Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.10040
Peer-review started: July 6, 2021
First decision: July 26, 2021
Revised: August 16, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: November 16, 2021
Processing time: 126 Days and 18.3 Hours
Ventricular tachycardia (VT) commonly occurs among patients with heart failure and can even cause sudden cardiac death. VT originating from the His bundle branch has been rarely reported. We present the case of a patient with VT from the His bundle branch.
A 58-year-old female complained of paroxysmal palpitations and dizziness for approximately 6 mo. She had a history of fatty liver and cholecystitis, and carotid atherosclerosis could not be excluded from the ultrasound results. An evaluation of the electrocardiogram obtained after admission showed spontaneous conversion between two different morphologies. The possible electrophysiologic mechanism suggested that the dual-source VT originated from the same source, the His bundle branch. Finally, the His bundle branch was ablated, and a dual-chamber pacemaker was inserted into the patient’s heart. No further VT occurred during the 3-year follow-up after hospital discharge.
The diagnosis of VT originating from the His bundle is rare and difficult to establish. The results of this study showed VT originating from the His bundle based on a careful evaluation of the electrocardiogram, and the diagnosis was confirmed by an intracardiac electrophysiologic examination.
Core Tip: This case report involves ventricular tachycardia originating from the His bundle, which is very rare. The diagnosis of the originating site was based on a careful electrocardiogram analysis and physician clinical experience. This case report provides a reference for the clinical diagnosis of such cases.
- Citation: Zhang LY, Dong SJ, Yu HJ, Chu YJ. Ventricular tachycardia originating from the His bundle: A case report. World J Clin Cases 2021; 9(32): 10040-10045
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/10040.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.10040
Heart failure is major cause of human mortality and is commonly caused by ventricular tachycardia (VT), a tachyarrhythmia[1]. The most feared consequence of VT is sudden cardiac death[2]. It is very important that cardiac surgeons recognize and manage VT promptly[3]. VT frequently originates from the right ventricular outflow tracts, the left ventricular outflow tracts, and the paravalvular aortic cusps. Less common sites of origin for VT include the His-Purkinje network and the cardiac crux[3-5]. The His-Purkinje system, however, is a very rare site of VT origination[6-8]. We report a case of VT originating from the His bundle and the underlying electrophysiologic mechanism. This case report may provide a reference for the clinical diagnosis of such special cases.
A 58-year-old woman was admitted to the Cardiovascular Department on September 24, 2017 for paroxysmal palpitations, chest tightness, and dizziness of approximately 6 mo duration without a history of syncope or structural heart disease.
Fatty liver, a rough gallbladder wall, and plaque formation in the left internal carotid artery were demonstrated by ultrasonography, which suggested liver disease, cholecystitis, and carotid atherosclerosis. The left ventricle was slightly enlarged, and the pulmonary artery was widened. The cardiac examination revealed mild mitral regurgitation. The ejection fraction was normal. Chest computed tomography (CT) showed a few old lesions in the middle lobe of the right lung and small nodules in the lower lobe of the right lung. A possible “drop effect” was noted in the lower lobe of the left lung. Thickening of the pleura was present bilaterally. Mediastinal lymphadenopathy was also detected. Double-phase angiography of the left atrium pulmonary vein showed no abnormalities. The patient was not treated for any of these findings before the current hospitalization because no imaging studies had ever been performed on the patient.
The patient denied cigarette smoking and consumption of alcohol. She had a history of hypertension for 20 years, for which the highest recorded blood pressure was 220/110 mmHg; she was treated with valsartan and amlodipine. The blood pressure was well-controlled.
The patient denied family history.
A physical examination was performed once on the patient after hospitalization. The patient had shortness of breath with a respiratory rate of 30 per min. Auscultation of both lungs was significant for thick breath sounds without dry rales, wet rales, or a pleural friction rub. Percussion of the heart was normal. Palpation of the apex beat was normal with a heart rate of 179 per min, and the rhythm was irregular. No heart murmur was heard in each valve auscultation area, and there was no pericardial friction rub. There was no edema in the lower limbs.
All laboratory tests were normal, including liver and kidney function, electrolytes, myocardial enzymes, a routine hemogram, thyroid function, glycosylated hemoglobin level, and coagulation profile. Blood test results suggest dyslipidemia and cardiac insufficiency, as follows: Triglycerides, 1.99 mmol/L (0–1.7 mmol/L); and pro-BNP, 2506 ng/L (133–900 ng/L).
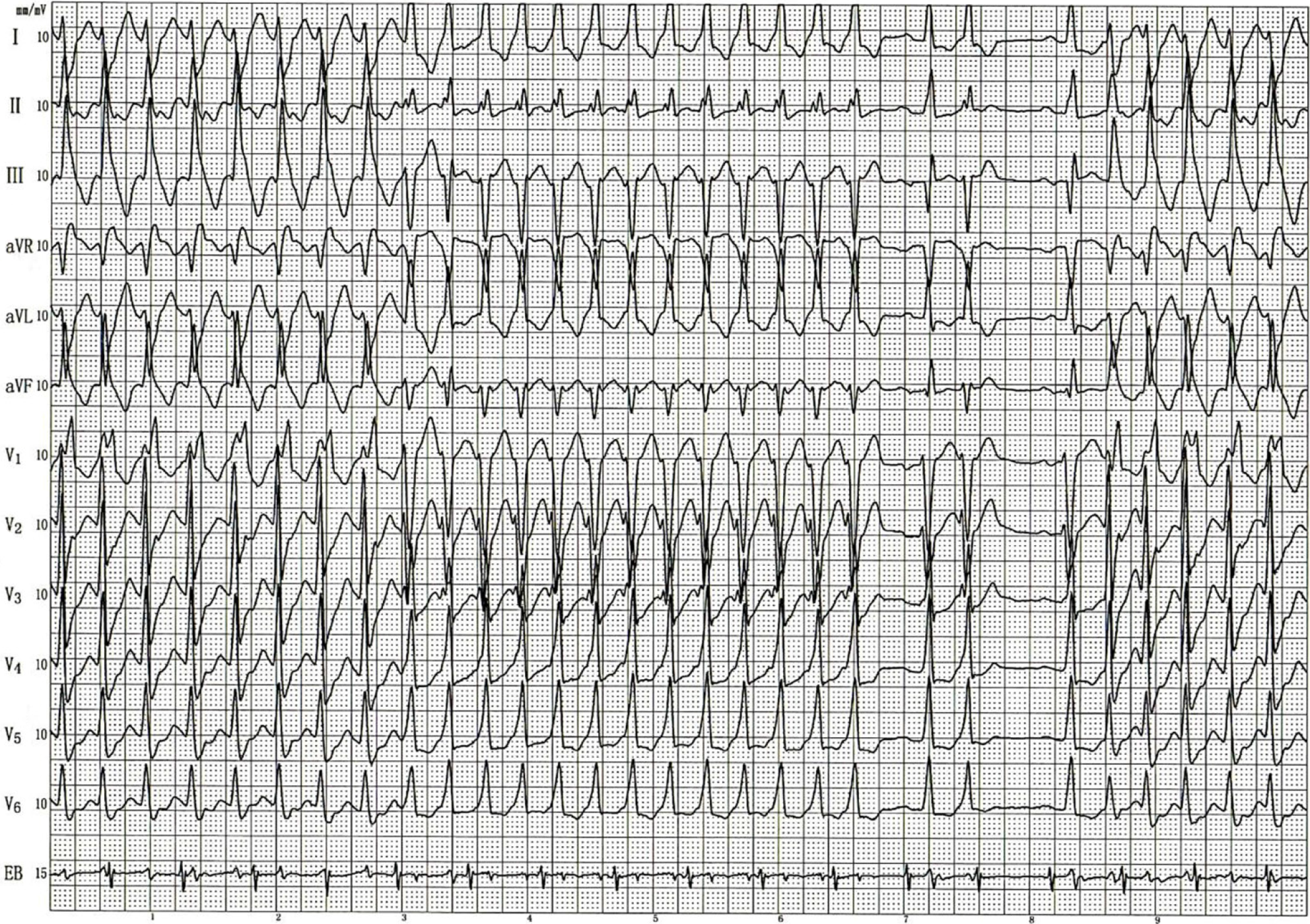
An electrocardiogram (ECG) obtained within 10 min after admission showed VT. After intravenous administration of amiodarone (0.15 g), the VT converted to a sinus rhythm, and the patient’s symptoms of discomfort were gradually relieved. The patient was transferred to the Cardiology Department for radiofrequency ablation. Before ablation, a preoperative transesophageal electrophysiologic examination was performed. Figure 1 shows the ECG induced by S1S1 200 bpm atrial pacing during the transesophageal electrophysiologic examination. Atrioventricular separation was observed in the EB lead, confirming VT. Transesophageal ventricular pacing with S1S1 graded incremental stimulation showed a normal shape and time limit for the QRS downloaded from S1. VT induced by S1S1 200 bpm stimulation showed patterns for blocks of the right bundle branch and left anterior branch. The ventricular rate was 187 bpm, and the atrial rate was 98 bpm. The dysrhythmia then changed to another form of VT. Lead Vl showed an rS pattern. RS moved to leads V3, I, and II. The lead aVL main wave was upward. The lead III and the aVF main wave were downward. The ventricular rate was 202 bpm, and the atrial frequency was 98 bpm. The two forms of tachycardia alternated. After being captured by a sinus heartbeat, sustained VT recurred spontaneously. The ECG indicated normal sinus node function and accelerated conduction in the atrioventricular node, and persistent dual-source VT was diagnosed by physicians in the ECG department. It was concluded that there were two different kinds of tachycardia, as shown in Figure 1 that could convert to each other spontaneously. The first form of VT was identified as a right-bundle-branch block, for which the morphology indicated the left posterior branch was much more likely to be blocked than the left anterior branch, and this prompted that VT originated from the left anterior branch. Whereas, the second form of VT had a left-bundle-branch-block morphology, similar to sinus rhythm, which prompted that VT originated from the septum of the right bundle branch. From the perspective of monism, the anatomical closeness of the origins of the two forms of VT suggested a single origin. Indeed, it was more likely that VT originated from the His bundle branch, which needed to be confirmed by a cardiac electrophysiologic examination.
The final diagnosis was VT originating from the His bundle branch in this case.
During hospitalization, the patient was given aspirin (100 mg qd) and atorvastatin (10 mg qn) to reduce cardiac preload and other cardiovascular risk factors as carotid plaque and elevated blood lipids were found during the hospital stay. Furosemide (20 mg qd) and spironolactone (20 mg qd) were administered to facilitate urine excretion, reduce cardiac preload and improve cardiac function. Irbesartan hydrochlorothiazide (1 tablet qd) was administered to lower the patient’s blood pressure. As the preoperative ECG indicated the His bundle as a potential origin of VT, the surgeon had a preoperative discussion with the patient and her family members. The patient and her family members were informed that if the patient had repeated episodes of VT during the operation, ablation would be ineffective, and the His bundle might need to be ablated, which would cause a third-degree atrioventricular block. A conduction block would thus require permanent pacemaker implantation at the same time. The patient and her family members agreed to the treatment strategy. During catheter ablation, VT recurred, and we mapped the earliest excitation of the ventricle near the His bundle branch, which confirmed the preoperative diagnosis. To avoid injuring the His bundle branch, an area near the His bundle branch was ablated, but the VT did not terminate and became severe with hemodynamic instability. We had another discussion with the patient’s relatives, who agreed to ablation of the His bundle branch and implantation of a pacemaker. When the ablated catheter was inserted and made contact with the His bundle, the VT terminated temporarily, and when the His bundle branch was ablated, the VT stopped, and the change in the heart rhythm indicated a complete atrioventricular block. Eventually, a dual-chamber pacemaker was inserted into the patient’s heart. The patient recovered uneventfully after discharge, and no further episodes of VT occurred. After 1 wk of continued treatment with the above medications, the patient was discharged.
After being discharged from the hospital, the patient continued oral administration of atorvastatin (10 mg qn), furosemide (20 mg qd), spironolactone (20 mg qd), and irbesartan hydrochlorothiazide (1 tablet qd) to address her comorbidities of carotid plaque, elevated blood lipids and hypertension. The pacemaker program did not record VT. Color Doppler ultrasound results showed that the wall motion of the left ventricle was uncoordinated, the left ventricle was large, a decreased EF value of the left ventricle, and decreased relaxation function of the left ventricle.
Sixteen months after discharge, the patient came to see a physician in our hospital due to cardiac insufficiency. A chest CT showed bilateral pneumonia, mild pulmonary edema, changes after cardiac pacemaker implantation, an increased heart shadow, coronary calcifications, and pericardial effusion. Ultrasound results showed mixed resolution of the thyroid bilaterally and a fatty liver. Laboratory tests showed normal liver and kidney function, electrolytes, blood lipids, thyroid function, and glycosylated hemoglobin. Pro-BNP was 2750 ng/L. No VT was observed upon review of Holter monitoring for 24 h. The patient gradually improved after being treated with diuretics.
The prevalence of VT from the His bundle branch is not known. Previous reports have confirmed that VT from the His bundle is rare and that the His bundle plays a very important role in the mechanism underlying this form of VT[6-8]. In this case report, no history of syncope or structural heart disease was reported, but paroxysmal palpitations and dizziness occurred, which were exceptional. After diagnosis, the cause for VT from the His bundle was not apparent. Fatty liver is a potential cause of VT. A previous study showed that fatty liver is independently related to an increased risk of prevalent VT[9]. The formation of plaque in the left internal carotid artery may have added to hemodynamic instability, which may be closely related to VT[10]. Hypertension in our patient raises the likelihood for VT to occur, as reported in another study[11].
The atria and ventricles play important roles in atrioventricular node re-entry tachycardia and atrioventricular re-entry tachycardia[12]. The ECG of our patient showed that the atria and ventricles were separated, which excluded supra VT. The loop for bundle branch re-entry VT is the macroreentry of the His-Purkinje system, which involves the His bundle, both bundle branches, and the ventricular myocardium in the circuit[13]. Wide QRS complex tachycardia with atrioventricular separation and the His bundle potential (H) before the ventricular wave (V) suggests bundle branch re-entrant VT[14]. Most cases of bundle branch re-entrant VT are of the left-bundle-branch-block type, with the right-bundle-branch-block type being a persistent monomorphic VT[14]. The ECG of our patient showed two tachycardia patterns but not persistent monomorphic VT. The first pattern of tachycardia pattern showed a right-bundle-branch block. Careful observation showed the morphology was considerably more consistent with a block of the left posterior branch than the left anterior branch. The second tachycardia pattern showed a left-bundle-branch-block morphology. The most important electrophysiologic feature of the proximal VT of the His-Purkinje system is that the His bundle potential is the earliest event during tachycardia. The right-bundle-branch potential, the left-anterior-branch potential, and the left-posterior-branch potential are all conducted away from the His bundle but do not form a re-entrant loop[15]. The ECG excluded bundle-branch re-entrant VT. Dual-source ventricular velocities correspond to ventricular velocities with two different origins. The two VT patterns for our patient converted to each other spontaneously and were polymorphic. Thus, origination from the same site was considered. When the His bundle branch was ablated, the VT stopped, and the change in the heart rhythm indicated a complete atrioventricular block. The characteristics of the ECG showed that VT originated from the His bundle branch, which was a very special case.
The interval between the His bundle potential (H) and the ventricular wave (V) is the most important electrophysiologic feature for diagnosing proximal VT of the His-Purkinje system. The HV interval is approximately 0 ms for typical bundle-branch re-entrant VT and approximately 30 ms for the proximal ventricular velocity of the His-Purkinje system. The longer the HV interval is, the further the site is from the His bundle, whereas the shorter the HV interval is, the closer the site is to the His bundle. A portion of the intraoperative electrophysiologic examination data was lost, making it difficult to clearly identify the HV interval. This uncertainty was the limitation of this study.
Radiofrequency ablation of VT from the His bundle is commonly applied and has been effective in previous cases[16]. Pacemaker implantation has been found to improve patient health and quality of life in other studies[17,18]. The VT in the current patient was from the His bundle; thus, pacemaker implantation was chosen for treatment. In the follow-up after discharge, VT did not recur, which indicated that the treatment was appropriate. Pacemaker implantation has been reported to cause atrial fibrillation during a 7–8-year follow-up[18]. The follow-up evaluation of the patient 16 mo after discharge showed changes after cardiac pacemaker implantation with an increased heart shadow, coronary calcifications, and pericardial effusion. Pacemaker implantation caused heart failure in the patient. In subsequent years, we continued to follow this patient to ensure her health.
In summary, the diagnosis of VT originating from the His bundle is difficult to establish. The ECG of this form of VT needs to be studied carefully to make a correct diagnosis. This case report provides valuable experience to facilitate a clinical diagnosis; however, neither a clinical examination nor imaging studies can clearly confirm the cause of VT from the His bundle. Therefore, the diagnosis of this form of VT warrants further research. Pacemaker implantation has disadvantages, and disease progression should be followed for a considerably long time after implantation.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oley MH S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Alvarez CK, Cronin E, Baker WL, Kluger J. Heart failure as a substrate and trigger for ventricular tachycardia. J Interv Card Electrophysiol. 2019;56:229-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Markman TM, Nazarian S. Treatment of ventricular arrhythmias: What's New? Trends Cardiovasc Med. 2019;29:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | AlMahameed ST, Ziv O. Ventricular Arrhythmias. Med Clin North Am. 2019;103:881-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Park KM, Kim YH, Marchlinski FE. Using the surface electrocardiogram to localize the origin of idiopathic ventricular tachycardia. Pacing Clin Electrophysiol. 2012;35:1516-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Lerman BB. Mechanism, diagnosis, and treatment of outflow tract tachycardia. Nat Rev Cardiol. 2015;12:597-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | He BJ, Boyden P, Scheinman M. Ventricular arrhythmias involving the His-Purkinje system in the structurally abnormal heart. Pacing Clin Electrophysiol. 2018;41:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Padanilam BJ, Ahmed AS, Clark BA, Gilge JL, Patel PJ, Prystowsky EN, Steinberg LA. Differentiating Atrioventricular Reentry Tachycardia and Atrioventricular Node Reentry Tachycardia Using Premature His Bundle Complexes. Circ Arrhythm Electrophysiol. 2020;13:e007796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Phlips T, Ramchurn H, De Roy L. Reverse BBRVT in a structurally normal heart. Acta Cardiol. 2012;67:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Mantovani A, Rigamonti A, Bonapace S, Bolzan B, Pernigo M, Morani G, Franceschini L, Bergamini C, Bertolini L, Valbusa F, Rigolon R, Pichiri I, Zoppini G, Bonora E, Violi F, Targher G. Nonalcoholic Fatty Liver Disease Is Associated With Ventricular Arrhythmias in Patients With Type 2 Diabetes Referred for Clinically Indicated 24-Hour Holter Monitoring. Diabetes Care. 2016;39:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Giacomino BD, Giudici MC. Carotid Dissection Complicated by Polymorphic Ventricular Tachycardia. J Stroke Cerebrovasc Dis. 2017;26:e99-e101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Afzal MR, Savona S, Mohamed O, Mohamed-Osman A, Kalbfleisch SJ. Hypertension and Arrhythmias. Heart Fail Clin. 2019;15:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Elbatran AI, Zarif JK, Tawfik M. Anterograde His bundle activation during right ventricular overdrive pacing in supraventricular tachycardia. Pacing Clin Electrophysiol. 2019;42:1374-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Balasundaram R, Rao HB, Kalavakolanu S, Narasimhan C. Catheter ablation of bundle branch reentrant ventricular tachycardia. Heart Rhythm. 2008;5:S68-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Chen H, Shi L, Yang B, Ju W, Zhang F, Yang G, Gu K, Li M, Cao K, Ouyang F, Chen M. Electrophysiological Characteristics of Bundle Branch Reentry Ventricular Tachycardia in Patients Without Structural Heart Disease. Circ Arrhythm Electrophysiol. 2018;11:e006049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Hayashi M, Kobayashi Y, Iwasaki YK, Morita N, Miyauchi Y, Kato T, Takano T. Novel mechanism of postinfarction ventricular tachycardia originating in surviving left posterior Purkinje fibers. Heart Rhythm. 2006;3:908-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Futyma P, Ciąpała K, Sander J, Głuszczyk R, Futyma M, Kułakowski P. Bipolar Radiofrequency Ablation of Ventricular Arrhythmias Originating in the Vicinity of His Bundle. Circ Arrhythm Electrophysiol. 2020;13:e008165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Dębski M, Maniecka-Bryła I, Dziankowska-Zaborszczyk E, Ulman M, Ząbek A, Boczar K, Haberka K, Kuniewicz M, Lelakowski J, Małecka B. Years of life lost as a measure of premature death among dualchamber pacemaker recipients from Małopolska Province. Kardiol Pol. 2019;77:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Hesselson AB, Parsonnet V, Bernstein AD, Bonavita GJ. Deleterious effects of long-term single-chamber ventricular pacing in patients with sick sinus syndrome: the hidden benefits of dual-chamber pacing. J Am Coll Cardiol. 1992;19:1542-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 147] [Article Influence: 4.5] [Reference Citation Analysis (0)] |