Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9770
Peer-review started: May 25, 2021
First decision: July 3, 2021
Revised: July 13, 2021
Accepted: September 22, 2021
Article in press: September 22, 2021
Published online: November 16, 2021
Processing time: 169 Days and 0.2 Hours
Radiological detection of small liver metastasis or peritoneal metastasis is still difficult, and some patients with biliary tract cancer (BTC) are unresectable after laparotomy. Staging laparoscopy may help avoid unnecessary laparotomy. However, which category of BTC is amenable with staging laparoscopy remains unclear.
To clarify the risk factors for occult metastasis in patients with BTC.
Medical records of patients with BTC who underwent surgery at our institution between January 2008 and June 2014 were retrospectively reviewed. The patients were divided into two groups, according to resection or exploratory laparotomy (EL). Preoperative laboratory data, including inflammation-based prognostic scores and tumor markers, were compared between the two groups. Prognostic importance of detected risk factors was also evaluated.
A total of 236 patients were enrolled in this study. Twenty-six (11%) patients underwent EL. Among the EL patients, there were 16 cases of occult metastasis (7 liver metastases and 9 abdominal disseminations). Serum carcinoembryonic antigen level, carbohydrate antigen 19-9 level, neutrophil-lymphocyte ratio and modified Glasgow prognostic score were significantly higher in the EL group than in the resected group, and these factors were prognostic. Among these factors, carcinoembryonic antigen > 7 ng/mL was the most useful to predict occult metastasis in BTC. When patients have more than three of these positive factors, the rate of occult metastasis increases.
Inflammation-based prognostic scores and tumor markers are useful in detecting occult metastasis in BTC; based on these factors, staging laparoscopy may reduce the rate of EL.
Core Tip: This is a retrospective study to clarify the risk factors for occult metastasis in patients with biliary tract cancer (BTC). Radiological detection of small liver metastasis or peritoneal metastasis is difficult, and 11% BTC patients resulted in exploratory laparotomy in 7 years. Serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 levels, neutrophil-lymphocyte ratio and modified Glasgow prognostic score were significantly higher in the exploratory laparotomy group than in resected group. In these, CEA > 7 ng/mL and a combination of these factors were useful for predicting occult metastasis in BTC. Based on these factors, selective staging laparoscopy may reduce the rate of exploratory laparotomy.
- Citation: Hashimoto Y, Ajiki T, Yanagimoto H, Tsugawa D, Shinozaki K, Toyama H, Kido M, Fukumoto T. Risk factors for occult metastasis detected by inflammation-based prognostic scores and tumor markers in biliary tract cancer. World J Clin Cases 2021; 9(32): 9770-9782
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9770.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9770
Biliary tract cancer (BTC) has a poor prognosis. Curative resection is the most promising therapy, but many patients present with advanced or unresectable disease at the time of diagnosis[1,2]. It is widely accepted that metastases to the liver, peritoneum, distant lymph nodes, lung and bone are definite unresectable factors[3]. Advances in radiological imaging techniques have enabled us to understand the details of BTC preoperatively. Detecting small liver metastasis or peritoneal metastasis remains difficult, and some patients are decided to be unresectable after laparotomy[2,4]. Staging laparoscopy (SL) may help avoid unnecessary laparotomy due to these occult metastases, and it is widely performed in patients with various solid cancer types [5-8].
Inflammation-based prognostic scores and serum carbohydrate antigen 19-9 (CA19-9) level are useful for the selection of SL in pancreatic head and periampullary cancer[9,10]. In BTCs, serum levels of tumor markers, including CA19-9 and carcinoembryonic antigen (CEA), and inflammation-based prognostic scores are associated with prognostic outcomes [11-13]. However, it remains unclear whether tumor markers and inflammation-based prognostic scores are associated with resectability or distant metastases in BTC and whether resection should be performed in certain categories of patients with BTC. This study aimed to elucidate the risk factors for exploratory laparotomy (EL) in patients with BTC using tumor markers and inflammation-based prognostic scores, to estimate the reduction rate of EL due to occult metastases, and to evaluate the prognosis of the risk factors.
This retrospective study included 236 patients with BTC who underwent open surgery between January 2008 and June 2014 at the Kobe University Hospital (Kobe, Hyogo, Japan). All BTC cases were histologically confirmed. For each patient, the following clinical information was collected: Age, sex, body mass index (BMI), location of tumor, operative procedure, prognosis, and preoperative laboratory and imaging findings. The patients were divided the following into two groups: Resected (R) and EL.
This study protocol was approved by the Ethics Committee of the Kobe University Graduate School of Medicine (#160163). Informed consent was obtained using the opt-out principle.
Preoperative laboratory tests and imaging studies including dynamic multidetector row computed tomography (MDCT), endoscopic retrograde cholangiography, magnetic resonance cholangiopancreatography, gadolinium ethoxybenzyl-diethylenetriamine pentaacetic acid contrast-enhanced magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), were performed routinely. Tumor marker, serum CA19-9 and CEA levels were assessed just before operation. Endoscopic biopsy and brushing cytology for pathological diagnosis were also performed. Endoscopic biliary drainage was performed, if necessary.
The patients were diagnosed with unresectable diseases when the imaging study findings showed definite distant metastases, far extended disease, or several locally advanced diseases such as arterial and/or portal vein encasement. Patients with unresectable disease received systemic chemotherapy or best supportive care. In the absence of radiologically unresectable disease, patients underwent open surgery. At the time of laparotomy, we routinely explored abnormalities in the abdominal cavity. EL was selected when we found liver or peritoneal metastasis and macroscopic bulky distant lymph node metastasis. When histological distant lymph node metastases (para-aortic lymph node metastases) were detected in patients with gallbladder cancer, we also performed EL according to a previous study[14]. When surgeons judged locally advanced tumor more than expected at laparotomy, EL was occasionally selected. In the absence of unresectable factors, complete tumor resection with lymphadenectomy was performed.
The neutrophil-lymphocyte ratio (NLR) was defined as the circulating blood neutrophil/lymphocyte count ratio. The platelet-lymphocyte ratio (PLR) was defined as the circulating blood platelet/lymphocyte count ratio. Modified Glasgow prognostic score (mGPS) was defined as follows: Group A, albumin ≥ 3.5 g/dL and C-reactive protein (CRP) < 0.5 mg/dL; group B, albumin < 3.5 g/dL and CRP < 0.5 mg/dL; group C, albumin ≥ 3.5 g/dL and CRP ≥ 0.5 mg/dL; and group D, albumin < 3.5 g/dL and CRP < 0.5 mg/dL[15,16]. The prognostic nutrition index was defined as: “10 × albumin levels (g/dL) + 0.005 × total lymphocyte count (/ μL)”[12].
Continuous variables were analyzed using the Wilcoxon rank-sum test. Fisher’s exact test was used to compare categorical variables. To elucidate the risk factors for EL in patients with BTC, tumor markers and inflammation-based prognostic scores differences between EL and R groups were compared. A receiver operating characteristic (ROC) curve was generated and the area under the curve [and its 95% confidence interval (CI)] was calculated to determine the best cutoff values for continuous parameter to discriminate the level of the EL and R group.
Next, the reduction rate of EL was estimated by the identified risk factors to assess how many patients with unresectable factors avoid EL when SL is performed. The reduction rate was calculated by the number of patients with occult liver or peritoneal metastasis divided by the number of patients.
Finally, to evaluate the prognosis of the risk factors, the Kaplan-Meier analysis with the log-rank test was used to compare survival curves by the identified risk factors.
All statistical tests were two-sided and performed at a significance level of 0.05.
These analyses were performed using JMP version 12.0 (SAS Institute Inc. Cary, NC, United States).
As shown in Table 1, there were 134 men and 102 women with a median age of 70 years. The tumor locations were as follows: Intrahepatic bile duct (n = 39), extrahepatic bile duct (n = 110) [perihilar bile duct (n = 55) and distal bile duct (n = 55)], gallbladder (n = 50), and papilla of Vater (n = 37). Twenty-six patients (11%) had an EL. The unresectable factors in the EL group were liver metastasis (n = 7), peritoneal metastasis (n = 9), distant lymph node metastasis (n = 4), and locally advanced disease (n = 6), including duplications.
| Type of operations | |||
| Characteristics | EL (n = 26) | R (n = 210) | P value |
| Age, yr1 | 69 (55-83) | 70 (25-89) | 0.57 |
| Sex (male / female) | 12/14 | 122/88 | 0.29 |
| BMI (kg/m2)1 | 21.8 (18-31.6) | 21.8 (15.6-40.1) | 0.98 |
| Location of the tumor, n | |||
| Intrahepatic bile duct (n = 39) | 6 | 33 | |
| Perihilar bile duct (n = 55) | 3 | 52 | |
| Distal bile duct (n = 55) | 8 | 47 | |
| Gallbladder (n = 50) | 9 | 41 | |
| Papilla of Vater (n = 37) | 0 | 37 | |
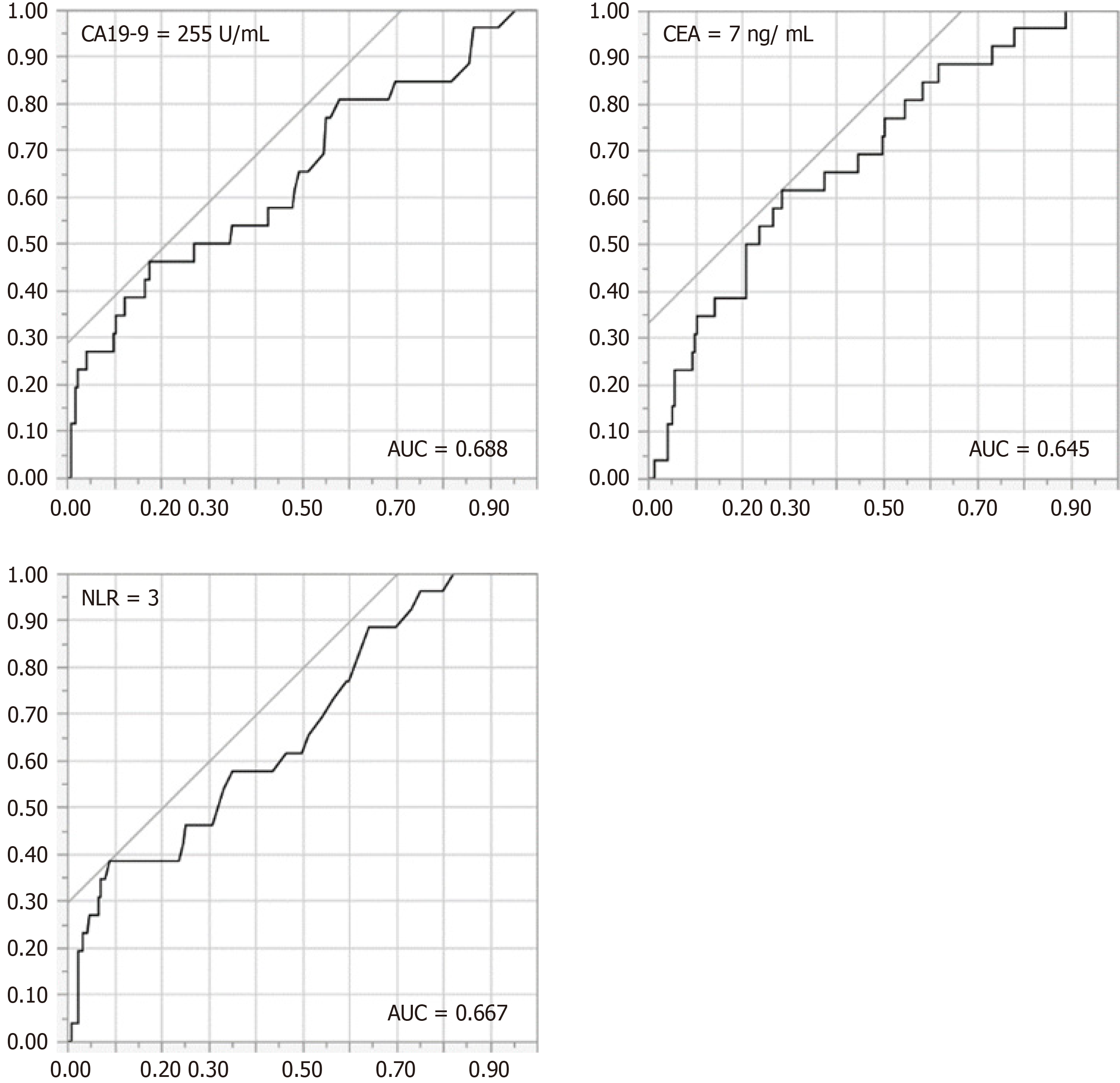
There were no significant differences between the EL and R groups for the individual indices of inflammation and nutrition. However, CA19-9 (5198 ± 2767 U/mL vs 777 ± 357 U/mL, P = 0.014), CEA (12.3 ± 3.1 ng/mL vs 5.1 ± 1.0 ng/mL, P = 0.0054), NLR (3.92 ± 0.40 vs 2.81 ± 0.14, P = 0.0018), and mGPS (P = 0.012) were significantly higher in the EL group than in the R group, respectively (Table 2). Consequently, we determined the cutoff values of CA19-9, CEA, and NLR as 255 U/mL, 7 ng/mL, and 3, respectively, using the ROC curve for all BTCs (Table 3, Figure 1). The area under the curve at each cutoff value was 0.688 (95%CI: 0.488-0.740), 0.645 (95%CI: 0.544-0.767), and 0.667 (95%CI: 0.565-0.780), respectively.
| Type of operations | |||
| Variables | EL (n = 26) | R (n = 210) | P value |
| Hb (g/dL)1 | 12.0 ± 0.33 | 12.3 ± 0.11 | 0.78 |
| Plt (10000/μL)1 | 24.4 ± 1.53 | 22.1 ± 0.54 | 0.079 |
| PT-INR1 | 0.97 ± 0.03 | 0.99 ± 0.01 | 0.72 |
| Albumin (mg/dL)1 | 3.73 ± 0.11 | 3.79 ± 0.04 | 0.66 |
| ALP (IU/L)1 | 641 ± 98 | 564 ± 34 | 0.22 |
| γ-GTP (IU/L)1 | 223 ± 51 | 192 ± 17 | 0.28 |
| T-bil (mg/dL)1 | 1.74 ± 0.34 | 1.24 ± 0.11 | 0.083 |
| LDH (IU/L)1 | 212 ± 16 | 187 ± 5.6 | 0.079 |
| CRP (mg/dL)1 | 0.88 ± 0.93 | 1.23 ± 0.33 | 0.63 |
| Creatinine clearance (mL/min)1 | 212 ± 16 | 187 ± 5.6 | 0.079 |
| Tumor markers | |||
| CA19-9 (IU/mL)1 | 5198 ± 2767 | 777 ± 357 | 0.014 |
| CEA (ng/mL)1 | 12.3 ± 3.1 | 5.1 ± 1.0 | 0.0054 |
| Inflammation-based prognostic scores | |||
| NLR1 | 3.92 ± 0.40 | 2.81 ± 0.14 | 0.0018 |
| PLR1 | 190 ± 18 | 166 ± 6.4 | 0.15 |
| PNI1 | 43.4 ± 3.4 | 46.7 ± 1.2 | 0.82 |
| mGPS (A / B, C, D) | 9 / 17 | 126 / 84 | 0.012 |
| Variables | Cut-off value | Sensitivity | Specificity | PPV | AUC |
| CA19-9 | 255 IU/mL | 0.423 | 0.823 | 0.229 | 0.688 |
| CEA | 7 ng/mL | 0.346 | 0.919 | 0.346 | 0.645 |
| NLR | 3 | 0.615 | 0.71 | 0.208 | 0.667 |
Table 4 shows the tumor markers, inflammation-based prognostic scores, and cumulative survival time for each biliary cancer. CA19-9 was high in intrahepatic and extrahepatic bile duct cancer and gallbladder cancer, but low in ampullary cancer. Inflammation-based prognostic scores were similar for each BTC. In terms of postoperative survival time, ampullary cancer showed a good prognosis compared to other BTCs.
| Variables | Type of cancer | ||||
| IBDC | EBDC | GBC | Vater C | ||
| (n = 39) | (n = 110) | (n = 50) | (n = 37) | ||
| Tumor markers | |||||
| CA19-9 (IU/mL)1 | 7904 ± 3316 | 1356 ± 506 | 832 ± 371 | 22 ± 36 | |
| CEA (ng/mL) 1 | 12.2 ± 26.8 | 4.0 ± 7.5 | 8.4 ± 22.0 | 3.2 ± 3.5 | |
| Inflammation-based prognostic scores | |||||
| NLR1 | 3.04 ± 1.60 | 2.83 ± 2.20 | 3.22 ± 2.27 | 2.74 ± 2.04 | |
| PLR1 | 151.1 ± 56.9 | 174.0 ± 95.4 | 176.6 ± 99.2 | 163.2 ± 109.9 | |
| PNI1 | 48.0 ± 5.5 | 45.3 ± 24.5 | 46.7 ± 7.0 | 47.1 ± 7.1 | |
| mGPS (A / B, C, D) | 10/29 | 47/63 | 32/18 | 10/27 | |
| Cumulative survival time (%)2 | |||||
| 1-year | 65.0 | 80.1 | 82.6 | 94.6 | |
| 3-year | 48.3 | 35.7 | 63.2 | 75.3 | |
| 5-year | 31.3 | 26.4 | 45.8 | 75.3 | |
When we performed sensitivity analysis for each biliary cancer (excluding ampullary cancer since ampullary cancer had zero EL cases) by using the cutoff value shown in Table 3, we found a similar tendency to the result for all BTCs; that is, specificity was high but sensitivity was particularly low in each cancer (Table 5).
| Variables (cut-off value) | Sensitivity | Specificity | PPV |
| Intrahepatic bile duct cancer | |||
| CA19-9 (255 IU/mL) | 0.833 | 0.667 | 0.313 |
| CEA (7 ng/mL) | 0.500 | 0.849 | 0.333 |
| NLR (3) | 0.500 | 0.636 | 0.200 |
| Extrahepatic bile duct cancer | |||
| CA19-9 (255 IU/mL) | 0.273 | 0.787 | 0.125 |
| CEA (7 ng/mL) | 0.273 | 0.939 | 0.333 |
| NLR (3) | 0.455 | 0.727 | 0.152 |
| Gallbladder cancer | |||
| CA19-9 (255 IU/mL) | 0.444 | 0.878 | 0.444 |
| CEA (7 ng/mL) | 0.444 | 0.878 | 0.444 |
| NLR (3) | 0.889 | 0.659 | 0.364 |
The positive outcome of this examination was liver or peritoneal metastasis. The considered criteria were: CA19-9 > 255 U/mL, CEA > 7 ng/mL, NLR > 3, and mGPS ≥ B, as mentioned above. Among all 236 patients, the number of patients at each of these criteria were 48 (20%), 26 (11%), 77 (33%) and 101 (43%), and the number of the patients with positive criterion, positive outcomes were 7, 6, 11 and 5, respectively (Table 6). Estimated reduction rate of EL was 14.6% (CA19-9 > 255 U/mL), 23.1% (CEA > 7 ng/mL), 14.3% (NLR > 3) and 5.0% (mGPS ≥ B) of patients with BTC, respectively (Table 6).
| Factor | n of patients | No. of patients with occult liver or peritoneal metastasis | Estimated reduction rate of exploratory laparotomy |
| CA19-9 > 255 U/mL | 48 (20) | 7 | 7/48 (14.6) |
| CEA > 7 ng/mL | 26 (11) | 6 | 6/26 (23.1) |
| NLR > 3 | 77 (33) | 11 | 11/77 (14.3) |
| mGPS = B, C, D | 101 (43) | 5 | 5/101 (5.0) |
| Positive factors ≥ 2 | 73 (31) | 11 | 11/73 (15.1) |
| Positive factors ≥ 3 | 26 (11) | 7 | 7/26 (26.9) |
| Positive factors ≥ 4 | 6 (3) | 2 | 2/6 (33.3) |
The number of patients with two of these positive factors was 73 (31%). Among these 73 patients, 11 patients had occult metastasis, and the estimated reduction rate of EL was 15.1%. The estimated reduction rate of EL in patients with three and four positive factors was 26.9% and 33.3%, respectively (Table 6).
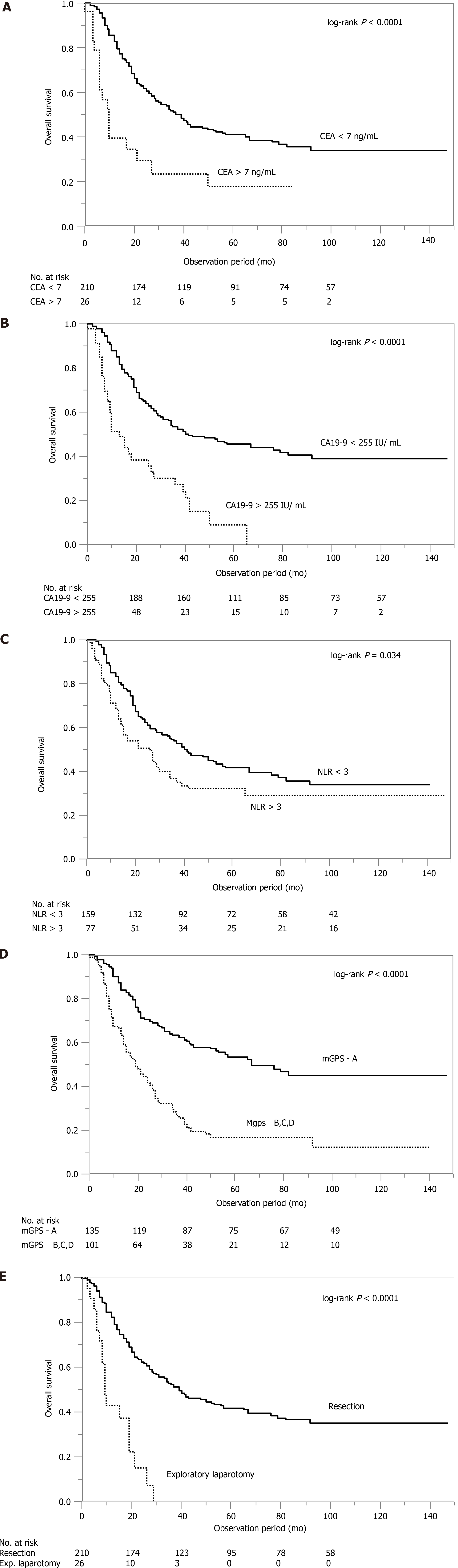
The overall survival curves between all the patients based on these four factors (CA19-9, CEA, NLR, and mGPS) and type of operation were compared (Figure 2). The prognoses were significantly worse in the cohorts with CA19-9 > 255 U/mL, CEA > 7 ng/mL, NLR > 3, mGPS = B, C, or D, and EL. This result suggests that these indices may be prognostic factors.
BTCs show poor prognosis and are often diagnosed at an advanced stage. The rate of resection of BTC with curative intent is only 70%[1,2]. In addition, approximately 30% of the patients preoperatively diagnosed with resectable diseases show unresectable diseases during laparotomy due to metastatic progression[2,4]. In our cohort, 11% of the patients diagnosed with resectable disease by imaging studies were eventually diagnosed with unresectable disease at laparotomy and underwent EL. This rate is better than that reported previously[2,4]. This discrepancy may be due to previous studies including cases from the 1990s, when the diagnostic modalities were limited and MDCT and FDG-PET were unavailable. Recently, advances in imaging techniques, especially MDCT, have enabled us to evaluate the local resectability of BTC. The limitations in detecting liver and peritoneal metastases have not yet been overcome[17-20]. As the lesions of liver and peritoneal metastasis first detected during the operation are often very small and solitary, they are difficult to detect even with contrast-based techniques. Even FDG-PET scanners have difficulty in delineating lesions < 8.8 mm in diameter[21].
Moreover, EL imparts physical strain on the patients and also has a negative impact on the optimization of medical resources. To perform an operation with curative intent, we have to secure the operation room for 8 h to 12 h along with the required number of surgeons and allied medical staff for each BTC patient. If the operation necessitates EL, then these resources would be wasted. For this problem, the utility of SL in patients with BTC has been reported[22-26]. SL ensures decreased blood loss, shorter duration of operation and faster induction of chemotherapy than EL[7]. However, as even SL may impose extra stress and costs for those whom SL is potentially unnecessary, it would not be appropriate to perform SL for all patients with BTC. In our cohort, if we had performed SL in all the patients, the outcome of SL would have been only 6.8% (occult metastasis, 16/236). Thus, clinicians should select patients who undergo SL efficiently. There are some previous reports describing the selection criteria for SL in pancreas and periampullary cancer[10,27,28]. These studies indicated that serum CA19-9 levels and the PLR are useful in selecting patients for SL. However, to the best of our knowledge, this is the first report discussing the selection criteria for SL in patients with BTC.
In this study, CEA, CA19-9, NLR and mGPS were detected as the factors predicting EL as well as prognostic factors. The prognostic usefulness of inflammation-based prognostic scores has been reported in various cancer types, such as hepatobiliary, colorectal, gastric, esophageal, lung, and renal cell carcinoma[21,29-36]. We also reported that NLR is an independent prognostic factor for resectable pancreatic ductal carcinoma and hepatocellular carcinoma[37]. The remarkable advantages of inflammation-based prognostic scores are their simplicity and convenience. We can examine blood cell count and biochemistry, including CRP and albumin, with low costs in daily clinical practice. There are no ethical issues, unlike with genetic examinations. Moreover, tumor markers, including CA19-9 and CEA, are widely used. CA19-9 is useful in selecting patients with pancreas and periampullary cancer for SL[10,27,28]. Here, we demonstrated that inflammation-based prognostic scores-NLR and mGPS, and tumor markers were good predictors of unresectable BTC in patients who were radiologically diagnosed with resectable disease. Recently, the utility of neoadjuvant chemotherapy (NAC) in patients with BTC has been discussed[38]. As one of the roles of NAC in BTC is to benefit the patients with distant micrometastasis who are potentially unresectable, inflammation-based prognostic scores and tumor markers may also be useful for selecting patients suitable for NAC.
In this study, CEA > 7 ng/mL was the most useful criteria for detecting occult metastasis. If SL was performed in cases with CEA > 7 ng/mL, unnecessary EL might be avoided in 23% of BTC patients. CA19-9, NLR and mGPS were also useful to decrease unnecessary laparotomy. In addition, when the number of positive factors increased, the estimated reduction rate of EL gradually increased. In this study, if all four factors were positive, the rate was 33.3%. However, these factors may not to be highly related for selective SL. Previous studies on BTC demonstrated that preoperative SL could detect liver or peritoneal metastasis in 14%–41% of the patients[23-26]. These studies discussed nonselective SL. Our data could be due to the relatively low rate of performing EL (11%) due to accurate preoperative imaging studies, including MDCT and FDG-PET.
This study has several limitations. First, BTCs are heterogeneous malignancies comprising intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, gallbladder carcinoma, and ampullary carcinoma. Although sensitivity analysis showed similar results for all BTCs, a study with a large number of tumors is required. However, due to the heterogeneity of BTCs, the results of this study may be suitable for evaluation of other solid malignancies. However, studies on other solid malignancies are also required. Second, this study included a limited number of patients with EL and no representation from primarily unresectable patients. Further studies including primarily unresectable patients are needed. Lastly, although we detected the factors predicting the EL or occult metastasis in BTC, the validation study has not been performed yet. To determine the selection criteria for SL future study is required.
The study indicates that planned SL may reduce requirement of EL when the inflammation-based prognostic scores and tumor markers or their combinations are used in selecting patients with BTC.
Advances in radiological imaging techniques have enabled us to understand the details of biliary tract cancer (BTC) preoperatively. However, detecting small liver metastasis or peritoneal metastasis remains difficult in BTCs.
Staging laparoscopy may help avoid unnecessary laparotomy due to the occult metastases. However, there are no standard methods for selecting staging laparoscopy in BTC.
This study aimed to elucidate the risk factors for exploratory laparotomy due to occult metastasis in patients with BTC using tumor markers and inflammation-based prognostic scores.
This was a retrospective study from the data of 236 BTC patients.
Twenty-six (11%) patients underwent exploratory laparotomy, and there were sixteen occult metastasis cases (7 liver metastasis and 9 abdominal disseminations). Serum carcinoembryonic antigen, carbohydrate antigen 19-9, neutrophil-lymphocyte ratio and modified Glasgow prognostic score were significantly higher in the exploratory laparotomy group (n = 26) than in the resected group (n = 210). Among these factors, carcinoembryonic antigen > 7 ng/mL was the most useful to predict occult metastasis. When patients had more than three of these positive factors, the rate of occult metastasis increased.
Inflammation-based prognostic scores and tumor markers are useful in detecting occult metastasis in patients with BTC, and based on a combination of these factors, selective staging laparoscopy may reduce the rate of exploratory laparotomy.
Since BTCs are heterogeneous malignancies, a study with a large number of tumors is required.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Surgery
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Saengboonmee C S-Editor: Liu M L-Editor: A P-Editor: Li JH
| 1. | Ishihara S, Horiguchi A, Miyakawa S, Endo I, Miyazaki M, Takada T. Biliary tract cancer registry in Japan from 2008 to 2013. J Hepatobiliary Pancreat Sci. 2016;23:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Shin DW, Kim MJ, Lee JC, Kim J, Woo SM, Lee WJ, Lee KH, Hwang JH. Gemcitabine Plus Cisplatin Chemotherapy Prolongs the Survival in Advanced Hilar Cholangiocarcinoma: A Large Multicenter Study. Am J Clin Oncol. 2020;43:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Miyazaki M, Yoshitomi H, Miyakawa S, Uesaka K, Unno M, Endo I, Ota T, Ohtsuka M, Kinoshita H, Shimada K, Shimizu H, Tabata M, Chijiiwa K, Nagino M, Hirano S, Wakai T, Wada K, Isayama H, Okusaka T, Tsuyuguchi T, Fujita N, Furuse J, Yamao K, Murakami K, Yamazaki H, Kijima H, Nakanuma Y, Yoshida M, Takayashiki T, Takada T. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepatobiliary Pancreat Sci. 2015;22:249-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Matsuo K, Rocha FG, Ito K, D'Angelica MI, Allen PJ, Fong Y, Dematteo RP, Gonen M, Endo I, Jarnagin WR. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg. 2012;215:343-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Brockbank EC, Harry V, Kolomainen D, Mukhopadhyay D, Sohaib A, Bridges JE, Nobbenhuis MA, Shepherd JH, Ind TE, Barton DP. Laparoscopic staging for apparent early stage ovarian or fallopian tube cancer. First case series from a UK cancer centre and systematic literature review. Eur J Surg Oncol. 2013;39:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Convie L, Thompson RJ, Kennedy R, Clements WD, Carey PD, Kennedy JA. The current role of staging laparoscopy in oesophagogastric cancer. Ann R Coll Surg Engl. 2015;97:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Hashimoto D, Chikamoto A, Sakata K, Nakagawa S, Hayashi H, Ohmuraya M, Hirota M, Yoshida N, Beppu T, Baba H. Staging laparoscopy leads to rapid induction of chemotherapy for unresectable pancreatobiliary cancers. Asian J Endosc Surg. 2015;8:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Ikoma N, Blum M, Chiang YJ, Estrella JS, Roy-Chowdhuri S, Fournier K, Mansfield P, Ajani JA, Badgwell BD. Yield of Staging Laparoscopy and Lavage Cytology for Radiologically Occult Peritoneal Carcinomatosis of Gastric Cancer. Ann Surg Oncol. 2016;23:4332-4337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Schlieman MG, Ho HS, Bold RJ. Utility of tumor markers in determining resectability of pancreatic cancer. Arch Surg. 2003;138:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Alexakis N, Gomatos IP, Sbarounis S, Toutouzas K, Katsaragakis S, Zografos G, Konstandoulakis MM. High serum CA 19-9 but not tumor size should select patients for staging laparoscopy in radiological resectable pancreas head and peri-ampullary cancer. Eur J Surg Oncol. 2015;41:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Oshiro Y, Sasaki R, Fukunaga K, Kondo T, Oda T, Takahashi H, Ohkohchi N. Inflammation-based prognostic score is a useful predictor of postoperative outcome in patients with extrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2013;20:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Okuno M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Yamaguchi J, Nagino M. Evaluation of inflammation-based prognostic scores in patients undergoing hepatobiliary resection for perihilar cholangiocarcinoma. J Gastroenterol. 2016;51:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Juntermanns B, Radunz S, Heuer M, Hertel S, Reis H, Neuhaus JP, Vernadakis S, Trarbach T, Paul A, Kaiser GM. Tumor markers as a diagnostic key for hilar cholangiocarcinoma. Eur J Med Res. 2010;15:357-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Fujiwara Y, Haruki K, Shiba H, Hamura R, Shirai Y, Furukawa K, Gocho T, Yanaga K. The Comparison of Inflammation-Based Prognostic Scores in Patients With Extrahepatic Bile Duct Cancer After Pancreaticoduodenectomy. J Surg Res. 2019;238:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Jansson H, Cornillet M, Björkström NK, Sturesson C, Sparrelid E. Prognostic value of preoperative inflammatory markers in resectable biliary tract cancer - Validation and comparison of the Glasgow Prognostic Score and Modified Glasgow Prognostic Score in a Western cohort. Eur J Surg Oncol. 2020;46:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Ni Q, Wang H, Zhang Y, Qian L, Chi J, Liang X, Chen T, Wang J. MDCT assessment of resectability in hilar cholangiocarcinoma. Abdom Radiol (NY). 2017;42:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Ajiki T, Fukumoto T, Ueno K, Okazaki T, Matsumoto I, Ku Y. Three-dimensional computed tomographic cholangiography as a novel diagnostic tool for evaluation of bile duct invasion of perihilar cholangiocarcinoma. Hepatogastroenterology. 2013;60:1833-1838. [PubMed] |
| 19. | Kawanaka Y, Kitajima K, Fukushima K, Mouri M, Doi H, Oshima T, Niwa H, Kaibe N, Sasako M, Tomita T, Miwa H, Hirota S. Added value of pretreatment (18)F-FDG PET/CT for staging of advanced gastric cancer: Comparison with contrast-enhanced MDCT. Eur J Radiol. 2016;85:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Yamada I, Ajiki T, Ueno K, Sawa H, Otsubo I, Yoshida Y, Shinzeki M, Toyama H, Matsumoto I, Fukumoto T, Nakao A, Kotani J, Ku Y. Feasibility of (18)F-fluorodeoxyglucose positron-emission tomography for preoperative evaluation of biliary tract cancer. Anticancer Res. 2012;32:5105-5110. [PubMed] |
| 21. | Wallstén E, Axelsson J, Sundström T, Riklund K, Larsson A. Subcentimeter tumor lesion delineation for high-resolution 18F-FDG PET images: optimizing correction for partial-volume effects. J Nucl Med Technol. 2013;41:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Bird N, Elmasry M, Jones R, Elniel M, Kelly M, Palmer D, Fenwick S, Poston G, Malik H. Role of staging laparoscopy in the stratification of patients with perihilar cholangiocarcinoma. Br J Surg. 2017;104:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Davidson JT 4th, Jin LX, Krasnick B, Ethun CG, Pawlik TM, Poultsides GA, Idrees K, Weber SM, Martin RCG, Shen P, Hatzaras I, Maithel SK, Fields RC; and the U. S. Extrahepatic Biliary Malignancy Consortium. Staging laparoscopy among three subtypes of extra-hepatic biliary malignancy: a 15-year experience from 10 institutions. J Surg Oncol. 2019;119:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Kobayashi S, Tomokuni A, Takahashi H, Akita H, Marubashi S, Gotoh K, Yanagimoto Y, Takahashi Y, Sugimura K, Miyoshi N, Moon JH, Yasui M, Omori T, Miyata H, Ohue M, Fujiwara Y, Yano M, Sakon M. Laparoscopic Hilar Lymph Node Sampling in Patients With Biliary Tract Cancers That are Rarely Associated With Nodal Metastasis. Surg Laparosc Endosc Percutan Tech. 2018;28:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Tilleman EH, de Castro SM, Busch OR, Bemelman WA, van Gulik TM, Obertop H, Gouma DJ. Diagnostic laparoscopy and laparoscopic ultrasound for staging of patients with malignant proximal bile duct obstruction. J Gastrointest Surg. 2002;6:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Bhardwaj N, Garcea G, Dennison AR, Maddern GJ. The Surgical Management of Klatskin Tumours: Has Anything Changed in the Last Decade? World J Surg. 2015;39:2748-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Smith RA, Bosonnet L, Ghaneh P, Sutton R, Evans J, Healey P, Garvey C, Hughes M, Raraty M, Campbell F, Neoptolemos JP. The platelet-lymphocyte ratio improves the predictive value of serum CA19-9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery. 2008;143:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. |
Suker M, Koerkamp BG, Coene PP, van der Harst E, Bonsing BA, Vahrmeijer AL, Mieog JSD, Swijnenburg RJ, Dwarkasing RS, Roos D, van Eijck CHJ.
Yield of |
| 29. | Goh BK, Chok AY, Allen JC Jr, Quek R, Teo MC, Chow PK, Chung AY, Ong HS, Wong WK. Blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are independent prognostic factors for surgically resected gastrointestinal stromal tumors. Surgery. 2016;159:1146-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Spolverato G, Maqsood H, Kim Y, Margonis G, Luo T, Ejaz A, Pawlik TM. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol. 2015;111:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H, Matsushima M. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22:803-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 330] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 32. | Pinato DJ, Shiner RJ, Seckl MJ, Stebbing J, Sharma R, Mauri FA. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer. 2014;110:1930-1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 33. | Aurello P, Tierno SM, Berardi G, Tomassini F, Magistri P, D'Angelo F, Ramacciato G. Value of preoperative inflammation-based prognostic scores in predicting overall survival and disease-free survival in patients with gastric cancer. Ann Surg Oncol. 2014;21:1998-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Moug SJ, McColl G, Lloyd SM, Wilson G, Saldanha JD, Diament RH. Comparison of positive lymph node ratio with an inflammation-based prognostic score in colorectal cancer. Br J Surg. 2011;98:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Hirahara N, Matsubara T, Hayashi H, Takai K, Fujii Y, Tajima Y. Impact of inflammation-based prognostic score on survival after curative thoracoscopic esophagectomy for esophageal cancer. Eur J Surg Oncol. 2015;41:1308-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Chang Y, An H, Xu L, Zhu Y, Yang Y, Lin Z, Xu J. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br J Cancer. 2015;113:626-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 37. | Asari S, Matsumoto I, Toyama H, Shinzeki M, Goto T, Ishida J, Ajiki T, Fukumoto T, Ku Y. Preoperative independent prognostic factors in patients with borderline resectable pancreatic ductal adenocarcinoma following curative resection: the neutrophil-lymphocyte and platelet-lymphocyte ratios. Surg Today. 2016;46:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Kobayashi S, Tomokuni A, Gotoh K, Takahashi H, Akita H, Marubashi S, Yamada T, Teshima T, Nishiyama K, Yano M, Ohigashi H, Ishikawa O, Sakon M. Evaluation of the safety and pathological effects of neoadjuvant full-dose gemcitabine combination radiation therapy in patients with biliary tract cancer. Cancer Chemother Pharmacol. 2015;76:1191-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |