Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9857
Peer-review started: June 22, 2021
First decision: July 26, 2021
Revised: July 28, 2021
Accepted: August 27, 2021
Article in press: August 27, 2021
Published online: November 16, 2021
Processing time: 140 Days and 20.3 Hours
Acute cholangitis is caused by bacterial infection and has high morbidity and mortality risk. The grade of cholangitis can guide clinical treatment from single antibiotic treatment to biliary drainage. With the introduction of white blood cell (WBC) count, C-reactive protein (CRP), and total bilirubin (T-Bil) into the diagnostic criteria and severity grading for acute cholangitis, the diagnosis rate and grading have significantly improved. However, early risk stratification assessments are challenging in the emergency department. Therefore, we hope to find an ideal predictive biomarker for cholangitis grade. Presepsin is a promising biomarker for the early diagnosis, severity, and prognosis of acute bacterial infections.
To assess the grading value of presepsin in patients with acute cholangitis.
This clinical study was conducted at the Beijing Friendship Hospital, a 2000-bed teaching hospital with approximately 200000 emergency admissions per year. In this prospective observational study, 336 patients with acute cholangitis meeting the Tokyo Guidelines 2018 diagnostic criteria in the emergency department from May 2019 to December 2020 were analyzed. WBC count, CRP, procalcitonin (PCT), presepsin, T-Bil, and blood culture results were collected. The values were compared using the Pearson χ2 test, Fisher’s exact test, or Mann-Whitney U test. The area under the receiver operating characteristic curve (AUC) of the value was examined using the Delong test. The correlations among the key research indicators were determined using Pearson correlation.
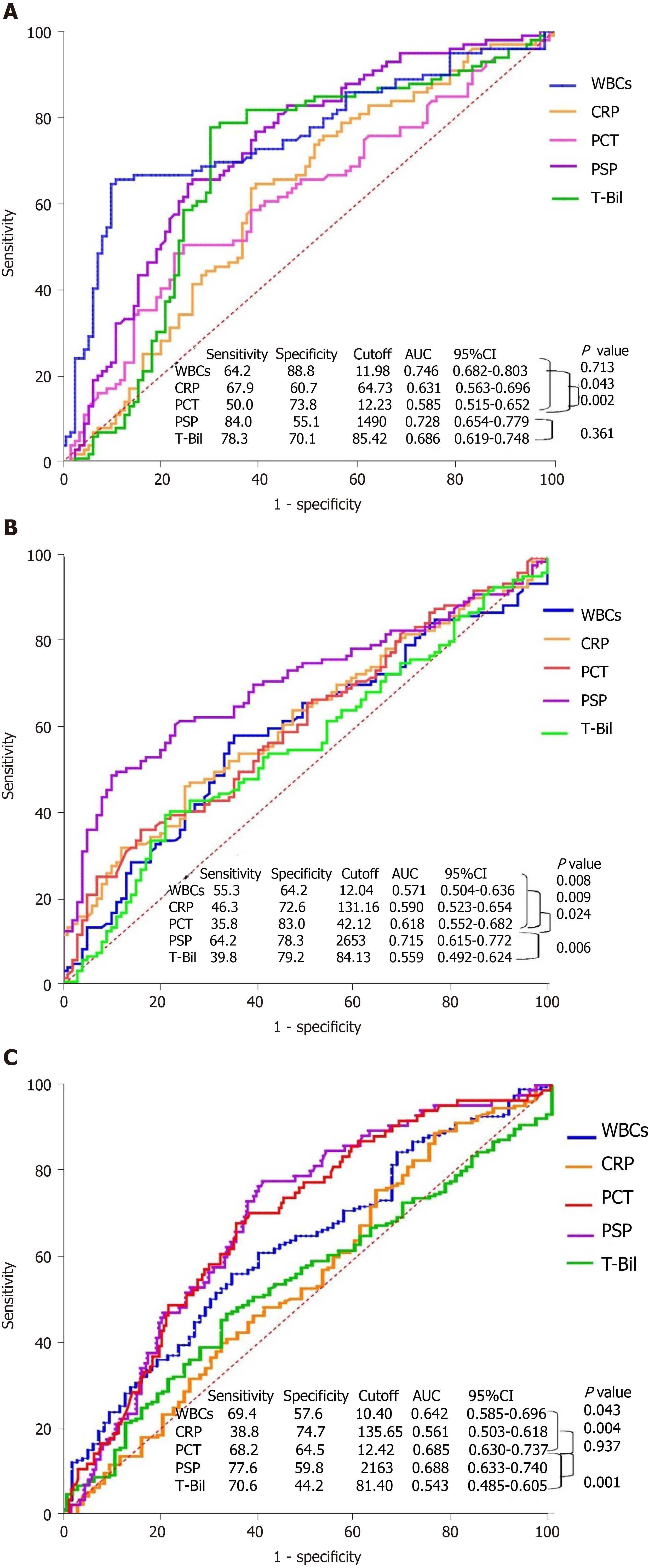
In total, 336 patients were examined, which included 107, 106, and 123 patients classified as having mild, moderate, and severe cholangitis, respectively. WBC count, CRP, PCT, presepsin, T-Bil, direct bilirubin, and sequential organ failure assessment scores of moderate and severe cholangitis patients were higher than those of mild cholangitis patients (P = 0.000). The AUC of presepsin in predicting moderate acute cholangitis was 0.728, which was higher than that of CRP (0.631, P = 0.043) and PCT (0.585, P = 0.002), and same as that of WBC count (0.746, P = 0.713) and T-Bil (0.686, P = 0.361). The AUC of presepsin in predicting severe acute cholangitis was 0.715, which was higher than that of WBC count (0.571, P = 0.008), CRP (0.590, P = 0.009), PCT (0.618, P = 0.024), and T-Bil (0.559, P = 0.006). The presepsin levels in the positive blood culture group were higher (2830.8pg/mLvs1987.8pg/mL, P = 0.000), and the AUC of presepsin (0.688) proved that it was a good biomarker for predicting positive bacterial culture.
Presepsin can predict positive blood culture in patients with acute cholangitis. It is superior to WBC count, CRP, PCT, and T-Bil for the risk stratification of acute cholangitis.
Core Tip: Although the mortality rate of acute cholangitis has decreased notably due to early drainage and antibiotic use, it remains higher in severe cholangitis. Early identification of severe cholangitis can reduce the mortality of patients with severe cholangitis. Presepsin is a promising biomarker for the early diagnosis and prognosis of bacterial infections. In this study, presepsin was found to be superior to white blood cell count, C-reactive protein, procalcitonin, and total bilirubin in predicting positive blood culture and assessing risk in patients with acute cholangitis in the emergency department.
- Citation: Zhang HY, Lu ZQ, Wang GX, Xie MR, Li CS. Presepsin as a biomarker for risk stratification for acute cholangitis in emergency department: A single-center study. World J Clin Cases 2021; 9(32): 9857-9868
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9857.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9857
Acute cholangitis is an acute inflammatory reaction caused by bacterial infection in the biliary tract[1]. It progresses rapidly and can develop into sepsis or septic shock[2]. Acute cholangitis is currently diagnosed based on clinical symptoms and signs, white blood cell (WBC) count, C-reactive protein (CRP), and total bilirubin (T-Bil) levels, and imaging findings. According to the Tokyo Guidelines 2018 (TG18): Diagnostic Criteria and Severity Grading for Acute Cholangitis, appropriate use of biliary drainage and antibiotics has observably reduced the mortality rate for acute cholangitis[1,3,4]; thus, early risk stratification is vital for the emergency treatment of acute cholangitis. However, TG18 is a comprehensive criterion and is cumbersome to use in the emergency department. Therefore, we hope to find an ideal predictive biomarker for cholangitis grade.
Presepsin, containing 64 amino acid residues cleaved from the monocyte/macro
Overall, this study enrolled all patients (age > 18 years) diagnosed with acute cholangitis from May 1, 2019 to December 20, 2020. All patients were administered with intravenous antibiotics according to the TG18 (imipenem and cilastatin sodium 0.5 g every 8 h, cefoperazone sodium and sulbactam sodium 3.0 g every 12 h, levofloxacin 0.5 g every 24 h, or tazobactam sodium and piperacillin sodium 4.5 g every 8 h) as soon as acute cholangitis was diagnosed.
All patients included in this study met the diagnostic criteria for acute cholangitis according to TG18. The diagnostic criteria were as follows: (1) Systemic inflammation (fever/chills, abnormal WBC count, or elevated CRP level); (2) cholestasis (T-Bil levels ≥ 34.2 µmol/mL or liver enzyme levels > 1.5 times that of the normal upper limit); and (3) supporting imaging findings (evidence of biliary dilatation, such as benign or malignant stenosis, stone, and/or stent). Participants were excluded if they had liver diseases such as viral or drug-induced hepatitis or chronic liver disease, chronic renal insufficiency, or any other infections on admission.
On admission, whole blood (30 mL) was collected from the patients’ cubital veins, and sequential organ failure assessment (SOFA) scores[8] were determined immediately. In addition, blood was tested for the following parameters: WBC counts, CRP, plate
Clinical data, including baseline demographic features, laboratory parameters, and blood culture, were collected on admission. Data regarding vital signs, mental status, oxygen flow, hepatic function, biomarkers, blood culture, urinary output, vasoactive drug administration (dopamine, dobutamine, adrenaline, and norepinephrine), arterial partial pressure of oxygen, and SOFA scores were all collected on admission.
Presepsin levels were measured using the PATHFAST analyzer (PATHFAST presepsin, Mitsubishi Chemical Medience Corporation, Tokyo, Japan), a chemiluminescence immunoassay of venous whole blood using EDTA as an anticoagulant[9]. The detection range of presepsin level was 20–200000 pg/mL. PCT levels were assayed with a BioMerieux Mini VIDAS immunoassay analyzer (Block Scientific, Bohemia, NY) in the plasma obtained from blood centrifuged for 15 min at 1000 g. The detection range of PCT level was 0.05–200 ng/mL. Information regarding other indicators was obtained from the clinical laboratory data.
Severe acute cholangitis was diagnosed if any dysfunction was noted in the cardiovascular, nervous, respiratory, or blood systems or in the kidneys or liver. Moderate acute cholangitis was diagnosed if any two of the following conditions were observed: Abnormal WBC counts, high fever, age > 75 years, hyperbilirubinemia, and/or hypoalbuminemia. Mild acute cholangitis was diagnosed if the criteria for the above two grades of acute cholangitis were not met.
A biomedical statistician performed the statistical review of the study before the submission for peer-review. All statistical analyses were performed using IBM SPSS Statistics for Windows 10, version 22.0 (IBM Corp., Armonk, NY, United States). Classified variables are expressed as counts and percentages and were compared using the Pearson χ2 test or Fisher’s exact test. Skewed distribution variables are expressed as medians (25th-75th percentiles), and Mann–Whitney U test was used to compare the groups. A two-sided P value of < 0.05 was considered statistically significant, and the significant level for pairwise comparisons among the three groups was set at 0.017 (P = 0.05/3). Diagnostic sensitivity, specificity, cutoff value, and area under the receiver operating characteristic (ROC) curve (AUC) were determined by performing ROC curve analyses to verify the role of presepsin levels on admission for acute cholangitis. The optimal cutoff value was calculated using the Youden index according to the ROC curves. Delong test was used to compare AUCs between the group by MedCalc Version 13 (MedCalc Software, Mariakerke, Belgium). The correlations among the main research indicators were determined using Pearson correlation.
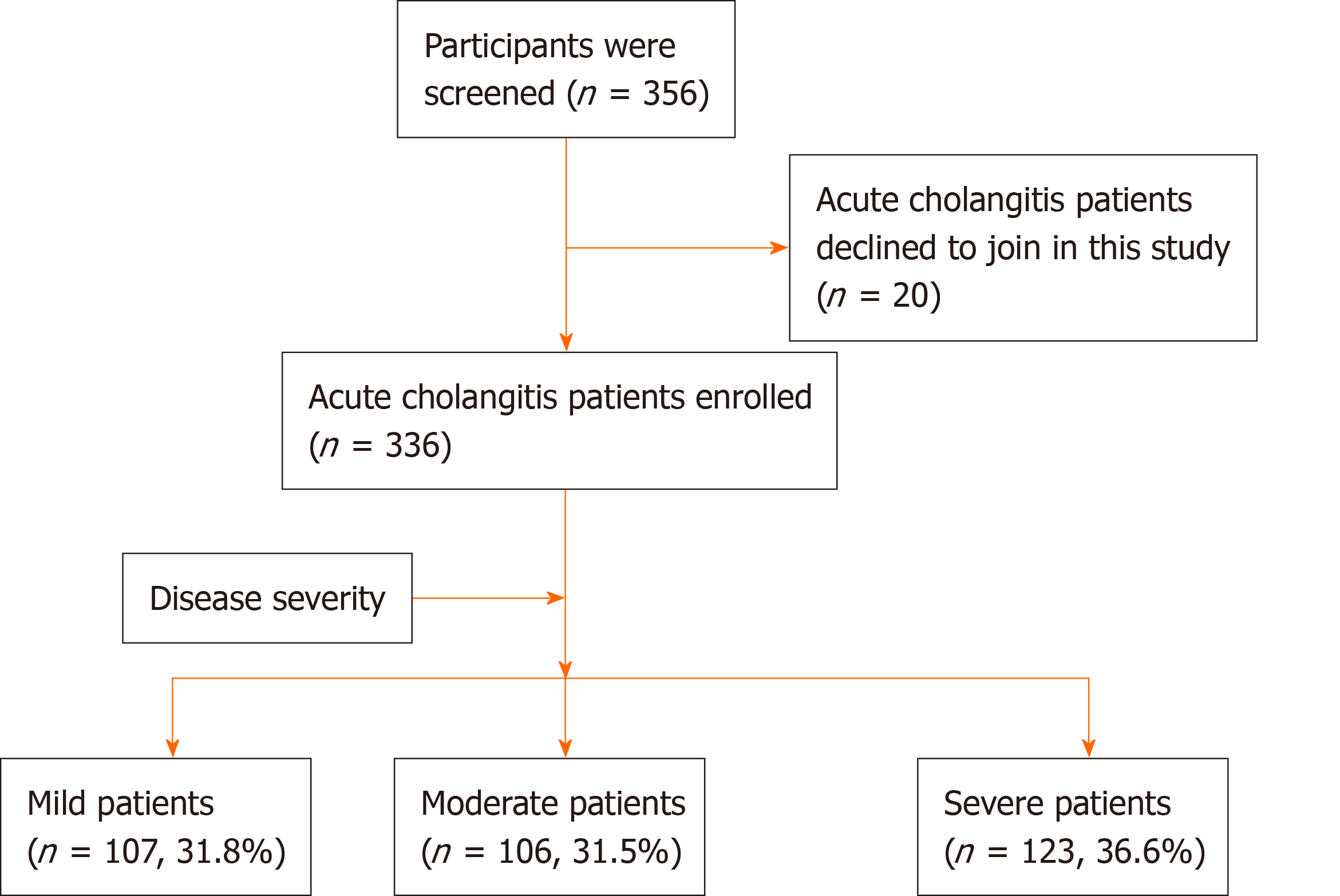
Among the 356 patients with acute cholangitis who were screened, 20 declined to participate in this study. This resulted in the final 336 patients (or their relatives) who signed an informed consent form before participating in this study. All patients with acute cholangitis on admission were divided into mild, moderate, and severe groups based on the TG18. The numbers of patients in the above three groups were 107 (31.8%), 106 (31.5%), and 123 (36.6%), respectively (Figure 1).
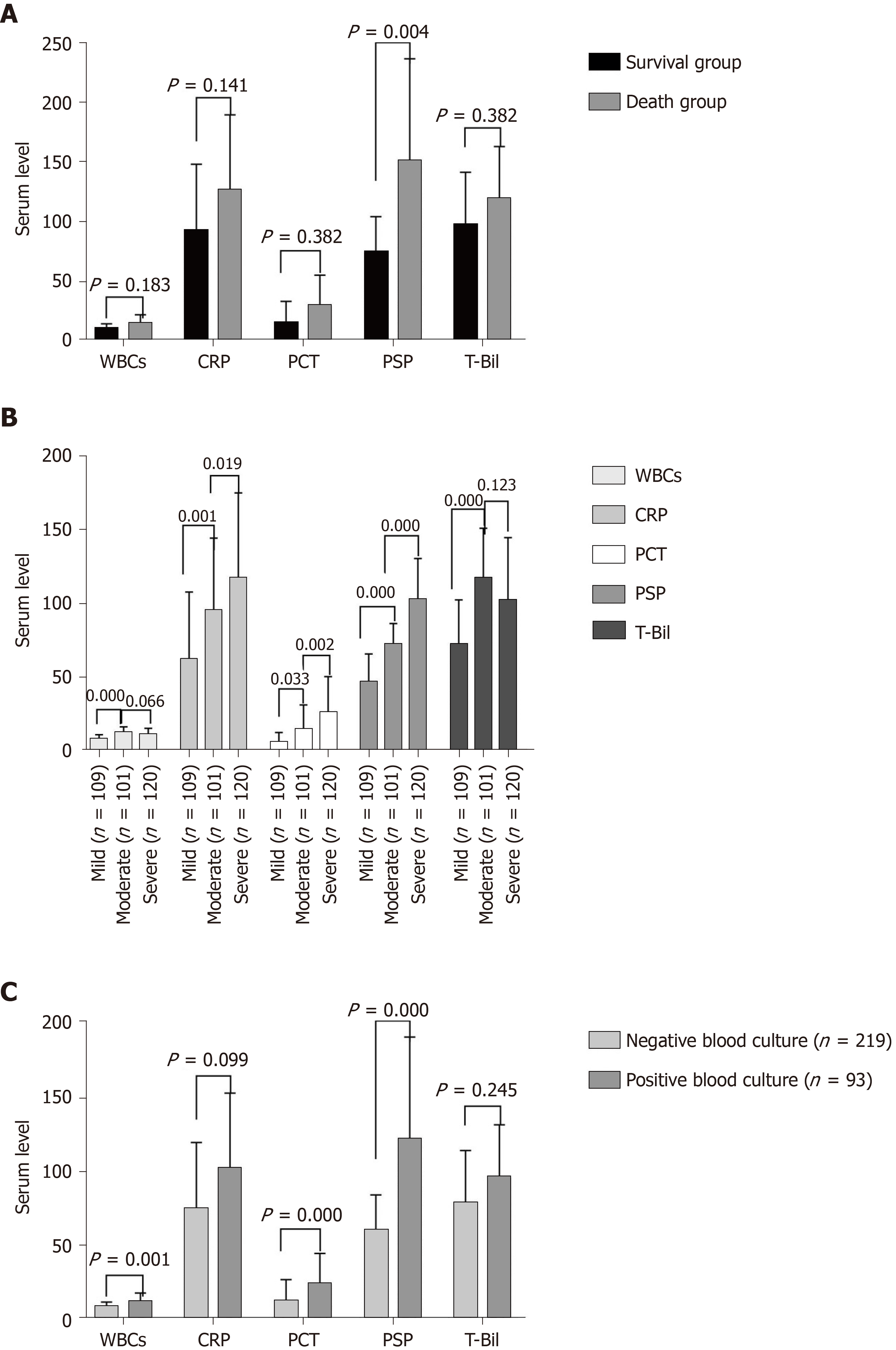
The features and outcomes of 336 patients with acute cholangitis are shown in Table 1. There were 215 men and 121 women, and the median age was 75 years. Age, WBC counts, CRP, PCT, presepsin, T-Bil, direct bilirubin, and SOFA scores of moderate and severe cholangitis patients were higher than those of mild cholangitis patients, whereas the opposite was true for total protein and albumin. Eight deaths occurred within 28 d of admission in patients with severe cholangitis. The 28-d mortality rate of all patients with acute cholangitis was 2.4%. Presepsin level in the survival group was lower than that in the death group (2122 pg/mL vs 3570 pg/mL, P = 0.004), although no prominent differences were observed in the WBC counts, PCT, or T-Bil levels between the two groups (P > 0.05) (Figure 2A).
| Acute cholangitis (n = 336) | Severity of acute cholangitis | P value | |||
| Mild (n = 107) | Moderate (n = 106) | Severe (n = 123) | |||
| Features | |||||
| Age (yr) | 75 (66-84) | 69 (64-79) | 81 (70-86) | 75 (67-85) | 0.000 |
| Male/female | 198/138 | 61/46 | 65/41 | 72/51 | 0.810 |
| Temperature (℃) | 38.5 (37.7-39.3) | 38.2 (37.5-39.0) | 38.6 (37.8-39.4) | 38.6 (37.7-39.7) | 0.021 |
| MAP (kPa) | 11.4 (10.1-12.7) | 11.6 (10.1-12.6) | 11.6 (10.5-12.8) | 11.2 (9.4-12.7) | 0.055 |
| Laboratory test | |||||
| WBCs (× 109/L) | 10.52 (7.32-14.78) | 8.70 (6.19-11.06) | 13.08 (8.97-16.27) | 11.24 (7.36-15.45) | 0.000 |
| CRP (mg/L) | 83.71 (43.17-152.86) | 54.86 (21.36-111.00) | 85.11 (53.17-148.70) | 116.92 (60.00-175.13) | 0.000 |
| PCT (ng/mL) | 9.93 (1.93-36.38) | 4.64 (0.96-13.31) | 9.58 (1.33-33.41) | 22.88 (4.15-51.70) | 0.000 |
| Presepsin (pg/mL) | 2159.50 (1477.25-3214.50) | 1376.00 (865.00-1989.00) | 2150.50 (1757.75-2603.00) | 3179.00 (2214.00-3862.00) | 0.000 |
| T-Bil (µmol/L) | 93.98 (57.06-144.26) | 64.06 (47.90-105.86) | 111.84 (88.06-153.27) | 101.81 (60.77-145.04) | 0.000 |
| D-Bil (µmol/L) | 66.89 (37.29-97.33) | 42.65 (30.30-78.95) | 76.60 (58.67-100.71 | 70.13 (40.37-98.56) | 0.000 |
| Total protein (g/L) | 66.5 (59.9-71.9) | 68.2 (63.5-72.3) | 67.8 (61.5-72.9) | 62.0 (57.1-68.8) | 0.000 |
| Albumin (g/L) | 33.9 (30.6-37.8) | 35.4 (31.7-39.8) | 35.4 (31.5-38.6) | 32.0 (29.2-36.1) | 0.000 |
| ALT (U/L) | 147.5 (75.0-266.5) | 153.0 (60.0-311.0 | 150.0 (88.4-298.3) | 139.0 (75.0-240.0 | 0.670 |
| AST (U/L) | 137.4 (72.1-279.4) | 126.5 (64.9-345.5) | 142.2 (96.6-276.8) | 128.3 (69.7-228.9) | 0.367 |
| LDH (U/L) | 243.0 (178.0-352.8) | 231.1 (167.0-416.0) | 235.5 (169.5-332.0) | 254.0 (192.0-356.5) | 0.183 |
| SOFA score | 5 (3-6) | 4 (3-5) | 4 (3-5) | 7 (5-9) | 0.000 |
| Blood culture | 312 (92.9%) | 97 (90.7% | 98 (92.5%) | 117 (95.1%) | 0.414 |
| Positive blood culture | 93 (29.8%) | 22 (22.7%) | 31 (31.6%) | 40 (34.2%) | 0.167 |
| G- bacteria | 76 (81.7%) | 20 (90.9%) | 25 (80.6%) | 31 (77.5%) | 0.418 |
| G+ bacteria | 11 (11.8%) | 0 | 4 (12.9%) | 7 (17.5%) | |
| Other bacteria | 6 (6.5%) | 2 (9.1% | 2 (6.5%) | 2 (5.0%) | 0.539 |
| Mortality in 28 d | 8 (2.4%) | 0 | 0 | 8 (6.5%) | |
After comparing the three groups, WBC counts, CRP, PCT, presepsin, T-Bil, and SOFA scores of patients with moderate and severe cholangitis were found to be higher than those of patients with mild cholangitis (P = 0.000; Table 1). Multiple comparison analysis was used to check the differences between particular pairs of groups. Presepsin had the lowest P-value in patients with mild-to-moderate and moderate-to-severe acute cholangitis (P = 0.000) (Figure 2B). The AUC of presepsin in predicting moderate acute cholangitis was 0.728, prominently higher than that of CRP (0.631, P = 0.043) and PCT (0.585, P = 0.002). No significant differences in the AUC of T-Bil (0.686, P = 0.361) and WBC counts (0.746, P = 0.713) were observed after the Delong test. The optimal cutoff value of presepsin for grading moderate acute cholangitis was 1490 pg/mL (sensitivity, 0.84; specificity, 0.56) (Figure 3A). The AUC of presepsin in predicting severe acute cholangitis was 0.715, which was higher than that of WBC count (0.571, P = 0.008), CRP (0.590, P = 0.009), PCT (0.618, P= 0.024), and T-Bil (0.559, P = 0.006). The optimal cutoff value of presepsin for grading severe acute cholangitis was 2653 pg/mL (sensitivity, 0.64; specificity, 0.78) (Figure 3B). The correlation between WBC counts, CRP, PCT, presepsin, T-Bil, SOFA scores, and TG18 grade revealed that WBC counts, CRP, presepsin, and SOFA scores were correlated with TG18 grade (P = 0.000), and the correlation coefficient of presepsin was the highest among WBC counts, CRP, PCT presepsin, and T-Bil (r = 0.324); presepsin was also correlated with SOFA scores (Table 2). The Kappa value of presepsin > 1490 pg/mL with TG18 in distinguishing moderate acute cholangitis was 0.508 (P = 0.000) by symmetric measures. The Kappa value of presepsin > 2653 pg/mL with TG18 in distinguishing severe acute cholangitis was 0.357 (P = 0.000) by symmetric measures.
| Correlation | TG18 grade | SOFA scores | ||
| Correlation coefficient | P value | Correlation coefficient | P value | |
| WBCs | 0.232 | 0.000 | 0.713 | 0.001 |
| CRP | 0.285 | 0.000 | 0.319 | 0.000 |
| PCT | 0.041 | 0.453 | 0.127 | 0.020 |
| Presespin | 0.324 | 0.000 | 0.384 | 0.000 |
| T-Bil | 0.057 | 0.301 | 0.173 | 0.001 |
| SOFA | 0.555 | 0.000 | ||
WBC counts, PCT, and presepsin levels in the positive blood culture group were greater than those in the negative blood culture group (P < 0.01); however, CRP and T-Bil levels did not show significant differences between the groups (P > 0.05). The ROC curves of biomarkers showed that presepsin had the highest AUC (0.688). This could indicate a positive blood culture when the presepsin level was > 2163 pg/mL (sensitivity, 0.78; specificity, 0.59) (Figures 2C and 3C).
For a long time, acute cholangitis has been diagnosed based on Charcot’s triad; however, its misdiagnosis rate can be as high as 30%–80%[10-12]. With the introdu
A SOFA score ≥ 2 is the diagnostic criterion for sepsis in the new sepsis 3.0 definitions, which show the number and severity of organ dysfunction[20]. As a biomarker of infection, presepsin was superior to CRP and PCT in predicting infection disease[21] and risk stratification[22]. In this study, presepsin levels in patients with acute cholangitis on admission were closely related to TG18 and SOFA scores. It implied that presepsin levels were associated with the number and severity of organ dysfunction during acute cholangitis. Some studies proposed PCT to evaluate the severity of acute cholangitis[23,24]. However, these studies used very strict inclusion criteria (for example, excluding patients who had received antibiotics or nonsteroidal drug before enrolment, or history of a percutaneous bile duct in situ or choledochojejunostomy, or a lower platelet or WBC count than normal). Some patients with cholangitis may take antipyretics or antibiotics before seeing a doctor. This may increase the limitations of the above research. This study included various conditions of cholangitis, and the results showed that presepsin had the best predictive value for determining the severity of cholangitis (P < 0.01). This suggests that presepsin levels could help determine the severity of acute cholangitis to some extent.
Most acute cholangitis cases are associated with intestinal bacterial infection and elevated bile acid and bilirubin levels, which play critical roles in innate immune function activation[25] and improper cytokine or antigen secretion in mononuclear/ Kupffer cells[26]. CRP and PCT levels are indirect acute-phase biomarkers of host-pathogen systemic responses because their syntheses require interleukin-1 and interleukin-6, secreted by monocytes/macrophages; however, their synthesis is suppressed in patients with acute cholangitis[26]. Presepsin is an infection-specific biomarker for innate immune responses that can directly cleave from the monocyte/macrophage-specific CD14 receptor complex once microbes or lipopolysaccharides invade the bloodstream of an organism[6,27]. In patients with acute cholangitis, presepsin may be excessively secreted in the peripheral blood because of monocyte endocytosis, which reduces the abundance of CD14 on the cell membrane[28].
In this study, the overall 28-d mortality rate was 2.4%, which was lower than the reported mortality rate of 2.7%–10% for acute severe cholangitis[1,3]. This may be related to the aggressive and comprehensive treatments as well as shorter observation times. Some studies showed that presepsin levels have significant prognostic value for predicting mortality risk in patients with sepsis, sepsis-related diseases, or bacterial infections[17,29,30]. Presepsin level in the death group was higher than that in the survival group in this study (P < 0.01). Only eight patients lost their lives in 28 d, and all of them were in the severe cholangitis group; therefore, statistical analysis of presepsin for the prognostic assessment was not conducted between the survival and death groups. A dataset with a larger number of deaths may help understand the importance of measuring presepsin levels on admission as it may help clinicians determine the risk of death in patients with cholangitis early.
Blood cultures are the gold standard for diagnosing bloodstream infections, especially when a serious infection is suspected; however, blood cultures are often negative, and the results usually arrive after several days[31]. In this study, presepsin was as effective as PCT in predicting positive bacterial cultures. Presepsin levels can be determined much sooner than blood culture, facilitating early risk stratification and treatment to some extent.
Some limitations should be recognized when interpreting the findings of this study. First, the study was performed at a single hospital using a relatively small sample size; furthermore, there could be some bias in terms of the enrolled patients. Thus, multicenter and large-sample studies should be conducted in the future. Second, the etiology and pathogens that were not statistically analyzed could interfere with the presepsin levels in our patients. Third, the duration of illness and the effect of previous treatment/medications were not considered when patients were enrolled, which could have affected the biomarkers.
In conclusion, presepsin levels are correlated with TG18 grade and SOFA scores in patients with acute cholangitis. Presepsin can help predict the positive blood culture, and is also superior to WBC count, CRP, PCT, and T-Bil in the risk stratification of acute cholangitis. Timely determination of presepsin levels can help clinicians identify the severity of patients with acute cholangitis early.
Acute cholangitis is an acute inflammatory reaction caused by bacterial infection in the biliary tract. It progresses rapidly and can develop into sepsis or septic shock. The grade of cholangitis can guide clinical treatment and predict the prognosis of patients. Acute cholangitis is currently graded based on age, temperature, white blood cell (WBC) count, total bilirubin (T-Bil) levels, hypoalbuminemia, and organ/system dysfunction.
Appropriate use of biliary drainage and antibiotics has observably reduced the mortality rate for acute cholangitis. However, the Tokyo Guidelines 2018 (TG18): Diagnostic Criteria and Severity Grading for Acute Cholangitis is a comprehensive criterion and is cumbersome to use in the emergency department.
Presepsin is a promising biomarker for the early diagnosis, severity, and prognosis of acute bacterial infections. To simplify grading, we wanted to evaluate the grading value of presepsin in patients with acute cholangitis.
Clinical observational trials were conducted in Beijing Friendship Hospital from 2019 to 2020. Whole blood was collected from patients with acute cholangitis for measuring WBC count, C-reactive protein (CRP), procalcitonin (PCT), presepsin, T-Bil and other clinical biochemical indices. Presepsin levels were measured using the PATHFAST analyzer.
A total of 336 patients were divided into mild, moderate, and severe groups based on the TG18. The area under the receiver operating characteristic curve (AUC) of presepsin in predicting moderate acute cholangitis was 0.728, prominently higher than that of CRP and PCT. The AUC of presepsin in predicting severe acute cholangitis was 0.715, which was higher than that of WBC count, CRP, PCT, and T-Bil. The WBC count, PCT, and presepsin levels in the positive blood culture group were greater than those in the negative blood culture group, and presepsin had the highest AUC.
Presepsin can help predict the positive blood culture, and is also superior to WBC count, CRP, PCT, and T-Bil in the risk stratification of acute cholangitis.
Timely determination of presepsin levels can help clinicians identify the severity of patients with acute cholangitis early. Presepsin may be an ideal biomarker for simplified grading of acute cholangitis.
We deeply appreciate the emergency staff, gastroenterologists, hepatobiliary surgeons, radiologists, sonographers, and interventional physicians for their assistance, as well as Na Zeng for biostatistical service. Authors are responsible for obtaining written permission to use any copyrighted text and illustrations.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Emergency medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Imaoka H S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Kimura Y, Takada T, Kawarada Y, Nimura Y, Hirata K, Sekimoto M, Yoshida M, Mayumi T, Wada K, Miura F, Yasuda H, Yamashita Y, Nagino M, Hirota M, Tanaka A, Tsuyuguchi T, Strasberg SM, Gadacz TR. Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:15-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 302] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 2. | Kimmings AN, van Deventer SJ, Rauws EAJ, Huibregtse K, Gouma DJ. Systemic inflammatory response in acute cholangitis and after subsequent treatment. Eur J Surg. 2000;166:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Welch JP, Donaldson GA. The urgency of diagnosis and surgical treatment of acute suppurative cholangitis. Am J Surg. 1976;131:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Huang WS, Kim MH, Okamoto K, Belli G, Dervenis C, Chan ACW, Lau WY, Endo I, Gomi H, Yoshida M, Mayumi T, Baron TH, de Santibañes E, Teoh AYB, Hwang TL, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Higuchi R, Kitano S, Inomata M, Deziel DJ, Jonas E, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 425] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 5. | Lin J, Sun H, Li J, Zheng Y, Shao C, Zhang YH, Chang H. Role of Presepsin for the Assessment of Acute Cholangitis Severity. Clin Lab. 2016;62:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Arai Y, Mizugishi K, Nonomura K, Naitoh K, Takaori-Kondo A, Yamashita K. Phagocytosis by human monocytes is required for the secretion of presepsin. J Infect Chemother. 2015;21:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Liu B, Yin Q, Chen YX, Zhao YZ, Li CS. Role of Presepsin (sCD14-ST) and the CURB65 scoring system in predicting severity and outcome of community-acquired pneumonia in an emergency department. Respir Med. 2014;108:1204-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Tavaré A, O'Flynn N. Recognition, diagnosis, and early management of sepsis: NICE guideline. Br J Gen Pract. 2017;67:185-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Novelli S, Morabito V, Ruberto F, Bini F, Marinozzi F, Pugliese F, Berloco P, Pretagostini R. Diagnostic Value of Presepsin for Bacterial Infection in Cirrhosis: A Pilot Study. Transplant Proc. 2020;52:1593-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Kiriyama S, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Yokoe M, Kimura Y, Tsuyuguchi T, Itoi T, Yoshida M, Miura F, Yamashita Y, Okamoto K, Gabata T, Hata J, Higuchi R, Windsor JA, Bornman PC, Fan ST, Singh H, de Santibanes E, Gomi H, Kusachi S, Murata A, Chen XP, Jagannath P, Lee S, Padbury R, Chen MF; Tokyo Guidelines Revision Committee. New diagnostic criteria and severity assessment of acute cholangitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci. 2012;19:548-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Kiriyama S, Takada T, Hwang TL, Akazawa K, Miura F, Gomi H, Mori R, Endo I, Itoi T, Yokoe M, Chen MF, Jan YY, Ker CG, Wang HP, Wada K, Yamaue H, Miyazaki M, Yamamoto M. Clinical application and verification of the TG13 diagnostic and severity grading criteria for acute cholangitis: an international multicenter observational study. J Hepatobiliary Pancreat Sci. 2017;24:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | O'Connor MJ, Schwartz ML, McQuarrie DG, Sumer HW. Acute bacterial cholangitis: an analysis of clinical manifestation. Arch Surg. 1982;117:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Gravito-Soares E, Gravito-Soares M, Gomes D, Almeida N, Tomé L. Clinical applicability of Tokyo guidelines 2018/2013 in diagnosis and severity evaluation of acute cholangitis and determination of a new severity model. Scand J Gastroenterol. 2018;53:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Benoist JF, Mimoz O, Assicot M. [Procalcitonin in severe trauma]. Ann Biol Clin (Paris). 1998;56:571-574. [PubMed] |
| 15. | Hatherill M, Jones G, Lim E, Tibby SM, Murdoch IA. Procalcitonin aids diagnosis of adrenocortical failure. Lancet. 1997;350:1749-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Baumgartner A, Schuetz P. [Laboratory results in clinical practice: importance of interpretation in the clinical context]. Ther Umsch. 2015;72:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Zhao J, Tan Y, Wang L, Shi Y. Discriminatory ability and prognostic evaluation of presepsin for sepsis-related acute respiratory distress syndrome. Sci Rep. 2020;10:9114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Venugopalan DP, Pillai G, Krishnan S. Diagnostic Value and Prognostic Use of Presepsin Versus Procalcitonin in Sepsis. Cureus. 2019;11:e5151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Unuma K, Makino Y, Sasaki Y, Iwase H, Uemura K. Presepsin: A potential superior diagnostic biomarker for the postmortem differentiation of sepsis based on the Sepsis-3 criteria. Forensic Sci Int. 2019;299:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Aliu-Bejta A, Atelj A, Kurshumliu M, Dreshaj S, Baršić B. Presepsin values as markers of severity of sepsis. Int J Infect Dis. 2020;95:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Yao S, Kaido T, Uozumi R, Hirata M, Iwamura S, Miyachi Y, Macshut M, Sharshar M, Yagi S, Uemoto S. Diagnostic potential of presepsin in bacterial infection following hepato-biliary-pancreatic surgery: A prospective observational study. J Hepatobiliary Pancreat Sci. 2020;27:756-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Chen M, Zhu Y. Utility of sTREM-1 and Presepsin (sCD14-ST) as Diagnostic and Prognostic Markers of Sepsis. Clin Lab. 2020;66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Liu R, Li X, Huang Z, Zhao D, Ganesh BS, Lai G, Pandak WM, Hylemon PB, Bajaj JS, Sanyal AJ, Zhou H. C/EBP homologous protein-induced loss of intestinal epithelial stemness contributes to bile duct ligation-induced cholestatic liver injury in mice. Hepatology. 2018;67:1441-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Scott-Conner CE, Grogan JB. The pathophysiology of biliary obstruction and its effect on phagocytic and immune function. J Surg Res. 1994;57:316-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Chenevier-Gobeaux C, Borderie D, Weiss N, Mallet-Coste T, Claessens YE. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta. 2015;450:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 26. | Li J, Tang Z, Xie M, Hang C, Yu Y, Li C. Association between elevation of plasma biomarkers and monocyte dysfunction and their combination in predicting sepsis: An observational single-centre cohort study. Innate Immun. 2020;26:514-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Drăgoescu AN, Pădureanu V, Stănculescu AD, Chiuțu LC, Florescu DN, Gheonea IA, Pădureanu R, Stepan A, Streba CT, Drocaș AI, Ciocâlteu-Ionescu AM, Șurlin VM, Drăgoescu OP. Presepsin as a Potential Prognostic Marker for Sepsis According to Actual Practice Guidelines. J Pers Med. 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Ferrarese A, Frigo AC, Mion MM, Plebani M, Russo FP, Germani G, Gambato M, Cillo U, Cattelan A, Burra P, Senzolo M. Diagnostic and prognostic role of presepsin in patients with cirrhosis and bacterial infection. Clin Chem Lab Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Zadroga R, Williams DN, Gottschall R, Hanson K, Nordberg V, Deike M, Kuskowski M, Carlson L, Nicolau DP, Sutherland C, Hansen GT. Comparison of 2 blood culture media shows significant differences in bacterial recovery for patients on antimicrobial therapy. Clin Infect Dis. 2013;56:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Webb AL, Kramer N, Stead TG, Mangal R, Lebowitz D, Dub L, Rosario J, Tak M, Reddy S, Lee JR, Adams J, Banerjee PR, Wallen M, Ganti L. Serum Procalcitonin Level Is Associated with Positive Blood Cultures, In-hospital Mortality, and Septic Shock in Emergency Department Sepsis Patients. Cureus. 2020;12:e7812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Yu Y, Li XX, Jiang LX, Du M, Liu ZG, Cen ZR, Wang H, Guo ZH, Chang P. Procalcitonin levels in patients with positive blood culture, positive body fluid culture, sepsis, and severe sepsis: a cross-sectional study. Infect Dis (Lond). 2016;48:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |