Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9680
Peer-review started: July 13, 2021
First decision: August 19, 2021
Revised: August 25, 2021
Accepted: September 10, 2021
Article in press: September 10, 2021
Published online: November 6, 2021
Processing time: 108 Days and 1.1 Hours
Squamous cell carcinoma (SCC) in pancreas and stomach is a rare histologic subtype with aggressive behavior, poor prognosis, and no standardized therapy. Pancreatic SCC or gastric SCC has been previously reported. However, case of SCC occurring in both the pancreas and the stomach has not been reported yet.
A 75-year-old female with prior history of hypertension and diabetes mellitus visited our hospital with complaint of abdominal pain that started three months ago. Computed tomography (CT) scan of the abdomen showed 3.3 cm mass at the distal pancreas. She received surgical resection which was histologically found to be SCC of the pancreas with clear resection margins. After she was discharged, she no longer visited the hospital. Three years later, she was referred to our hospital after showing abnormal findings on a gastroscopy performed at another hospital. Gastroscopy revealed a single, 2cm sized, ill-defined irregular flat and hyperemic mass at high body. Histologic finding of the mass was SCC. CT scan and positive emission tomography CT showed metastatic lesions to the liver and the peritoneum. She received combination chemotherapy with capecitabine and oxaliplatin. However, she passed away 6 mo after diagnosis of gastric SCC.
To the best of our knowledge, this is the first case of metachronous SCC of stomach occurring after diagnosis of pancreatic SCC.
Core Tip: Pancreatic squamous cell carcinoma (SCC) and gastric SCC are very rare malignancies with aggressive behavior, poor prognosis, and no standardized therapy. To the best of our knowledge, this is the first case report of metachronous gastric SCC occurring at three years after curative resection of pancreatic cancer.
- Citation: Kim JH, Kang CD, Lee K, Lim KH. Metachronous squamous cell carcinoma of pancreas and stomach in an elderly female patient: A case report. World J Clin Cases 2021; 9(31): 9680-9685
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9680.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9680
Squamous cell carcinoma (SCC) is most frequently found in skin, head and neck region, oral cavity, esophagus, lung, cervix, and anus. Cells lining stomach and pancreas are glandular epithelium. Thus, adenocarcinoma is the most common histopathologic type. It has been reported in up to 90% of all gastric cancer and pancreatic cancer[1,2]. Gastric SCC is very rare, accounting for about 0.04% to 0.2% of all gastric carcinomas[3-5]. About 100 cases have been reported since its first report in 1905[6,7]. Pancreatic SCC is another rare neoplasm. About 50 cases have been reported, accounting for about 0.5% to 5% of all pancreatic neoplasms[8]. For both gastric SCC and pancreatic SCC, clinicopathologic characteristics and pathogenesis of are poorly understood. Therefore, currently there is no established standard treatment for them. The prognosis is known to be very poor for both gastric SCC and pancreatic SCC[6-8].
Metachronous cancers are defined as those occurring more than 6 mo later after the first primary cancer. Since both pancreatic SCC and gastric SCC are very rare, there has been no report of metachronous occurrence of these cancers. Here, we report an unusual case of metachronous gastric SCC that occurring three years after the diagnosis of pancreatic SCC.
A 75-year-old female with complaint of abdominal pain visited our hospital in August, 2015.
Abdominal pain was mainly located on the epigastric area which started three months ago accompanied by dyspepsia. She denied diarrhea, vomiting, weight loss, and fever.
The patient had hypertension and diabetes. She had no history of surgery.
She had no prior history of smoking or drinking alcohol. No family history was noted.
Her vital sign was stable, with blood pressure of 130/90 mmHg, heart rate of 98 beats per minute, and body temperature of 36.8 ℃. Physical examination of the abdomen revealed sharp tenderness at the epigastric area.
Routine laboratory test showed slight elevation of white blood cell (13600/mm3), C-reactive protein (5.199 mg/dL), and tumor marker CA 19-9 (488 U/mL). Other laboratory test results were within reference ranges. Her aspartate aminotransferase, alanine aminotransferase, and total bilirubin levels were also within reference ranges.
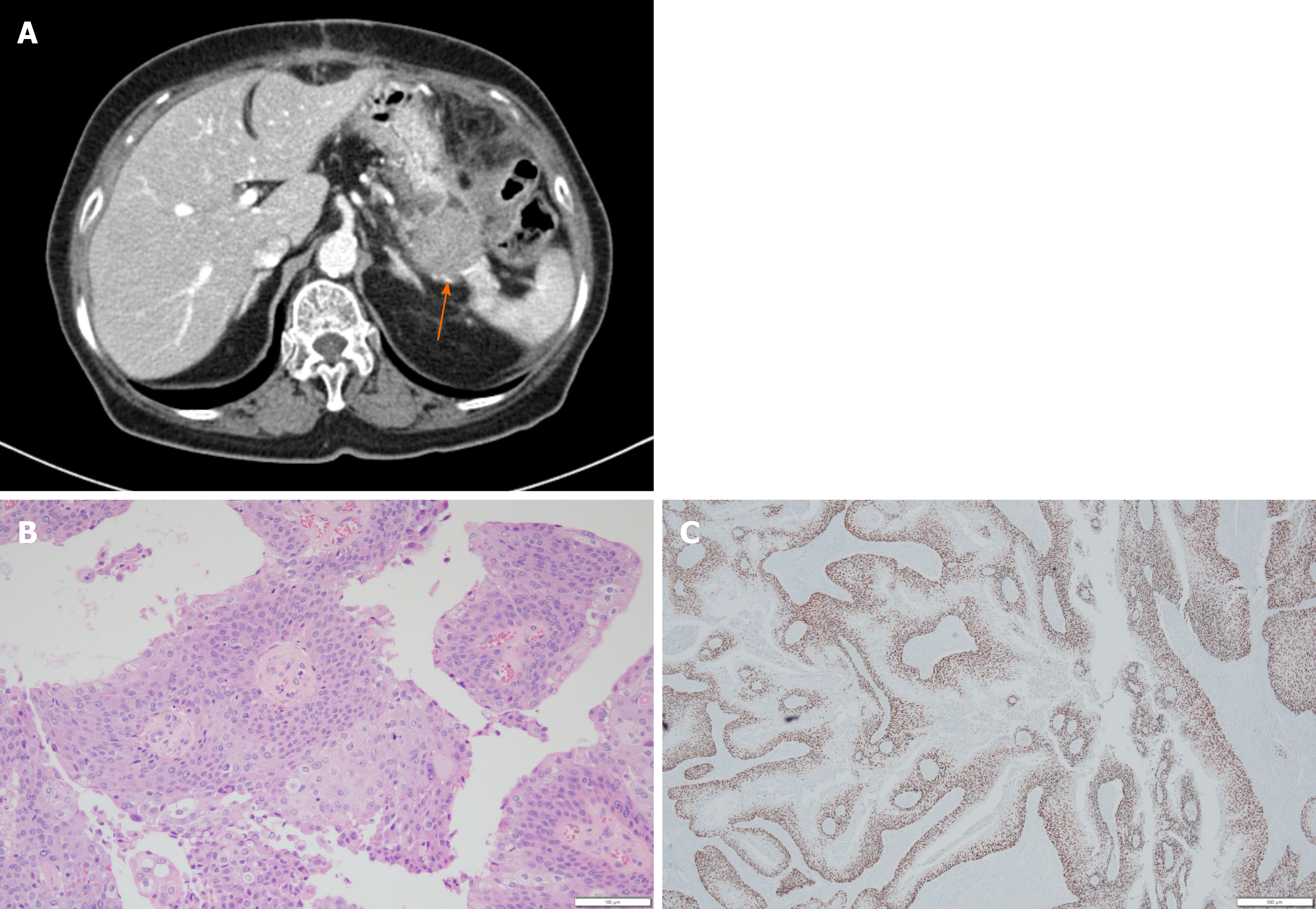
Computed tomography (CT) scan was done to further evaluate the cause of pain. It revealed a 3.3 cm sized mass at the tail of the pancreas (Figure 1A). Under clinical impression of resectable pancreatic cancer, she underwent distal pancreatectomy. Histopathologic finding of the surgical specimen was moderately differentiated SCC with papillary growth pattern and clear resection margin (Figure 1B). Immunohistochemical staining showed that tumor cells were positive for p63 (Figure 1C) but negative for CK7 and CK20.
The patient recovered without complications after surgery. She was discharged from our hospital. After discharge, she needed periodic outpatient follow-up to check for recurrence. However, she no longer visited the hospital.
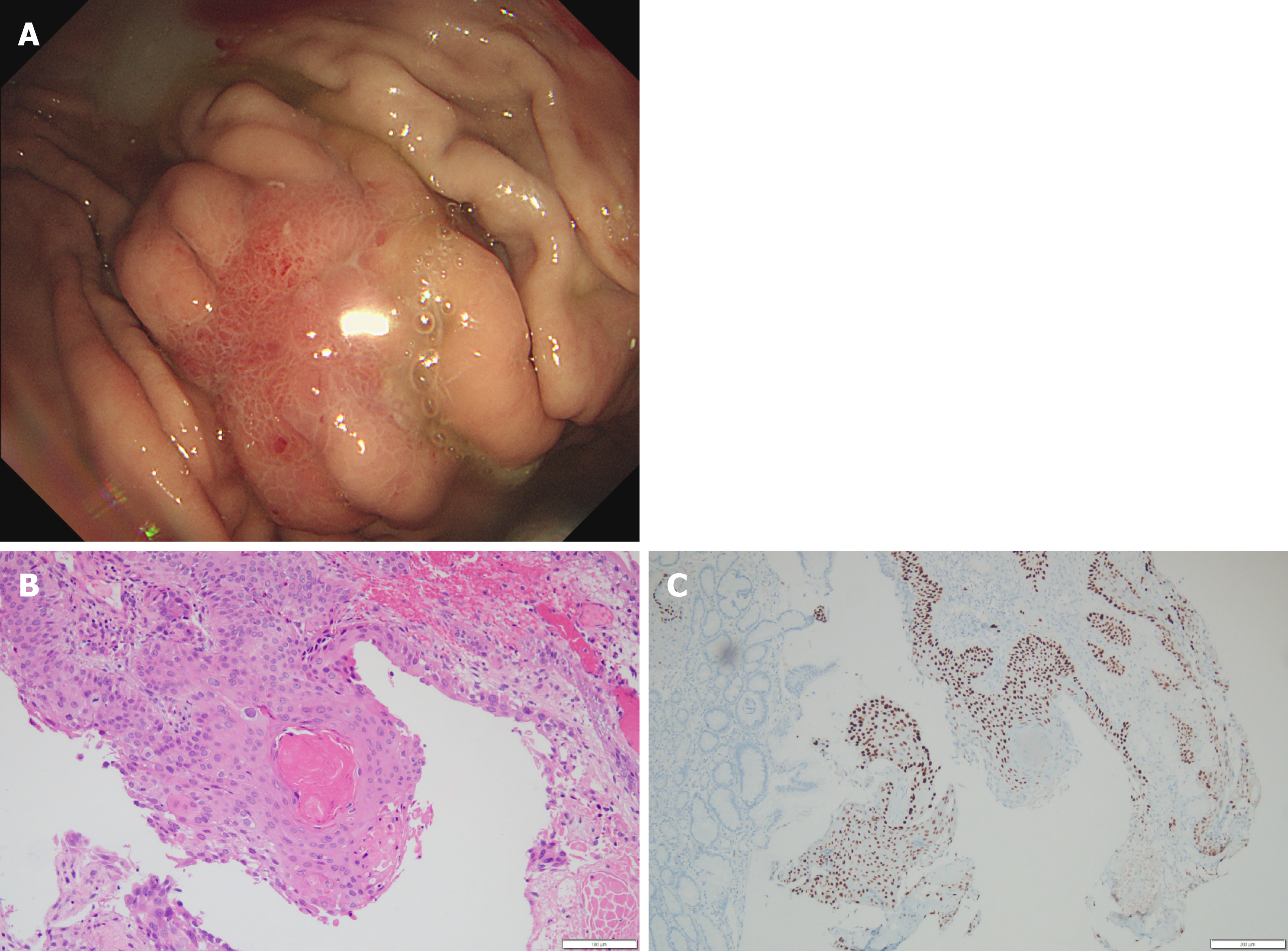
Three years later, in 2018, she received a gastroscopy for regular check-up at another hospital. She was referred to our hospital with clinical impression of gastric cancer. Gastroscopy showed a single, 2 cm sized, ill-defined irregular flat and hyperemic mass at the greater curvature side of high body with mucosa friability (Figure 2A). Biopsy was performed. Moderately differentiated SCC was noted in the biopsy specimen (Figure 2B). Tumor cells were positive for p63 (Figure 2C) but negative for CK7 and CK20. These histopathologic findings in the stomach were consistent with those of the pancreas that had previously undergone surgery. CT scan & positive emission tomography CT scan for staging work-up revealed multiple hepatic and peritoneal metastasis.
The final diagnosis of the presented case was metachronous gastric SCC that occurred after the diagnosis of pancreatic SCC.
Under the clinical diagnosis of advanced stage gastric SCC, she received combination chemotherapy with capecitabine and oxaliplatin.
After receiving three cycles of combination chemotherapy, CT scan for response evaluation showed a stable disease. During additional chemotherapy, she wanted to stop the chemotherapy due to chemotherapy induced mucositis and general weakness. Since then, she received conservative treatment for symptom control. She died 6 mo later in 2019 from disease progression.
It has been suggested that patients diagnosed with one type of cancer have higher likelihood of developing another type of cancer compared to the general population[9]. Although the reason is yet to be identified, factors to be considered include more frequent screening methods, recent improvement of imaging modalities, exposure to radiotherapy and hormonal therapy, environmental factors, and genetic factors. We report the first case of metachronous gastric SCC following pancreatic SCC.
p63 is a known marker of squamous differentiation. It is normally found in progenitor cells of stratified epithelia. Expression of p63 is diffuse in SCC. Pancreatic SCC is a rare neoplasm accounting for up to 5% of pancreatic neoplasms[3-5]. The pathogenesis of pancreatic SCC is unclear. Proposed theories include squamous metaplasia from chronic inflammation leading to malignant transformation, transition from previous adenocarcinoma to SCC, or arising from precursor progenitor cell[8]. Although there is no standardized treatment for pancreatic SCC, treatment options include surgical resection, chemotherapy, and radiotherapy[10]. The biologic behavior of pancreatic SCC from published literature highlights its propensity to affect elderly patients, metastasis at the time of diagnosis, poor response to chemotherapy and radiation therapy, and an extremely short survival period[11]. Several studies have suggested an improved survival with chemotherapeutic agents using a combination of 5-fluorouracil, cisplatin, and vinblastine, gemcitabine, or nanosomal paclitaxel[11-14]. Prognosis still remains dismal with most cases undergoing dissemination at the time of diagnosis. The median overall survival for patients who had undergone curative resection and those who had not were 7 mo (range: 6-16 mo) and 3 mo (range: 0.25-9 mo), respectively[10].
Gastric SCC is another rare disease accounting for 0.04% to 0.2% of all gastric carcinomas[3-5]. According to the Japanese classification, primary gastric SCC can be diagnosed if all tumor cells are identified as squamous cell and if there is evidence that carcinoma arises from gastric mucosa[13]. Although the pathogenesis of gastric SCC is yet to be elucidated, hypothesis includes squamous differentiation of existing adenocarcinoma, squamous metaplasia of gastric mucosa prior to malignant transformation, differentiation from multipotent stem cells, translocation of ectopic squamous cell in gastric mucosa, gastric vascular endothelial cell, and effect of viruses such as Epstein-Barr virus and human papilloma virus. Similar to pancreatic SCC, there is no established standardized treatment. For cases with resectable gastric SCC, radical resection is recommended to improve survival[14]. However, it is usually impossible as most gastric SCCs are found at advanced stages. For metastatic gastric SCC, chemotherapy may be considered as a treatment option. Single or combination regimens of agents such as 5-fluorouracil, platinum, and taxane is usually used[15-17]. However, currently there is no established optimal regimen. Overall survival of metastatic gastric SCC has been reported to be 7 mo[16], which is shorter than that (12-16 mo) of gastric adenocarcinoma.
The patient had received curative resection for pancreatic SCC. Three years later, she was diagnosed with gastric SCC which had metastasized to the liver and the peritoneum. As direct invasion from pancreatic SCC to the stomach has been reported[18,19], it can be argued that the gastric SCC might be relapsed lesion of the previous pancreatic SCC. We think it is more reasonable to judge it as a metachronous case for the following reasons. First, given the aggressive nature of pancreatic SCC, median overall survival of patients with curative resection of pancreatic SCC is 7 mo (range: 6-16 mo), with 1 year survival rate of 4.8%[8]. However, our patient survived for 3 years after the diagnosis of PSCC. Second, according to gastroscopy findings, it is a single mucosal lesion in the stomach, not a direct invasion of other organs or multiple mucosal lesions. Third, stomach is an unusual site for metastasis. Primary origins of cancers associated with metastasis to the stomach are known to be lung, breast, esophagus, and melanoma.
For pancreatic SCC and gastric SCC, each of them is an extremely rare and aggressive tumor. To the best of our knowledge, this is the first case report of metachronous SCC on stomach occurring after the diagnosis of pancreatic SCC. Based on the rare incidence of this histologic subtype in the pancreas and the stomach, there is a need for ongoing research on their diagnosis and treatment options.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li JL, Zhao J S-Editor: Yan JP L-Editor: A P-Editor: Xing YX
| 1. | Thomas RM, Sobin LH. Gastrointestinal cancer. Cancer. 1995;75:154-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Capella C, Albarello L, Capelli P, Sessa F, Zamboni G; Gruppo Italiano Patologi Apparato Digerente (GIPAD); Società Italiana di Anatomia Patologica e Citopatologia Diagnostica/International Academy of Pathology, Italian division (SIAPEC/IAP). Carcinoma of the exocrine pancreas: the histology report. Dig Liver Dis. 2011;43 Suppl 4:S282-S292. [PubMed] |
| 3. | Chang YS, Kim MS, Kim DH, Park S, You JY, Han JK, Kim SH, Lee HJ. Primary Squamous Cell Carcinoma of the Remnant Stomach after Subtotal Gastrectomy. J Gastric Cancer. 2016;16:120-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Dong C, Jiang M, Tan Y, Kong Y, Yang Z, Zhong C, Li D, Yuan Y. The clinicopathological features and prognostic factors of gastric squamous cell carcinoma. Medicine (Baltimore). 2016;95:e4720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Gao S, Chen D, Huang L, Dai R, Shan Y. Primary squamous cell carcinoma of the stomach: a case report and literature review. Int J Clin Exp Pathol. 2015;8:9667-9671. [PubMed] |
| 6. | Bonnheim DC, Sarac OK, Fett W. Primary squamous cell carcinoma of the stomach. Am J Gastroenterol. 1985;80:91-94. [PubMed] |
| 7. | Callacondo D, Ganoza-Salas A, Anicama-Lima W, Quispe-Mauricio A, Longacre TA. Primary squamous cell carcinoma of the stomach with paraneoplastic leukocytosis: a case report and review of literature. Hum Pathol. 2009;40:1494-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Ntanasis-Stathopoulos I, Tsilimigras DI, Georgiadou D, Kanavidis P, Riccioni O, Salla C, Psaltopoulou T, Sergentanis TN. Squamous cell carcinoma of the pancreas: A systematic review and pooled survival analysis. Eur J Cancer. 2017;79:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Torina TB, Hudspeth EL, Chun JM, Zaloga W, Alderink C, Abdeen Y. An Unusual Occurrence of Multiple Metachronous and Synchronous Primary Cancers in a Female Patient. Case Rep Oncol Med. 2020;2020:5691732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Brown HA, Dotto J, Robert M, Salem RR. Squamous cell carcinoma of the pancreas. J Clin Gastroenterol. 2005;39:915-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Al-Shehri A, Silverman S, King KM. Squamous cell carcinoma of the pancreas. Curr Oncol. 2008;15:293-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Anagnostopoulos GK, Aithal GP, Ragunath K, Kaye P, Rowlands BJ. Squamous cell carcinoma of the pancreas: report of a case and review of the literature. JOP. 2006;7:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 960] [Reference Citation Analysis (0)] |
| 14. | Schmidt C, Schmid A, Lüttges JE, Kremer B, Henne-Bruns D. Primary squamous cell carcinoma of the stomach. Report of a case and review of literature. Hepatogastroenterology. 2001;48:1033-1036. [PubMed] |
| 15. | Guzman Rojas P, Parikh J, Vishnubhotla P, Oharriz JJ. Primary Gastric Squamous Cell Carcinoma. Cureus. 2018;10:e2389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Meng Y, Zhang J, Wang H, Zhang Y, Sun R, Zhang Z, Gao F, Huang C, Zhang S. Poorer prognosis in patients with advanced gastric squamous cell carcinoma compared with adenocarcinoma of the stomach: Case report. Medicine (Baltimore). 2017;96:e9224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Vailas MG, Syllaios A, Hasemaki N, Sotiropoulou M, Mpaili E, Sarlanis H, Felekouras E, Papalampros A. A type of neoplasia deadlier than gastric adenocarcinoma? World J Surg Oncol. 2019;17:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Rana SS, Bhasin DK, Jain K, Nada R, Sinha SK, Singh K. Endoscopic diagnosis of squamous cell carcinoma of the pancreas invading the stomach. JOP. 2009;10:181-183. [PubMed] |
| 19. | De Palma GD, Masone S, Rega M, Simeoli I, Donisi M, Addeo P, Iannone L, Pilone V, Persico G. Metastatic tumors to the stomach: clinical and endoscopic features. World J Gastroenterol. 2006;12:7326-7328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |