of synchronous multiple advanced primary malignancies and the importance of comprehensive assessment in the population at high risk for cancer.
Core Tip: This article presents a case with synchronous multiple primary malignancies, including esophageal squamous cell carcinoma, gastric adenocarcinoma, and jejunal stromal tumor. This patient had many cancer-related risk factors like heavy drinking, smoking, and family history. The high-risk jejunal stromal tumor was found during operation. Despite radical surgery and adjuvant chemotherapy, the patient died of widespread metastases 33 mo later. This case suggests a poor prognosis of synchronous multiple advanced primary malignancies and the importance of comprehensive assess
- Citation: Li Y, Ye LS, Hu B. Synchronous multiple primary malignancies of the esophagus, stomach, and jejunum: A case report. World J Clin Cases 2021; 9(32): 9889-9895
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9889.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9889
Multiple primary malignancies are relatively common, accounting for about 2.4%-17.2% within 20 years of follow-up in a cancer population[1-2]. The prognosis of multiple primary malignancy varies much due to cancer type and stage at initial diagnosis. The treatment of patients with synchronous multiple primary malignancy, especially advanced ones, is still difficult[3]. Herein, we report one case with syn
A 50-year-old man sought treatment for dysphagia lasting for 4 mo.
The patient had difficulty when swallowing solid and semiliquid food, without nausea, vomiting, thoracalgia, acid reflux, heartburn, abdominal pain, abdominal distension, melena, constipation, or weight loss, etc.
The patient had no previous medical history.
The patient smoked 10 cigarettes per day for 30 years, drank alcohol 100 g/d for 30 years, and often ate hot food. His father died of lung cancer.
The patient’s height and body weight were 169 cm and 61 kg, respectively. His general condition was good and physical examination revealed no special abnormalities.
There were no abnormal laboratory data findings, including blood test, hepatic function parameters, renal function parameters, tumor markers, etc.
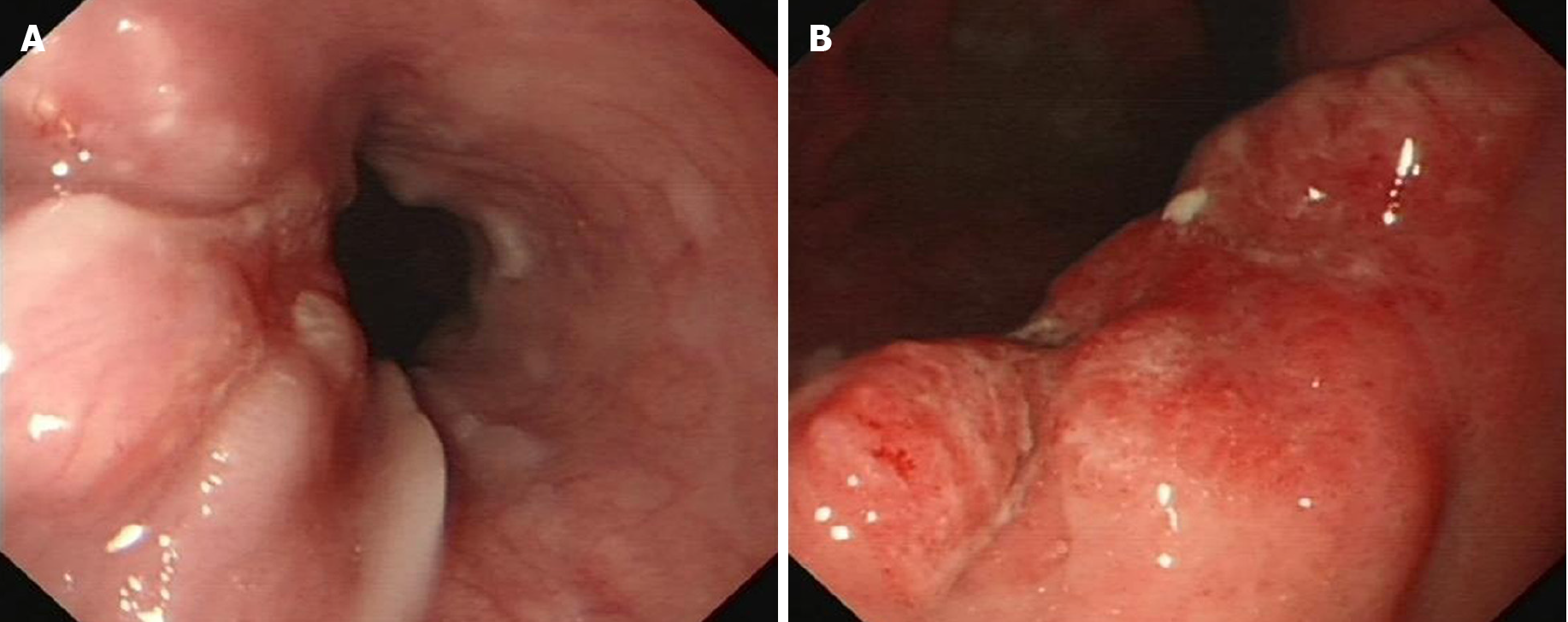
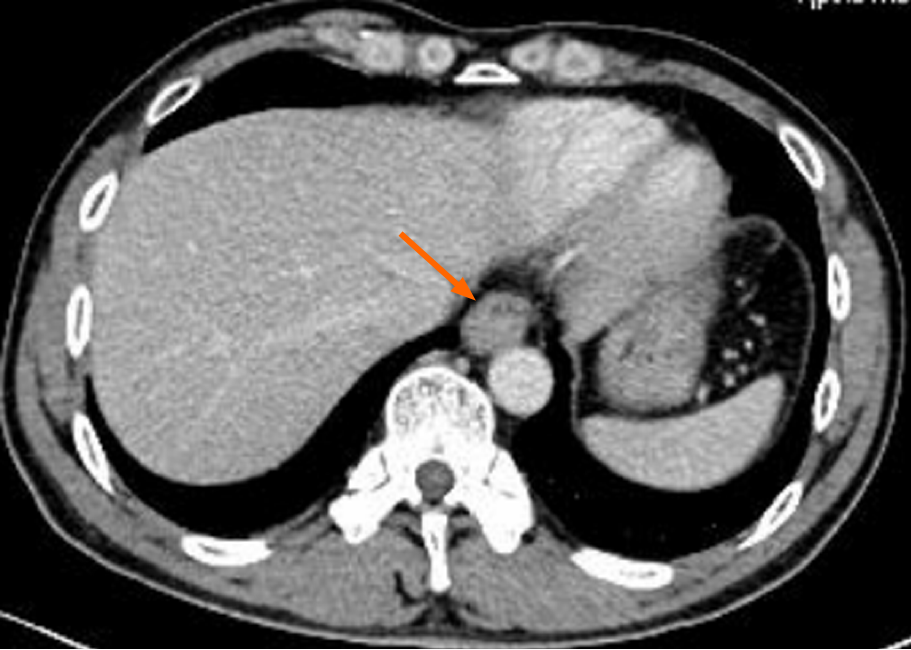
White light endoscopy revealed one soft nodular neoplasm with superficial erosion along the lower esophagus 35-37 cm from the incisors (Figure 1A), which was identified as squamous cell carcinoma following biopsy, and one hard nodular neoplasm with superficial erosion and irregular bound in the stomach angular notch (Figure 1B) that was identified as adenocarcinoma following biopsy. Chest and upper abdomen enhanced computed tomography (CT) showed eccentrically enhanced thickening of the lower esophagus (Figure 2), without enlarged lymph nodes and distant metastasis.
The final diagnosis was lower esophageal squamous cell carcinoma (pT3N2M0, G2, stage IIIB), gastric angular adenocarcinoma (pT3N2M0, G2-G3, stage IIIA) with greater omental lymph node metastasis, and jejunal stromal tumor (high risk).
Lower esophageal carcinoma resection and total gastrectomy were first planned. Entering the thoracic cavity through the right posterolateral 5th intercostal space, we found a fungating 3 cm × 3 cm × 2 cm tumor in the lower esophagus and enlarged lymph nodes. Entering the abdomen through the midline incision of the upper ab
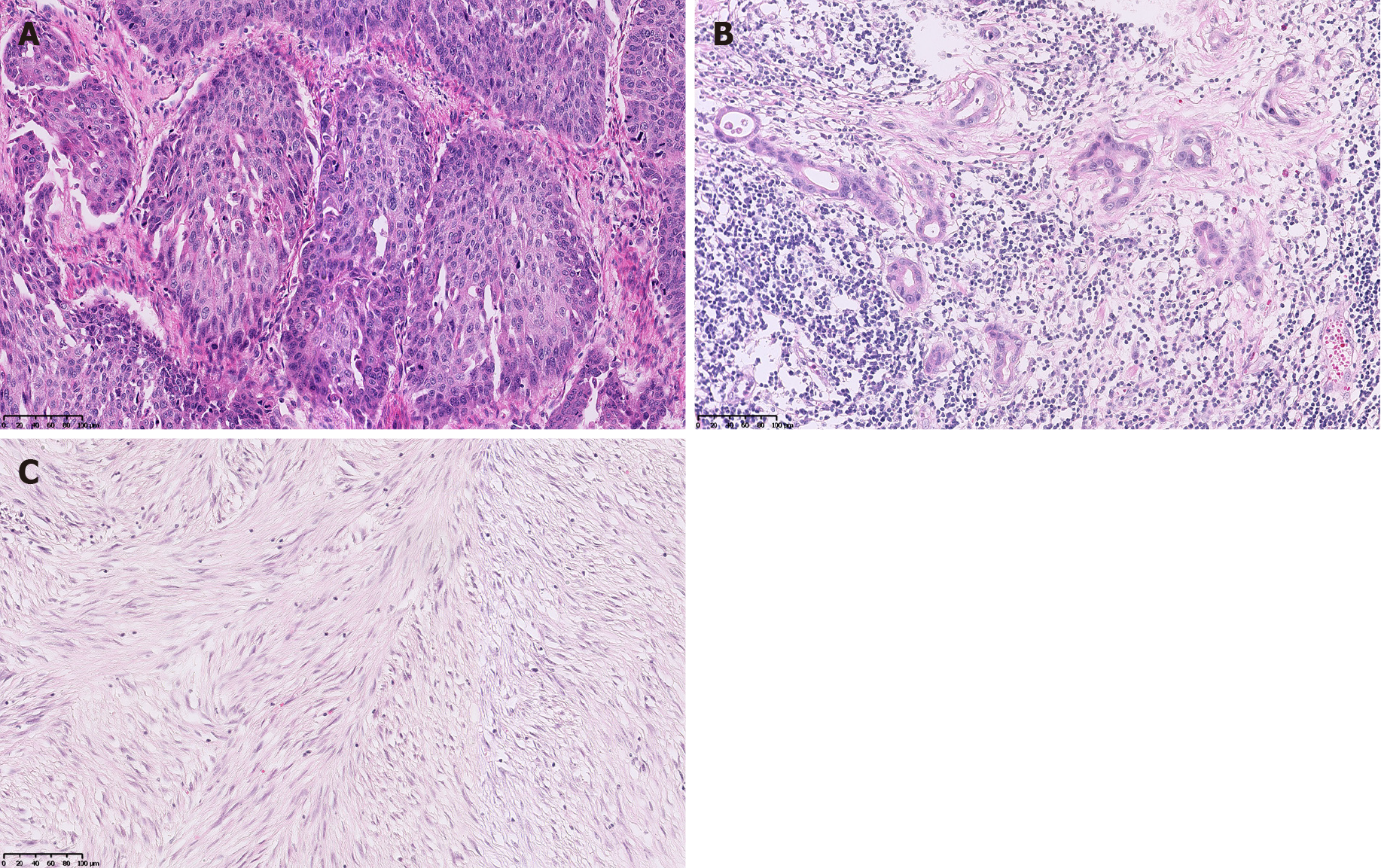
The esophageal tumor turned out to be a moderately differentiated squamous cell carcinoma (Figure 3A) invading to outer membrane, with positive vascular cancer thrombus and negative margin. Metastasis of squamous cell carcinoma was detected in groups 8, 10, and 16 lymph nodes. The gastric tumor was a moderately to poorly differentiated adenocarcinoma (tubular adenocarcinoma and signet-ring cell car
Enhanced head, neck, chest, abdomen, and pelvis CT and bone SPECT before adjuvant therapy showed no organic metastasis. A blood test showed a decreased white cell count at 2.54 × 109/L. Hepatic function and renal function parameters were normal. The patient was treated adjuvantly with six cycles of Oxaliplatin 200 mg d1 combined with Tegafur Gimeracil Oteracil Potassium capsules 50 mg bid d1-14 (SOX regimen). Re-examinations taken every 3 mo showed no relapse or metastasis within 18 mo postoperatively.
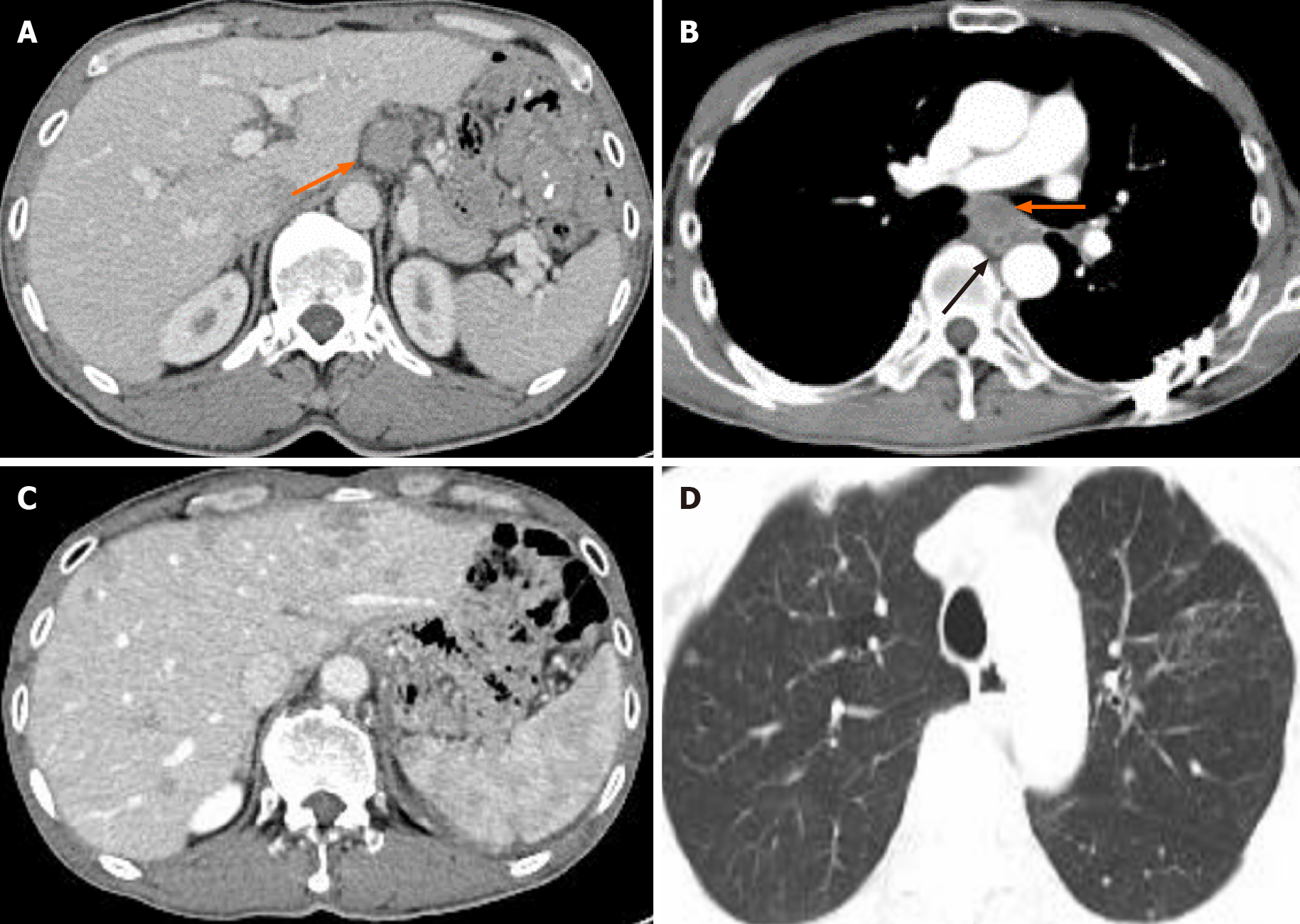
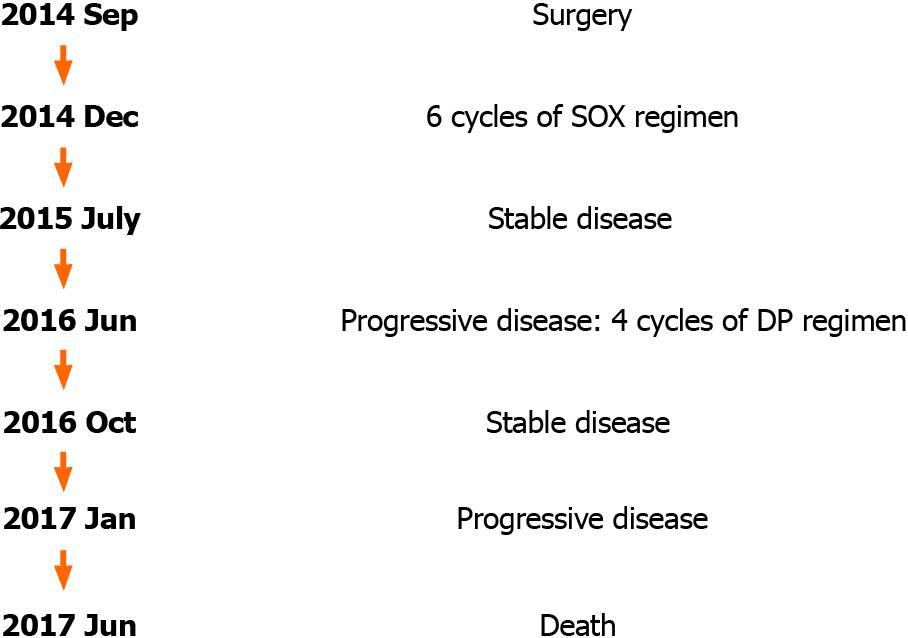
At the 21st mo after surgery, abdominal enhanced CT showed that the para-aortic and hepatogastric ligamentous lymph nodes enlarged (Figure 4A), and the concentration of CA72-4 was elevated to 10.18 U/mL. Additional Docetaxel 110 mg d1 combined with Cisplatin 35 mg d1-d3 (DP regimen) was given; however, nausea reached grade III at the first cycle and the latter three cycles were dose-reduced DP regimen (Docetaxel 90 mg d1 combined with Cisplatin 35 mg d1-d3). At the 24th mo after surgery, abdominal lymph nodes were further enlarged and the concentration of CA72-4 elevated to 17.9 U/mL. The patient refused biopsy and further treatment. At the 28th mo after surgery, local thickening of esophageal anastomotic site, multiple nodules in the liver, pancreatic invasion, small nodules scattered in both lungs, enlarged hepatogastric ligamentous and para-aortic lymph nodes, and enlarged subcarinal lymph nodes under enhanced CT (Figures 4B, 4C and 4D), and further elevated serum tumor markers (CA199 > 1000 U/mL, CEA 141.2 ng/mL, and CA72-4 > 300 U/mL) indicated rapid disease progression. The patient finally died of widespread metastases 33 mo after surgery. The timeline of his treatment is shown in Figure 5.
Multiple primary malignancies relate to more than one independent primary ma
Inherited predisposition to cancer, cancer promoting aspects of lifestyle (heavy drinking, smoking, high salt diet, frequent hot food, obesity, etc.), and hormonal and environmental factors have been associated with the occurrence of multiple primary neoplasms[4-6]. In this case, the patient had multiple risk factors like heavy drinking, smoking, frequent hot food, and family history. Cancer type and stage at initial diagnosis are related to the prognosis of synchronous multiple primary malignancies. This patient was diagnosed with esophageal squamous cell carcinoma at stage IIIB, gastric adenocarcinoma at stage IIIA, and high-risk jejunal stromal tumor. Despite radical resection and adjuvant chemotherapy, he died of multi-organ metastases 33 mo postoperatively. The 5-year overall survival (OS) of esophageal cancer and gastric cancer were 14.7%-23.5% and 20.4%-32.8%, respectively[7]. But the 5-year OS of early esophageal and gastric carcinoma may reach up to 63.2%-84%[8-10]. This sheds light on a poor prognosis of synchronous multiple advanced primary malignancies and the importance of comprehensive screening for high-risk population.
GIST is the most common mesenchymal tumor of the gastrointestinal tract, but many patients are asymptomatic because the tumor can grow inside the abdominal and pelvic cavity. Patients with gastrointestinal stromal tumor have a higher risk of additional cancers than the general population, reaching about 16.4%-37.9%[11-13]. In this case, the high-risk jejunal stromal tumor concealed in the pelvic cavity and we did not discover this lesion until performing esophagojejunostomy. GISTs in the small intestine have a poor prognosis, especially when the tumor size is larger than 5 cm, the mitotic rate is over 5/50 high-power fields, or the tumor is ruptured[14]. Also, surgery is the optimal therapy for localized primary GISTs. If we missed this GIST during this operation, the patient would have to encounter another major surgery. Therefore, for populations at high risk for malignancy, comprehensive cancer screening is crucial to avoid omission.
This case report had some limitations. First, it was unclear which malignancy the widespread metastases originated from because the patient refused to take biopsy after tumor recurrence and metastasis. Second, no genetic testing was performed to detect possible oncogene(s) for multiple primary malignancies.
Patients with synchronous multiple advanced primary malignancies have a poor prognosis, and comprehensive assessment of multiple primary malignancies is critical for patients at high risk for cancer.
We wish to thank the pathology department and the radiology department at our hospital for their assistance in providing materials related to this case.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen L, Gallo G S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Buiatti E, Crocetti E, Acciai S, Gafà L, Falcini F, Milandri C, La Rosa M. Incidence of second primary cancers in three Italian population-based cancer registries. Eur J Cancer. 1997;33:1829-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Amer MH. Multiple neoplasms, single primaries, and patient survival. Cancer Manag Res. 2014;6:119-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, Omlin A. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2:e000172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 368] [Article Influence: 46.0] [Reference Citation Analysis (1)] |
| 4. | Soerjomataram I, Coebergh JW. Epidemiology of multiple primary cancers. Methods Mol Biol. 2009;471:85-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Engel LS, Chow WH, Vaughan TL, Gammon MD, Risch HA, Stanford JL, Schoenberg JB, Mayne ST, Dubrow R, Rotterdam H, West AB, Blaser M, Blot WJ, Gail MH, Fraumeni JF Jr. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 518] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 6. | Wu M, Liu AM, Kampman E, Zhang ZF, Van't Veer P, Wu DL, Wang PH, Yang J, Qin Y, Mu LN, Kok FJ, Zhao JK. Green tea drinking, high tea temperature and esophageal cancer in high- and low-risk areas of Jiangsu Province, China: a population-based case-control study. Int J Cancer. 2009;124:1907-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust TÅ, Tervonen H, Thursfield V, Ransom D, Shack L, Woods RR, Turner D, Leonfellner S, Ryan S, Saint-Jacques N, De P, McClure C, Ramanakumar AV, Stuart-Panko H, Engholm G, Walsh PM, Jackson C, Vernon S, Morgan E, Gavin A, Morrison DS, Huws DW, Porter G, Butler J, Bryant H, Currow DC, Hiom S, Parkin DM, Sasieni P, Lambert PC, Møller B, Soerjomataram I, Bray F. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 725] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 8. | Uedo N, Iishi H, Tatsuta M, Ishihara R, Higashino K, Takeuchi Y, Imanaka K, Yamada T, Yamamoto S, Tsukuma H, Ishiguro S. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Kosaka T, Endo M, Toya Y, Abiko Y, Kudara N, Inomata M, Chiba T, Takikawa Y, Suzuki K, Sugai T. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center retrospective study. Dig Endosc. 2014;26:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Berry MF, Zeyer-Brunner J, Castleberry AW, Martin JT, Gloor B, Pietrobon R, D'Amico TA, Worni M. Treatment modalities for T1N0 esophageal cancers: a comparative analysis of local therapy versus surgical resection. J Thorac Oncol. 2013;8:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Shen C, Chen H, Yin Y, Chen J, Han L, Zhang B, Chen Z. Synchronous occurrence of gastrointestinal stromal tumors and other digestive tract malignancies in the elderly. Oncotarget. 2015;6:8397-8406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Murphy JD, Ma GL, Baumgartner JM, Madlensky L, Burgoyne AM, Tang CM, Martinez ME, Sicklick JK. Increased risk of additional cancers among patients with gastrointestinal stromal tumors: A population-based study. Cancer. 2015;121:2960-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Du J, Shen N, He HS, Fu XL, Wang JZ, Mao CZ. Synchronous gastrointestinal cancer and gastrointestinal stromal tumors: a single-institution experience. World J Surg Oncol. 2016;14:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (33)] |