Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9815
Peer-review started: June 27, 2021
First decision: July 14, 2021
Revised: July 19, 2021
Accepted: September 1, 2021
Article in press: September 1, 2021
Published online: November 16, 2021
Processing time: 135 Days and 13.4 Hours
Helicobacter pylori (H. pylori) has been found to be associated with extragastroin
To explore the relationship between H. pylori infection and food-specific IgG
We retrospectively analyzed the physical examination data of 21822 subjects from February 2014 to December 2018 in this study. H. pylori infection was detected using the 13C urea breath test. Food-specific IgG of eggs, milk and wheat in serum was assessed. Subjects were grouped according to H. pylori positivity, and the positive rates of three kinds of food-specific IgG were compared between the two groups. Multivariable logistic regression analysis was performed to elucidate the association between H. pylori infection and food-specific IgG.
The total infection rate of H. pylori was 39.3%, and the total food-specific IgG-positive rates of eggs, milk and wheat were 25.2%, 9.0% and 4.9%, respectively. The infection rate of H. pylori was higher in males than in females, while the positive rates of food-specific IgG were lower in males than in females. The positive rates of food-specific IgG decreased with age in both males and females. In the H. pylori-positive groups, the positive rates of food-specific IgG of eggs, milk and wheat were all lower than those in the H. pylori-negative groups. Multivariate logistic regression analysis revealed that H. pylori infection was negatively correlated with the food-specific IgG-positive rates of eggs, milk and wheat (odds ratio value of eggs 0.844-0.873, milk 0.741-0.751 and wheat 0.755-0.788, in different models).
H. pylori infection was found to be negatively associated with the food-specific IgG of eggs, milk and wheat in Southwest China.
Core Tip: This is a retrospective study to evaluate the association of Helicobacter pylori (H. pylori) infection and food-specific immunoglobulin G. We analyzed the data of 21822 subjects who underwent H. pylori infection assessment by the urea breath test and testing for food-specific immunoglobulin G of eggs, milk and wheat. The key finding was that H. pylori infection was associated with lower positivity for food-specific immunoglobulin G. If the negative correlation could be further confirmed and the mechanism could be clarified, it would provide some advisable suggestions for medical decisions regarding asymptomatic H. pylori infection.
- Citation: Liu Y, Shuai P, Liu YP, Li DY. Association between Helicobacter pylori infection and food-specific immunoglobulin G in Southwest China. World J Clin Cases 2021; 9(32): 9815-9824
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9815.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9815
Helicobacter pylori (H. pylori) infection, which is considered a major pathogenic factor in chronic gastritis, gastric ulcer and gastric cancer, is an important public health issue worldwide[1]. However, growing evidence suggests that H. pylori infection affects not only the gastrointestinal tract but also extragastrointestinal function, which has become a research hotspot. In contrast to the traditional view that H. pylori is a risk factor for disease, some studies have found a negative correlation between H. pylori infection and the development of certain diseases. For example, H. pylori infection showed a negative correlation with the development of some allergic diseases, such as asthma and eosinophilic esophagitis, especially in children and young people with early allergic reactions[2].
Notably, the relationship between food and chronic diseases has received increasing attention. Specific epitopes of food can be used as specific antigens to induce the immune response of the body, thus producing food-specific antibodies. Food allergy related to the classic pathway, which can be mediated by food-specific immunoglobulin (Ig)E, is well known by scholars. Few studies have researched the relationship between H. pylori infection and food allergy, and the results remain controversial[3]. In recent years, the correlation between food-specific IgG and a variety of allergic diseases or symptoms has attracted the attention of scholars and has been found to be related to irritable bowel syndrome[4], inflammatory bowel disease[5], eosinophilic esophagitis[6] and other autoimmune diseases[7]. The role of food-specific IgG in food allergy has also been discussed, and its application value in non-IgE-mediated detection of food adverse reactions has been affirmed by international authoritative guidelines[8].
Food intolerance is another common adverse food reaction. Although the pathogenesis of food intolerance is not directly related to immunity, some scholars indicate increased gut permeability in patients with food intolerance, which permits food substances to gain access to the circulation and trigger food-specific IgG production; thus, a correlation may also exist between food intolerance and food-specific IgG. Fewer studies have directly discussed the relationship between H. pylori infection and food intolerance. A study of 12765 people in North China by Sai et al[9] suggested that crab intolerance may be related to H. pylori infection.
In China, adverse reactions to food may be affected by various socioeconomic factors, eating habits, food types, geographical climates and so on[10]. Our study focused on food types and serum food-specific IgG. The three types of food—egg, milk and wheat—are widely consumed in Southwest China, where there is a relatively high positive rate of serum food-specific IgG. In this study, we used these three foods to explore the association between H. pylori infection and serum food-specific IgG in Southwest China.
The physical examination data of the subjects were obtained from the Health Management Center, Sichuan Provincial People’s Hospital (Chengdu, Sichuan Province). All the subjects completed the medical history questionnaire. Physical examinations, which included height, body weight and blood pressure, were performed by trained nurses. All subjects underwent laboratory examinations (routine blood tests and measurement of alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transpeptidase, serum creatinine, fasting blood glucose, hemoglobin A1c, total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and uric acid), abdominal ultrasonography, chest imaging (X-ray or computed tomography), 13C urea breath tests and testing for food-specific IgG of eggs, milk and wheat.
Subjects were excluded if they had: (1) A history of gastrectomy or subtotal gastrectomy; (2) Organic diseases that have been identified to affect gastrointestinal digestion and absorption; (3) An inability to perform the 13C urea breath tests due to pregnancy, lactation or other reasons; (4) Immune system diseases, severe heart, liver or kidney dysfunction or tumors; or (5) A history of anti-H. pylori therapy in the past 6 mo.
All methods were carried out based on relevant guidelines and regulations. Ethics approval was obtained from the Ethical Committee of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital. Approval No. 408(2020).
H. pylori infection was detected using 13C urea breath testing (Beijing Boran Pharmaceutical Co., Ltd. Beijing, China), according to the recommendation of the Fifth Chinese National Consensus Report on the management of H. pylori infection[11]. All subjects fasted overnight for more than 8 h, maintained normal breathing, inserted a straw into the bottom of one sample tube, and exhaled slowly into the sample tube through the straw for 4 to 5 s. Thereafter, they pulled the straw out and tightened the cap immediately; this was considered a sample of zero points. Then, the subjects took another bottle with urea 13C granules and 80 mL to 100 mL cold drinking water, rested for 30 min, and then collected another breath sample. The two collected gas samples were tested for 13CO2, and δ‰ was used to represent the result: δ‰ = (isotopic abundance of 13C for the test sample - isotopic abundance of 13C for the reference sample) × 1000/isotopic abundance of 13C for the reference sample. The detection value was defined as the δ‰ measured at 30 min subtracted from that measured at 0 min. H. pylori infection was considered positive when the detection value was ≥ 4.0.
A food-specific IgG screening enzyme-linked immunosorbent assay kit (HOB Biotech Co., Ltd. Jiangsu, China) was used. Serum samples were collected from the subjects, the amount used was 5 μL, and the test was carried out according to the operation manual. A blank well was used to calibrate the zero value of the enzyme analyzer [Thermo Fisher Scientific (China) Co., Ltd. Shanghai, China] at a wavelength of 450 nm, and the absorbance value Y of each tested sample was read. The standardized activity value X (U/mL) was obtained with the formula Y = AX3 + BX2 + CX + D calculated from the standard curve. An activity value of X ≥ 50 U/mL was defined as food-specific IgG positive.
Statistical analysis was performed using IBM SPSS 21.0 (IBM Corp., Armonk, NY, United States). Continuous data were expressed as the mean ± standard deviation for normally distributed data or the median with 25th and 75th percentiles for non-normally distributed data. Categorical data were described as percentages. Student’s t-test was used to analyze continuous variables, and the χ2 test was used to analyze categorical variables. Univariable and multivariable regression models were performed using logistic regression analysis to identify the association between H. pylori infection and food-specific IgG. Various covariates, such as age, sex, body mass index, hemoglobin A1c, total cholesterol, triglycerides, alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transpeptidase, serum creatinine, uric acid, blood pressure, smoking and drinking, were used to adjust the confounding factors, with the results expressed as odds ratios (ORs) and 95% confidence intervals. A P value < 0.05 was considered statistically significant.
The demographic and laboratory baseline characteristics of 21822 subjects (12396 males and 9426 females) are shown in Table 1. The average age was 43.82 ± 10.98 years (range: 18-89 years). The total infection rate of H. pylori was 39.3%, and the food-specific IgG-positive rates of eggs, milk and wheat were 25.2%, 9.0% and 4.9%, respectively. The infection rate of H. pylori was higher in males than in females (39.9% vs 38.6%, P = 0.043). The food-specific IgG-positive rates of the three foods in males were all significantly lower than those in females (20.4% vs 31.5% for eggs, 7.9% vs 10.5% for milk and 4.0% vs 6.2% for wheat, all P < 0.001).
| Variables | Total, n = 21822 | H. pylori negative, n = 13239 | H. pylori positive, n = 8583 | P value | |
| Demographic data | |||||
| Sex (female), n (%) | 9426 (43.2) | 5791 (43.7) | 3635 (42.4) | 0.043 | |
| Age (yr) | 43.82 ± 10.98 | 43.49 ± 11.10 | 44.32 ± 10.76 | < 0.001 | |
| Drinking, n (%) | 2295 (10.5) | 1304 (9.8) | 991 (11.5) | < 0.001 | |
| Smoking, n (%) | 4578 (21.0) | 2661 (20.1) | 1917 (22.3) | < 0.001 | |
| Anthropometric data | |||||
| Body weight (kg) | 64.08 ± 12.02 | 63.52 ± 11.83 | 64.94 ± 12.26 | < 0.001 | |
| Height (cm) | 163.65 ± 8.23 | 163.48 ± 8.26 | 163.90 ± 8.18 | < 0.001 | |
| BMI (kg/m2) | 23.81 ± 3.37 | 23.65 ± 3.33 | 24.05 ± 3.41 | < 0.001 | |
| SBP (mmHg) | 117.43 ± 17.08 | 117.28 ± 16.92 | 117.67 ± 17.31 | 0.099 | |
| DBP (mmHg) | 72.86 ± 11.39 | 72.76 ± 11.24 | 73.01 ± 11.62 | 0.109 | |
| Laboratory data | |||||
| ALT (U/L) | 23 (16, 36) | 23 (16, 36) | 24 (16, 38) | < 0.001 | |
| AST (U/L) | 27.20 ± 19.03 | 27.26 ± 21.89 | 27.10 ± 13.47 | 0.530 | |
| GGT (U/L) | 24 (15, 42) | 23 (15, 41) | 24 (15, 45) | < 0.001 | |
| Creatinine (μmol/L) | 67.24 ± 21.41 | 67.01 ± 23.54 | 67.58 ± 17.62 | 0.058 | |
| Fasting glucose (mmol/L) | 5.11 ± 1.33 | 5.07 ± 1.24 | 5.16 ± 1.46 | < 0.001 | |
| HbA1c (%) | 5.54 ± 0.79 | 5.51 ± 0.74 | 5.58 ± 0.87 | < 0.001 | |
| Total cholesterol (mmol/L) | 4.86 ± 0.95 | 4.84 ± 0.94 | 4.89 ± 0.96 | < 0.001 | |
| Triglycerides (mmol/L) | 1.38 (0.95, 2.08) | 1.67 (1.15, 2.45) | 1.09 (0.80, 1.56) | < 0.001 | |
| LDL-C (mmol/L) | 2.87 ± 0.81 | 2.86 ± 0.81 | 2.90 ± 0.83 | < 0.001 | |
| HDL-C (mmol/L) | 1.33 ± 0.33 | 1.33 ± 0.33 | 1.31 ± 0.33 | < 0.001 | |
| Uric acid (μmol/L) | 345.40 ± 90.58 | 343.64 ± 90.40 | 348.11 ± 90.80 | < 0.001 | |
The subjects were further stratified by age to investigate the prevalence of H. pylori infection and positive rates of food-specific IgG. The results revealed that the positive rates of the three food-specific IgG antibodies all decreased with age in both males and females (Table 2).
| Age in yr | Number | H. pylori infection, n (%) | Food-specific IgG positivity, n (%) | ||
| Egg | Milk | Wheat | |||
| Male | |||||
| 18-29 | 903 | 296 (32.8) | 409 (45.3) | 188 (20.8) | 126 (14.0) |
| 30-39 | 3258 | 1292 (39.7) | 858 (26.3) | 350 (10.7) | 167 (5.1) |
| 40-49 | 4714 | 1835 (38.9) | 746 (15.8) | 256 (5.4) | 126 (2.7) |
| ≥ 50 | 3521 | 1525 (43.3) | 512 (14.5) | 188 (5.3) | 71 (2.0) |
| Total | 12396 | 4948 (39.9) | 2525 (20.4) | 982 (7.9) | 490 (4.0) |
| Linear by linear association value | 26.981 | 436.396 | 260.529 | 212.714 | |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Female | |||||
| 18-29 | 978 | 311 (31.8) | 535 (54.7) | 199 (20.3) | 100 (10.2) |
| 30-39 | 2747 | 1031 (37.5) | 1102 (40.1) | 361 (13.1) | 197 (7.2) |
| 40-49 | 3263 | 1355 (41.5) | 795 (24.4) | 250 (7.7) | 198 (6.1) |
| ≥ 50 | 1587 | 938 (38.5) | 535 (21.9) | 181 (7.4) | 91 (3.7) |
| Total | 9426 | 3635 (38.6) | 2967 (31.5) | 991 (10.5) | 586 (6.2) |
| Linear by linear association value | 12.391 | 462.821 | 143.539 | 54.716 | |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
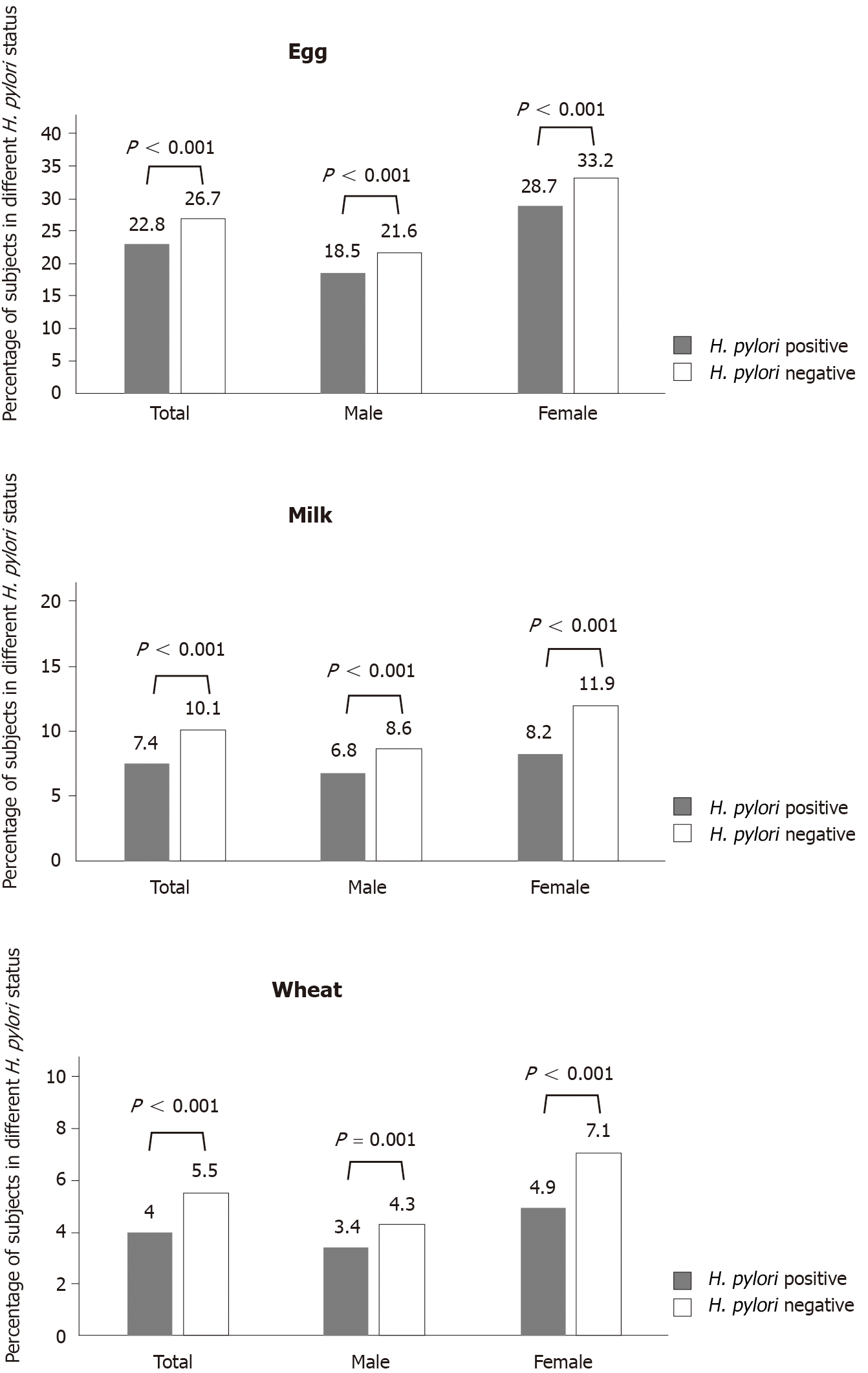
Whether in the general population or between sexes, the positive rates of the three food-specific IgG antibodies in the H. pylori-positive group were all significantly lower than those in the H. pylori-negative group (22.8% vs 26.7% for eggs, 7.4% vs 10.1% for milk and 3.9% vs 5.3% for wheat) (Figure 1).
Logistic regression analysis was performed to explore the independent association between H. pylori infection and food-specific IgG. In univariate analysis, the results revealed that H. pylori infection was associated with a lower risk of food-specific IgG (OR = 0.814, P < 0.001 for eggs; OR = 0.714, P < 0.001 for milk; and OR = 0.720, P < 0.001 for wheat). After adjusting for confounding factors in different models, the results remained significant (OR value of egg 0.844-0.873, milk 0.741-0.751 and wheat 0.755-0.788, P < 0.001) (Table 3).
| Non-adjusted | Model 1 | Model 2 | Model 3 | |
| Eggs | 0.814 (0.764-0.867) | 0.844 (0.791-0.901) | 0.873 (0.812-0.938) | 0.871 (0.810-0.936) |
| Milk | 0.714 (0.647-0.788) | 0.741 (0.671-0.818) | 0.751 (0.669-0.842) | 0.746 (0.665-0.838) |
| Wheat | 0.720 (0.631-0.820) | 0.755 (0.662-0.861) | 0.787 (0.681-0.909) | 0.788 (0.682-0.910) |
The infection rate of H. pylori is high worldwide and is 50% in China[12]. However, compared with the high H. pylori infection rate, only 15%-20% of infected subjects have peptic ulcers, 5%-10% have H. pylori-related dyspepsia, and approximately 1% have gastric cancer, mucosa-associated lymphoid tissue lymphoma and other gastric malignant tumors[13-15]. Most of the infected subjects are asymptomatic and do not receive drug treatment. Scholars have focused on exploring the chronic process in such a large number of asymptomatic carriers. Moreover, the influence of H. pylori infection is not limited to the gastrointestinal tract itself. In 1994, Mendall et al[16] first reported the relationship between H. pylori infection and extragastric diseases. Subsequently, neurological, cardiovascular, hematologic, dermatological, ocular, metabolic and allergic diseases were found to be associated with H. pylori infection[17]. Immune mechanisms may play an important role in the relationship between H. pylori infection and extragastrointestinal diseases[18]. In consideration of the high H. pylori infection rate, the relationship between H. pylori and many other extragastrointestinal diseases cannot be ignored.
Over the years, adverse food reactions, which can be classified as food allergy or intolerance, have been increasing and have received more attention. Immune factors are very important in the pathogenesis of adverse food reactions. As an immune-based disease, food allergy is estimated to affect 5% of children under the age of 5 years and 4% of teens and adults in the United States[19]. Classic food allergy is usually identified as IgE-mediated immediate hypersensitivity reactions. However, with the development of research, delayed non-IgE-mediated reactions have also been included in the mechanism of food allergy[20]. IgG is the immunoglobulin with the highest serum content, accounting for 70%-75%; IgG can be divided into IgG1, IgG2, IgG3 and IgG4 subtypes, and the normal body content is approximately 66%, 23%, 7% and 4%, respectively[21]. Unlike IgE-mediated type I hypersensitivity (immediate hypersensitivity), IgG is mainly involved in type II (cytotoxic hypersensitivity) and type III hypersensitivity (immune complex-mediated hypersensitivity)[22]. The immune system can identify certain food molecules as harmful substances and produce an excessive protective immune response against these substances, generating food-specific IgG. Through antigen-antibody reactions, IgG antibodies form circulating immune complexes with food particles that are deposited in various organs or systems via blood circulation. Therefore, food-specific-IgG may participate in the mechanism of non-IgE-mediated adverse food reactions, which is related to food allergy[8].
Food intolerance is another common chronic disease with many extragas
To date, studies on the relationship between food allergy or intolerance and H. pylori infection in large samples are limited. In our study, we analyzed the physical examination data of more than 20000 subjects. We selected eggs, milk and wheat as the research objects, as they are commonly consumed in Southwest China where there is a relatively high positive rate of food-specific IgG. The results suggested that H. pylori infection seemed to help the body achieve a lower rate of food-specific IgG positivity. Interestingly, our results were in contrast to those of a similar study in China[9]. The differences might be related to the sample size, food types and geographical differences, which need to be further studied.
Previous studies have found that H. pylori infection affects immune regulation so that H. pylori can avoid immune surveillance to establish long-term colonization. This may also be the cause of its association with some extragastrointestinal diseases[25]. For example, asthma has been reported to be inversely associated with H. pylori infection. The protective effects of H. pylori depend on Foxp3+ regulatory T cells[26]. Regulatory T cells are a potently immunosuppressive CD4+ T cell subset and play a key role in immune tolerance by controlling the extent of the response to self- and non-self-antigens. These cells can promote the rapid recovery of immune homeostasis[27]. H. pylori also upregulates the expression of CD80 and interleukin 10 via toll-like receptors on B lymphocytes and then promotes regulatory T cell differentiation[28]. Idiopathic thrombocytopenic purpura, an autoimmune disorder, was found to be associated with H. pylori infection in 1999[29]. One of the mechanisms involves an enhanced phagocytic capacity and low levels of inhibitory FcγRIIB in monocytes from H. pylori-infected patients, leading to increased monocyte autoreactivity with B and T lymphocytes. This may cause B lymphocytes to produce autoantibodies against circulating platelets[18]. Therefore, H. pylori may be related to some extragas
A limitation of our study was that the subjects were from the health examination population rather than from a random sampling of the community, which led to sample deviation. Furthermore, our study lacked sociological data. Previous studies have revealed that the H. pylori infection rate is higher in developing countries[30]. Poor health conditions, low socioeconomic status and associated unhealthy dietary hygiene habits may facilitate exposure to more bacteria or antigens, which will promote immune tolerance to the corresponding antigens in the body and reduce the risk of adverse food reactions. Therefore, the two flowers—higher H. pylori infection rates and lower rates of food-specific IgG positivity—may both grow in the common soil of poor socioeconomic conditions mentioned above. Our study found that there may be a correlation between H. pylori infection and food-specific IgG, and whether there is a causal relationship and the mechanism between them require further study.
H. pylori is considered an important risk factor for gastric ulcer and gastric cancer. Aggressive drug therapy is recommended for patients who meet the indications[31]. However, our study found a negative correlation between H. pylori infection and food-specific IgG, which was not consistent with the commonly held perception of H. pylori. Considering the “beneficial protective effect” of H. pylori in some diseases as well as its high infection rate and the relatively limited proportion of symptomatic infected individuals in a population, some researchers have reassessed the role of such bacteria in the human body and proposed the question of whether H. pylori is a “commensal, symbiont or pathogen”[32]. Our results seem to provide a positive evaluation of H. pylori in discussing this issue and suggest that we need more individualized understanding of the effect of H. pylori on the body’s immunity. Further confirmation of the negative correlation found in our study and clarification of the mechanism in future studies would provide some advisable suggestions for medical decisions.
In conclusion, H. pylori infection was found to be negatively associated with the food-specific IgG of eggs, milk and wheat in Southwest China.
Helicobacter pylori (H. pylori) has been found to be associated with extragastrointestinal diseases, possibly including adverse food reactions (such as food allergy or intolerance). However, there are few studies on H. pylori and food allergy or intolerance, and the results are inconsistent.
Food-specific immunoglobulin (Ig) G has been revealed to be associated with food allergy or intolerance and can be used as a marker to explore the correlation between H. pylori infection and food allergy or intolerance.
To explore the relationship between H. pylori infection and food-specific IgG.
H. pylori infection was detected with the 13C urea breath test. Food-specific IgG of eggs, milk and wheat was detected in serum. Subjects were grouped according to H. pylori positivity, and the positive rates of three kinds of food-specific IgG were compared between the two groups. Multivariable logistic regression analysis was performed to identify the association between H. pylori infection and food-specific IgG.
In the H. pylori-positive groups, the positive rates of food-specific IgG of eggs, milk and wheat were all lower than those in the H. pylori-negative groups. Multivariate logistic regression analysis showed that H. pylori infection was negatively correlated with the food-specific IgG-positive rates of eggs, milk, and wheat.
H. pylori infection was negatively correlated with the food-specific IgG of eggs, milk and wheat in Southwest China.
Our study might reflect only a negative association between H. pylori infection and food-specific IgG rather than causality. Establishing relevant animal models and exploring the underlying mechanism based on immunity or a well-designed clinical intervention study may help to verify our findings. Moreover, finding additional similar “protective” effects in asymptomatic patients with H. pylori infection may help us reassess the role of H. pylori in the body and provide advisable suggestions for medical decisions.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reshetnyak VI, Toyoshima O S-Editor: Chang KL L-Editor: Filipodia P-Editor: Guo X
| 1. | Blaser MJ. Hypothesis: the changing relationships of Helicobacter pylori and humans: implications for health and disease. J Infect Dis. 1999;179:1523-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 210] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 2. | Blaser MJ, Chen Y, Reibman J. Does Helicobacter pylori protect against asthma and allergy? Gut. 2008;57:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Ma ZF, Majid NA, Yamaoka Y, Lee YY. Food Allergy and Helicobacter pylori Infection: A Systematic Review. Front Microbiol. 2016;7:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 344] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Cai C, Shen J, Zhao D, Qiao Y, Xu A, Jin S, Ran Z, Zheng Q. Serological investigation of food specific immunoglobulin G antibodies in patients with inflammatory bowel diseases. PLoS One. 2014;9:e112154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Schuyler AJ, Wilson JM, Tripathi A, Commins SP, Ogbogu PU, Kruzsewski PG, Barnes BH, McGowan EC, Workman LJ, Lidholm J, Rifas-Shiman SL, Oken E, Gold DR, Platts-Mills TAE, Erwin EA. Specific IgG4 antibodies to cow’s milk proteins in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142:139-148.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Coucke F. Food intolerance in patients with manifest autoimmunity. Observational study. Autoimmun Rev. 2018;17:1078-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | NIAID-Sponsored Expert Panel. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 585] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 9. | Sai XY, Zheng YS, Sun YF, Sun J. [A cross-sectional survey of crab intolerance positive rate and its determinants in healthy medical examination population in Beijing]. Zhonghua Yi Xue Za Zhi. 2012;92:1959-1962. [PubMed] |
| 10. | Lomer MC. Review article: the aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment Pharmacol Ther. 2015;41:262-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH; Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 330] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 12. | Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016;8:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 13. | Moayyedi P, Forman D, Braunholtz D, Feltbower R, Crocombe W, Liptrott M, Axon A. The proportion of upper gastrointestinal symptoms in the community associated with Helicobacter pylori, lifestyle factors, and nonsteroidal anti-inflammatory drugs. Leeds HELP Study Group. Am J Gastroenterol. 2000;95:1448-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Sipponen P. Natural history of gastritis and its relationship to peptic ulcer disease. Digestion. 1992;51 Suppl 1:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29:895-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Mendall MA, Goggin PM, Molineaux N, Levy J, Toosy T, Strachan D, Camm AJ, Northfield TC. Relation of Helicobacter pylori infection and coronary heart disease. Br Heart J. 1994;71:437-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 429] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 17. | Franceschi F, Covino M, Roubaud Baudron C. Review: Helicobacter pylori and extragastric diseases. Helicobacter. 2019;24 Suppl 1:e12636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M. Helicobacter pylori and extragastric diseases: A review. World J Gastroenterol. 2018;24:3204-3221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 244] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (6)] |
| 19. | Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, Sundaram V, Paige NM, Towfigh A, Hulley BJ, Shekelle PG. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 20. | Tordesillas L, Berin MC, Sampson HA. Immunology of Food Allergy. Immunity. 2017;47:32-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 21. | Gocki J, Bartuzi Z. Role of immunoglobulin G antibodies in diagnosis of food allergy. Postepy Dermatol Alergol. 2016;33:253-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Rajan TV. The Gell-Coombs classification of hypersensitivity reactions: a re-interpretation. Trends Immunol. 2003;24:376-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Shakoor Z, AlFaifi A, AlAmro B, AlTawil LN, AlOhaly RY. Prevalence of IgG-mediated food intolerance among patients with allergic symptoms. Ann Saudi Med. 2016;36:386-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Tan QH, Li XH. Progress in understanding the relationship between food intolerance and functional gastrointestinal disorders. Shijie Huaren Xiaohua Zazhi. 2013;21:2551-2556. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Lina TT, Alzahrani S, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Immune evasion strategies used by Helicobacter pylori. World J Gastroenterol. 2014;20:12753-12766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
| 26. | Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 27. | Alvarez F, Al-Aubodah TA, Yang YH, Piccirillo CA. Mechanisms of TREG cell adaptation to inflammation. J Leukoc Biol. 2020;108:559-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Reyes VE, Peniche AG. Helicobacter pylori Deregulates T and B Cell Signaling to Trigger Immune Evasion. Curr Top Microbiol Immunol. 2019;421:229-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | García Pérez A, Valverde de La Osa J, Giménez Samper M, Alonso García I. [Resolution of an autoimmune thrombocytopenic purpura after eradicating treatment of Helicobacter pylori]. Sangre (Barc). 1999;44:387-388. [PubMed] |
| 30. | Mandeville KL, Krabshuis J, Ladep NG, Mulder CJ, Quigley EM, Khan SA. Gastroenterology in developing countries: issues and advances. World J Gastroenterol. 2009;15:2839-2854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 1018] [Article Influence: 127.3] [Reference Citation Analysis (1)] |
| 32. | Reshetnyak VI, Burmistrov AI, Maev IV. Helicobacter pylori: Commensal, symbiont or pathogen? World J Gastroenterol. 2021;27:545-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (3)] |