Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.10018
Peer-review started: July 1, 2021
First decision: July 16, 2021
Revised: July 21, 2021
Accepted: September 10, 2021
Article in press: September 10, 2021
Published online: November 16, 2021
Processing time: 131 Days and 13.4 Hours
Caused by premature activation of the hypothalamic-pituitary-gonadal axis, there is increasing incidence of central precocious puberty (CPP), especially in girls. Makorin ring finger protein 3 (MKRN3), a maternal imprinted gene with a highly conserved sequence, is the most common genetic etiology associated with CPP. Approximately 50 different mutations in MKRN3 have been found in CPP.
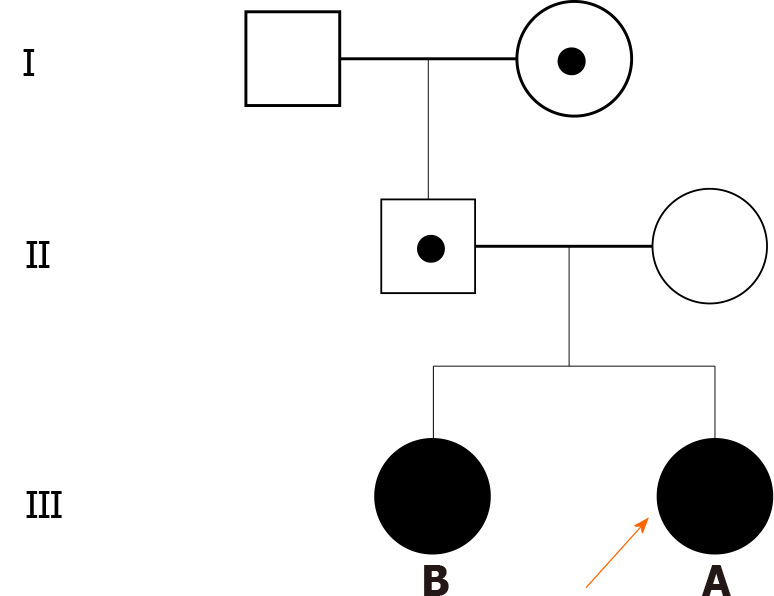
This case report involves identical twin sisters presenting with premature thelarche at the age of 6 years. The left hand bone age of both patients revealed advanced age (9 years). Pelvic B ultrasound indicated enlargement of the ovaries. Luteinizing hormone (LH) releasing hormone testing confirmed CPP. Whole-exome sequencing detected the c.841C>T mutation in MKRN3, leading to a single base substitution, in the twins. This mutation was inherited from the father and paternal grandmother. After 3 mo of treatment with a gonadotropin-releasing hormone analog, levels of LH, follicle-stimulating hormone, and estradiol in the proband’s sister returned to normal levels.
Here, we report a rare mutation (c.841C>T) in MKRN3 in identical twin sisters with CPP.
Core Tip: This report discusses the diagnosis and treatment of central precocious puberty caused by a new Makorin ring finger protein 3 gene mutation and includes a detailed clinical and laboratory analysis of the pathogenic principle, which provided the diagnosis and led to the treatment of central precocious puberty.
- Citation: Jiang LQ, Zhou YQ, Yuan K, Zhu JF, Fang YL, Wang CL. Rare mutation in MKRN3 in two twin sisters with central precocious puberty: Two case reports. World J Clin Cases 2021; 9(32): 10018-10023
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/10018.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.10018
Precocious puberty is defined by the development of breast enlargement before the age of 8 years or menarche before the age of 10 years in girls and testicular enlargement (≥ 4 mL) before the age of 9 years in boys. Central precocious puberty (CPP) is a specific type of precocious puberty that results from premature activation of the hypothalamic-pituitary-gonadal (HPG) axis[1]. CPP has critical short-term and long-term impacts on children, including increased risks of psychosocial stress, short stature, obesity, cardiovascular disease, and type 2 diabetes in adulthood[1]. The incidence of CPP is greater in girls than in boys, though the mechanism underlying this difference is not yet understood[2]. Boys with CPP are more likely to have specific pathological factors, such as thalamic hamartoma. In contrast, 90% of cases of CPP in girls are idiopathic[3].
Genetic testing of children diagnosed with CPP has led to the identification of several pathogenic genes. Multiple genes, including makorin ring finger protein 3 (MKRN3), kisspeptin (KISS1), kisspeptin receptor (KISS1R), and delta-like homolog 1 (DLK1), are associated with CPP. Loss-of-function mutations in MKRN3 are the most common genetic etiology contributing to CPP[4], and 115 cases of familial and sporadic CPP involving more than 20 different loss-of-function MKRN3 mutations were reported from 2013 to 2019[5].
Herein, we report a rare mutation in MKRN3 causing CPP in twin sisters. This is the first report of MKRN3 gene mutation in identical twins.
Patient A: A Chinese girl was referred to our hospital for premature thelarche at the age of 6 years and 9 mo.
Patient B: The identical twin elder sister of patient A was previously examined at the age of 6 years and 7 mo for premature thelarche in another hospital. Premature thelarche appeared when the patient was 6 years and 4 mo old.
At the age of 6 years and 3 mo, patient A showed breast development and progressive enlargement; at the age of 6 years and 4 mo, patient B showed breast development and progressive enlargement. Similar to patient A, no other obvious discomfort, misuse of contraceptives, or other abnormal performance was reported.
The girls were born at full term weighing 2400 g and 2690 g and with a length of 48 cm (both twins). Neither patient had a history of a significant medical illness. The patients' diet was normal. The growth and development of the children prior to thelarche were similar to those of Han girls of their age.
The patients were twin daughters of Han Chinese nonconsanguineous parents. There was no family history of immunodeficiency or recurrent infection. The parents had no genetic diseases. The menarche age of the mother and pubertal development of the father were normal. The father had a height of 170 cm (-0.4 SDS) and the mother 162 cm (0.3 SDS).
Patient A had a height of 119.6 cm (0.04 SDS) and weighed 23 kg: Tanner stage 2 in the breasts and Tanner stage 1 in pubic hair, with no axillary hair. The height of patient B was 122.6 cm (0.8 SDS), and her body weight was 26.7 kg; she showed breast Tanner stage 2, pubic hair stage 1, and no axillary hair.
The luteinizing hormone releasing hormone (LHRH) stimulation test for patient A indicated CPP (early stage); the peak luteinizing hormone (LH) value was 4.71 mIU/mL, the peak follicle-stimulating hormone (FSH) value was 16.48 mIU/mL, and the LH/FSH ratio value was 0.29. Laboratory results together with the typical clinical manifestations indicated CPP. The LHRH stimulation test of patient B revealed a peak LH value of 15.81 mIU/mL (Table 1).
| Patient A | Patient B | |
| Age at onset (yr) | 6.25 | 6.33 |
| Age at referral (yr) | 6.66 | 6.5 |
| Weight (kg) | 23 | 26.7 |
| Height (cm) | 119.6 | 122.6 |
| Bone age (yr) | 9 | 9 |
| Tanner stage | B2 | B2 |
| Pubarche stage | PH1 | PH1 |
| Basal LH (mIU/mL) | 0.13 | N/A |
| Peak LH (mIU/mL) | 4.71 | 15.81 |
| Basal FSH (mIU/mL) | 2.71 | N/A |
| Peak FSH (mIU/mL) | 16.48 | N/A |
| Estradiol (pg/ml) | 25.26 | N/A |
| Peak LH/FSH | 0.29 | N/A |
| Uterine (mL) | 2.9 | 5.3 |
| Left Ovary (mL) | 1.2 | 1.9 |
| Right Ovary (mL) | 1.7 | 1.8 |
| Brain magnetic resonance imaging | Normal | Normal |
The left hand bone age of patient A was 9 years. Pelvic ultrasound of patient A revealed enlargement of the ovary; the volume of the left ovary was 1.2 mL, while the volume of the right ovary was 1.7 mL. Pelvic ultrasound of patient B revealed larger ovaries than in patient A; the volume of the left ovary was 2 mL, and the volume of the right ovary was 3.8 mL. The left hand bone age of patient B was 9 years.
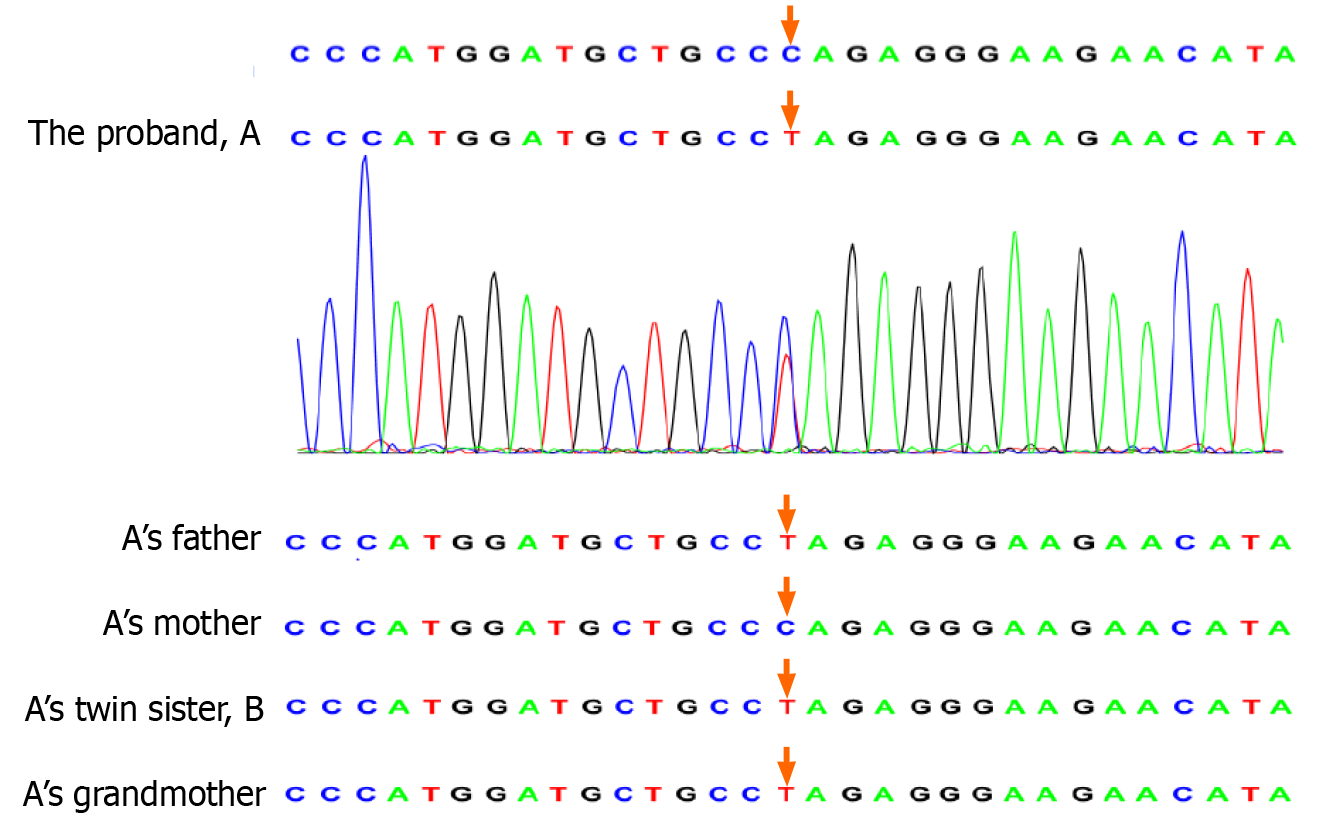
We performed trio-whole-exome sequencing on genomic DNA extracted from peripheral blood of the twins and their parents. The sequencing results revealed a rare mutation in MKRN3 in the twins resulting in the substitution of a single base (c.841C>T). The father was heterozygous for the mutation, whereas the mother carried wild-type MKRN3. We obtained blood samples from the grandparents, and DNA was extracted and subjected to reverse transcription polymerase chain reaction amplification. We then performed direct sequencing of the MKRN3 target site in the proband’s cDNA, which showed that the mutation was inherited from the paternal grandmother, who had normal development (Figures 1 and 2).
Central precocious puberty (MKRN3 c.841C>T).
Gonadotropin-releasing hormone (GnRH) analog (3.75 mg every 4 wk, subcutaneous route).
After 3 mo of treatment, levels of LH, FSH and estradiol (E2) decreased to normal levels.
MKRN3 is an intronless gene located in the Prader-Willi syndrome region on chromosome 15q11.2. Based on whole-exome sequencing of 40 members from 15 families diagnosed with CPP, Abreu et al[6] confirmed in 2013 that MKRN3 mutation is associated with CPP.
MKRN3 is a maternally imprinted or silenced gene and is expressed only from the paternal allele. MKRN3 is a member of the Makorin family of proteins that contain special zinc-finger motifs and have a highly conserved structure among multiple species[6]. MKRN family proteins MKRN1-4 contain three CH3 zinc-finger motifs, a characteristic RING finger domain (C2HC4 motif), and a CH motif that is rich in Cys-His. The C3H motifs are associated with RNA binding activity. The RING finger motif is present in many E3 ubiquitin ligases and is responsible for ubiquitin-ligase activity[5]. As with other Makorin family members, the transcript levels of MKRN3 in several species are maximal during the very first developmental stages and sharply decrease over time. Previous studies showed that MKRN3 transcripts progressively decrease in the mouse hypothalamus during the first two postnatal weeks[5]. This was further verified by reports of lower serum MKRN3 levels in girls with CPP than in healthy girls[4-6].
CPP is caused by premature activation of the HPG axis and an increase in the amplitude and frequency of GnRH pulses. GnRH is produced in the hypothalamus and regulated by kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are critical in pulse generation, steroid negative feedback, puberty and other functions. Previous human genetic studies have demonstrated the crucial role of kisspeptin and neurokinin B in stimulating GnRH secretion; dynorphin restrains secretion of GnRH[7,8]. One study reported that MKRN3 selectively inhibits the promoter activity of the KISS1 and TAC3 genes, which encode kisspeptin and tachykinin-3, in the arcuate nucleus independent of its RING finger domain[8]. Liu et al[9] found that the RING domain of MKRN3 interacts with Nptx1, one of the earliest activated extracellular signals responding upon GnRH neuron induction when puberty begins, leading to polyubiquitination and suppression of Nptx1 activity during the initiation of puberty. Both in vivo and in vitro experiments have shown that microRNA-30 targets the 3’-untranslated region of the MKRN3 mRNA to control pubertal initiation. Injection of microRNA-30 inhibitors during prepuberty reverses downregulation of the hypothalamic MKRN3 protein and delays the onset of female puberty[10]. Therefore, MKRN3 is extremely essential in the initiation of puberty, and this mechanism likely underlies its association with CPP.
We identified a rare missense mutation in MKRN3 (c.841C>T) in a 6 year and 9 mo-old girl with CPP with a twin sister who also showed premature thelarche. Whole-exome sequencing revealed that the mutation was inherited from the father, whose pubertal development was normal. The c.841C>T mutation is located in the CH motif and results in a single amino acid change in the MKRN3 protein. Thus far, 50 MKRN3 mutations associated with CPP have been reported, including 14 frameshift mutations, 27 missense mutations, 4 nonsense mutations and 5 variants in upstream promoter or regulatory regions[3,5]. The current case is only the second report of the c.841C>T mutation in MKRN3[11]. Notably, this is also the first case of MKRN3 mutation in twin sisters. It is worth noting that mutations in MKRN3 are more common in patients from Western countries than in those from Asian countries[3], which may result from genetic differences in ethnic groups or the less frequent use of gene testing among children with CPP in Asian countries.
A systematic review and meta-analysis of the clinical features of patients with CPP carrying MKRN3 mutations showed that MKRN3 mutations are associated with non-syndromic CPP. Girls are more severely affected by CPP than boys and often experience pubertal initiation at an early age, with higher basal FSH levels[12]. However, no studies have investigated associations between genotypes and clinical phenotypes of CPP.
Long-acting GnRH analogs are considered the gold standard treatment of CPP, and this treatment is typically administered by intramuscular injection or subcutaneous implantation[1]. Whether GnRH is effective for CPP patients with MKRN3 mutation has not been completely determined. A retrospective study revealed no significant differences in mean LH or target height between CPP patients with or without MKRN3 mutations after GnRH treatment[13]. In our study, after patient B was treated with three doses of GnRH analog (3.75 mg every 4 wk), LH, FSH and E2 decreased to normal levels, without any progression of puberty signs, suggesting that the GnRH analog is effective in the treatment of CPP patients with MKRN3 mutation.
MKRN3 mutation is the most frequent genetic etiology of CPP. Here, we report twin sisters who presented premature thelarche and were diagnosed with CPP with a rare mutation in MKRN3 (c.841C>T).
The authors would like to thank the probands and their family for agreeing to participate in this research.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abubakar MS, Cimen SG S-Editor: Wang JJ (Online Science Editor) L-Editor: A P-Editor: Ma YJ
| 1. | Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016;4:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 318] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 2. | Yoo JH. Effects of early menarche on physical and psychosocial health problems in adolescent girls and adult women. Korean J Pediatr. 2016;59:355-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Liu M, Fan L, Gong CX. A novel heterozygous MKRN3 nonsense mutation in a Chinese girl with idiopathic central precocious puberty: A case report. Medicine (Baltimore). 2020;99:e22295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Roberts SA, Kaiser UB. GENETICS IN ENDOCRINOLOGY: Genetic etiologies of central precocious puberty and the role of imprinted genes. Eur J Endocrinol. 2020;183:R107-R117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Maione L, Naulé L, Kaiser UB. Makorin RING finger protein 3 and central precocious puberty. Curr Opin Endocr Metab Res. 2020;14:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Abreu AP, Dauber A, Macedo DB, Noel SD, Brito VN, Gill JC, Cukier P, Thompson IR, Navarro VM, Gagliardi PC, Rodrigues T, Kochi C, Longui CA, Beckers D, de Zegher F, Montenegro LR, Mendonca BB, Carroll RS, Hirschhorn JN, Latronico AC, Kaiser UB. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368:2467-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 338] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 7. | Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy Cells Revisited. Endocrinology. 2018;159:3219-3234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Abreu AP, Toro CA, Song YB, Navarro VM, Bosch MA, Eren A, Liang JN, Carroll RS, Latronico AC, Rønnekleiv OK, Aylwin CF, Lomniczi A, Ojeda S, Kaiser UB. MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J Clin Invest. 2020;130:4486-4500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Liu H, Kong X, Chen F. Mkrn3 functions as a novel ubiquitin E3 ligase to inhibit Nptx1 during puberty initiation. Oncotarget. 2017;8:85102-85109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Heras V, Sangiao-Alvarellos S, Manfredi-Lozano M, Sanchez-Tapia MJ, Ruiz-Pino F, Roa J, Lara-Chica M, Morrugares-Carmona R, Jouy N, Abreu AP, Prevot V, Belsham D, Vazquez MJ, Calzado MA, Pinilla L, Gaytan F, Latronico AC, Kaiser UB, Castellano JM, Tena-Sempere M. Hypothalamic miR-30 regulates puberty onset via repression of the puberty-suppressing factor, Mkrn3. PLoS Biol. 2019;17:e3000532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Lee HS, Jin HS, Shim YS, Jeong HR, Kwon E, Choi V, Kim MC, Chung IS, Jeong SY, Hwang JS. Low Frequency of MKRN3 Mutations in Central Precocious Puberty Among Korean Girls. Horm Metab Res. 2016;48:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Valadares LP, Meireles CG, De Toledo IP, Santarem de Oliveira R, Gonçalves de Castro LC, Abreu AP, Carroll RS, Latronico AC, Kaiser UB, Guerra ENS, Lofrano-Porto A. MKRN3 Mutations in Central Precocious Puberty: A Systematic Review and Meta-Analysis. J Endocr Soc. 2019;3:979-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Ramos CO, Macedo DB, Canton APM, Cunha-Silva M, Antonini SRR, Stecchini MF, Seraphim CE, Rodrigues T, Mendonca BB, Latronico AC, Brito VN. Outcomes of Patients with Central Precocious Puberty Due to Loss-of-Function Mutations in the MKRN3 Gene after Treatment with Gonadotropin-Releasing Hormone Analog. Neuroendocrinology. 2020;110:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |