Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9509
Peer-review started: July 12, 2021
First decision: August 18, 2021
Revised: August 31, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: November 6, 2021
Processing time: 109 Days and 3 Hours
Osteoporosis with vertebral compression fractures is increasingly common in the elderly population. Cement augmentation is one of the effective surgical treatments for these patients. Currently, there are several different types of cement augmentation treatments. No studies have compared the safety and efficacy of different cement augmentation types for the treatment of such fractures; thus, we retrospectively compared vertebroplasty, balloon kyphoplasty, and kyphoplasty with SpineJack or an intravertebral expandable pillar.
To compare the postoperative safety and efficacy of each surgical intervention in treating vertebral compression fractures.
We retrospectively analyzed 354 patients with acute vertebral compression fractures, defined as signal changes in the T1 weighted magnetic resonance imaging, and randomly divided the patients into five groups. Their visual analog scale scores for pain, kyphotic angle, average body height, rate of cement leakage, and occurrence of adjacent vertebral compression fractures were followed for 1 year. One-way analysis of variance, the post hoc Bonferroni test, and Fisher exact probability test were used for statistical analyses.
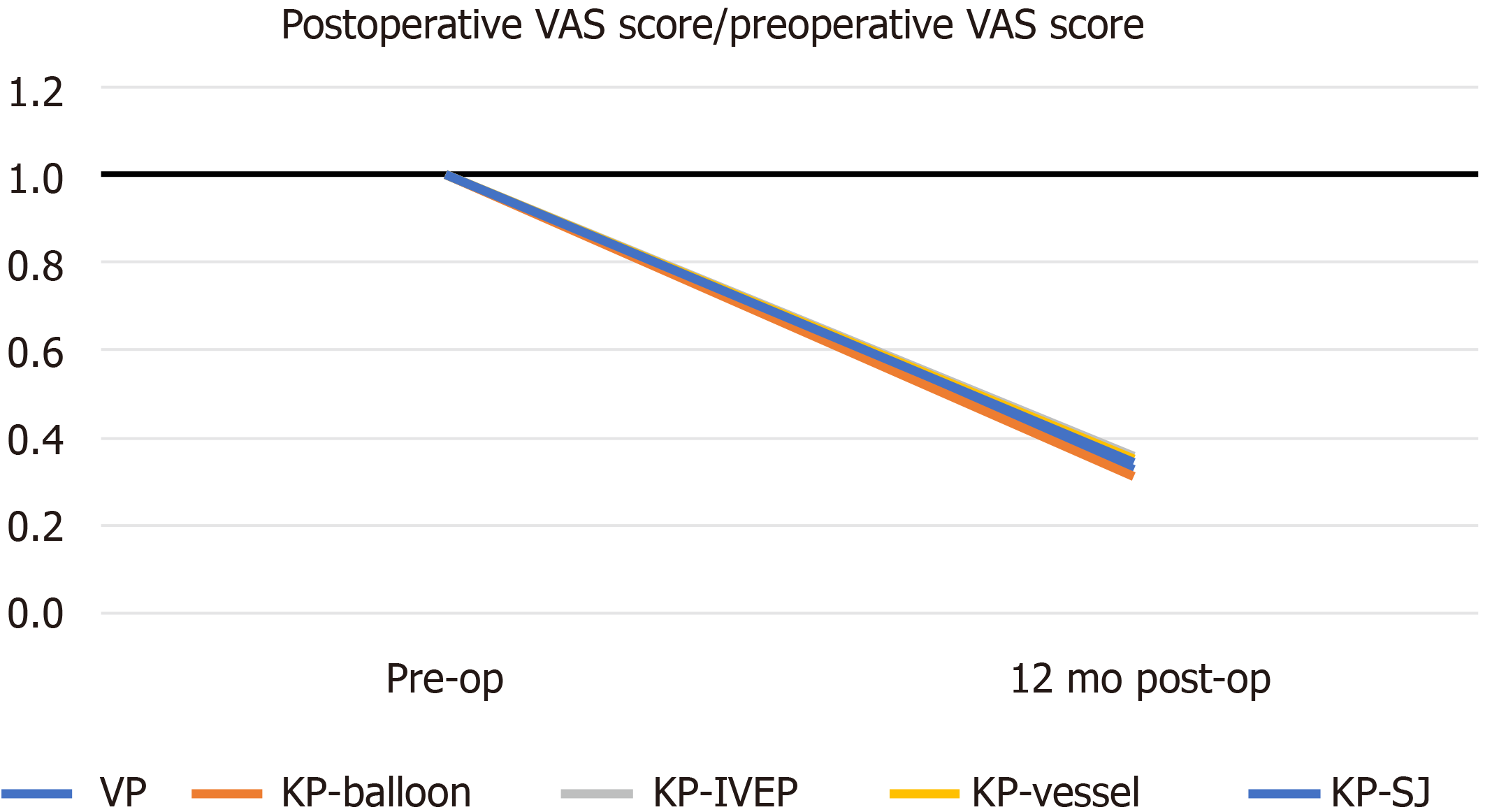
All pain scores significantly improved 12 mo postoperatively; however, there was no significant difference between the groups (P = 0.325). Kyphoplasty with SpineJack significantly reduced the kyphotic angle (P = 0.028) and restored the height of the vertebral body (P = 0.02). The rate of adjacent compression fractures was the highest in the vertebroplasty group, with a statistically significant difference according to the Fisher exact probability test (P = 0.02). The treatment with the lowest cement leakage rate cannot be identified because of the small sample size; however, kyphoplasty with SpineJack, an IVEP, and vesselplasty resulted in lower rates of cement leakage than balloon kyphoplasty and vertebroplasty.
Kyphoplasty with SpineJack has good outcomes in kyphotic angle reduction and body height restoration. Vertebroplasty has the highest cement leakage rate and adjacent compression fracture occurrence.
Core Tip: The outcomes of different surgical treatments for vertebral compression fractures are discussed in this study. Kyphoplasty with SpineJack was found to have the best kyphotic angle reduction, body height restoration, and rate of cement leakage. Nevertheless, vertebroplasty was associated with the highest rate of cement leakage and adjacent compression fracture occurrence. There was no significant difference between each surgical group in terms of pain relief, which was quantified using visual analog scale scores.
- Citation: Yeh KL, Wu SH, Liaw CK, Hou SM, Wu SS. Outcomes of different minimally invasive surgical treatments for vertebral compression fractures: An observational study. World J Clin Cases 2021; 9(31): 9509-9519
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9509.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9509
Osteoporosis in the elderly population is becoming increasingly common with the increasing age of the population and affects approximately 35% of women older than 65 years of age[1]. Vertebral compression fractures (VCFs) represent a common problem in osteoporosis[2]. VCFs destabilize the vertebral body, whereas back pain is attributed to macro- and micromotion at the fracture site. In addition to back pain, the sequelae of VCFs are sometimes related to kyphotic angulation of the spine, which can diminish forced vital capacity[3,4].
Management of VCFs includes both conservative treatments and surgical interventions. Conservative treatments include bed rest or activity modification, analgesics, and bracing; however, for most patients, conservative treatment is ineffective and unsatisfactory[5]. The kyphotic change in spine alignment reduces the lung cavity and shifts the center of gravity forward; thus, for elderly patients, bed rest for long periods is associated with complications, such as pneumonia, urinary infections, and deep vein thrombosis[6,7]. Ultimately, conservative treatment does not correct spinal malalignment.
Surgical treatments for VCFs are increasingly crucial as they result in improvements in back pain and function, as well as quality of life following treatment[8]. There are several reported real-world practice and minimally invasive management options for VCFs, such as vertebroplasty (VP), balloon kyphoplasty (KP), KP with SpineJack, KP with an intravertebral expandable pillar (IVEP), and KP with vesselplasty. However, to date, no studies have compared their efficacy and safety, which was the main purpose of the present retrospective observational study. All the patients underwent surgical treatment by the same doctor, Professor Shing-Sheng Wu. Emphasis was placed on the visual analog scale (VAS) scores for pain, kyphotic angle (KA) reductions, average body height (AVBH) restorations, complications, and occurrence of adjacent VCFs during the 1-year follow-up.
The inclusion criteria were as follows: (1) Patients aged ≥ 50 years; (2) those with bone mineral density < -1.0 [suggestive of osteopenia (between -1.0 and -2.5) or osteoporosis (less than -2.5) according to the definition by the World Health Organization]; (3) those with severe back pain (VAS score ≥ 6); (4) acute (< 2 wk), subacute (2 wk to 3 mo), or nonunion VCFs; (5) tenderness on physical examination; and (6) magnetic resonance imaging (MRI) showing bone marrow edema (T1-signal alteration in the vertebral body on MRI performed within 4 wk prior to hospitalization). All patients underwent surgical treatment by the same doctor, Professor Wu. The exclusion criteria were as follows: (1) Patients without regular postoperative follow-up examinations at our outpatient clinic for at least 1 year; (2) cancer-related VCFs, such as metastasis and multiple myeloma; and (3) VCF extending to the posterior vertebral cortex with retropulsed fragment, cord compression, or radiculopathy.
Therefore, we retrospectively collected the data of 354 patients with VCFs treated between 2009 and 2019. The levels of VCFs ranged from T6 to L4. The patients were randomly divided into five groups: VP (88 patients; 20 men, 68 women), balloon KP (124 patients; 45 men, 79 women), KP with an IVEP (46 patients; 13 men, 33 women), KP with vesselplasty (36 patients; 11 men, 25 women), and KP with SpineJack (Vexim, Balma, France) (60 patients; 18 men, 42 women) (Table 1). The patients were aged 45-86 years. All surgical treatments were performed bipedicularly.
| No. of patients | Age (yr) | Sex (M/F) | Volume of cement | |
| VP | 88 | 74.3 ± 6.4 (50-86) | 20/68 | 3.2 (1.0-7.0) |
| KP with a balloon | 124 | 72.3 ± 7.6 (50-85) | 45/79 | 5.5 ± 0.6 |
| KP with an IVEP | 46 | 72.7 ± 6.4 (50-90) | 13/33 | – |
| KP with vesselplasty | 36 | 77.9 ± 9.5 (65-89) | 11/25 | 5.7 ± 2.0 |
| KP with SJ | 60 | 65.9 ± 1.9 (45-87) | 18/42 | 4.8 ± 0.4 |
The VAS was used to indicate “pain at rest,” with 0 being no pain and 10 being the worst pain ever experienced. The VAS score was evaluated based on the patient’s subjective feelings. The ratio of the postoperative to preoperative VAS score was calculated, and the evaluation was performed 12 mo postoperatively.
We defined AVBH as the average of the anterior edge of the vertebral body height and posterior edge of the vertebral body height in the lateral view of spinal radiographs. The ratio of preoperative AVBH to that at 1 year postoperatively was calculated.
The KA was used to quantify the magnitude of vertebral body wedge deformities. We drew lines along the vertebral upper endplate and lower endplate using lateral spine radiographs. We then measured the angle between the two lines and defined it as the KA. The KA was measured 1 year postoperatively and preoperatively; the ratio of these two data points was then recorded.
We counted the occurrence of adjacent VCFs at 1 year postoperatively. The null hypothesis was that there would be no difference between the groups; once the P-value was less than 0.05, the null hypothesis was rejected.
In this study, we recorded the symptoms of patients with cement leakage events and divided them into symptomatic or asymptomatic episodes.
Analysis of variance (ANOVA) with the post hoc Bonferroni test was used to compare differences in baseline characteristics and percent changes in the VAS score and AVBH at 1 year between the four groups. One-way ANOVA was used to examine the significance of longitudinal changes in VAS scores at 3, 6, and 12 mo. A significance level of P < 0.05 was used for all comparisons.
The Fisher exact probability test was performed to determine the prevalence of adjacent VCFs. The null hypotheses were rejected if P-values were less than 0.05. Statistical analyses were performed using SPSS 12.0 statistical software (IBM Corp.).
A 1-year follow-up was completed for all patients. Compared with baseline scores, we found improvements in VAS scores after VP or KP 12 mo postoperatively. One-way ANOVA revealed no significant difference (P12th month = 0.325) among the various surgical treatments in terms of postoperative VAS scores (Figure 1).
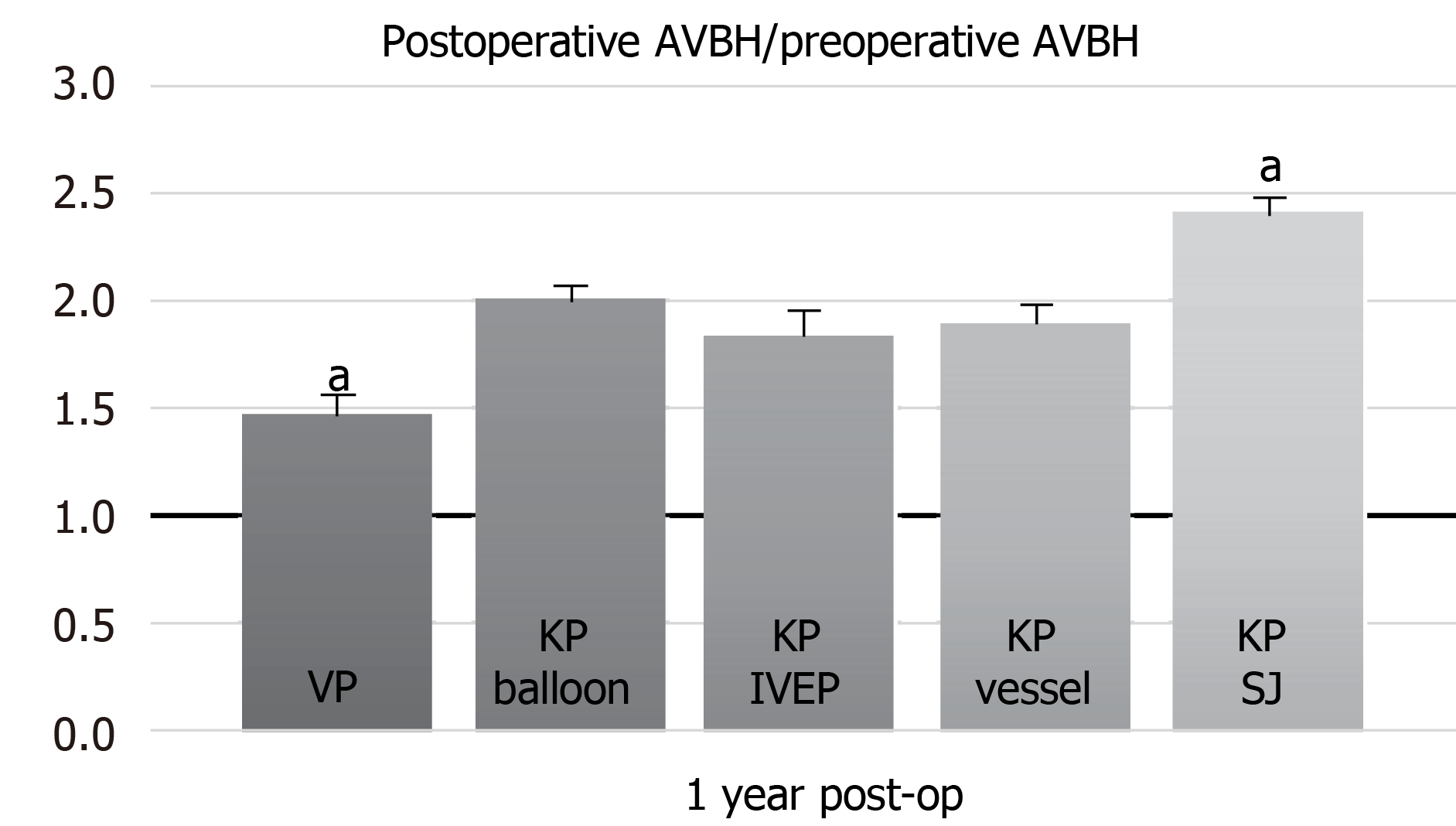
Statistical analysis for AVBH was performed using the ratio of the postoperative to preoperative AVBH via one-way ANOVA, which revealed a significant difference (P = 0.020), followed by post hoc Bonferroni analysis. Significant differences were observed in the VP and KP with SpineJack groups when compared with other groups in the post hoc analysis (P < 0.05) (Figure 2).
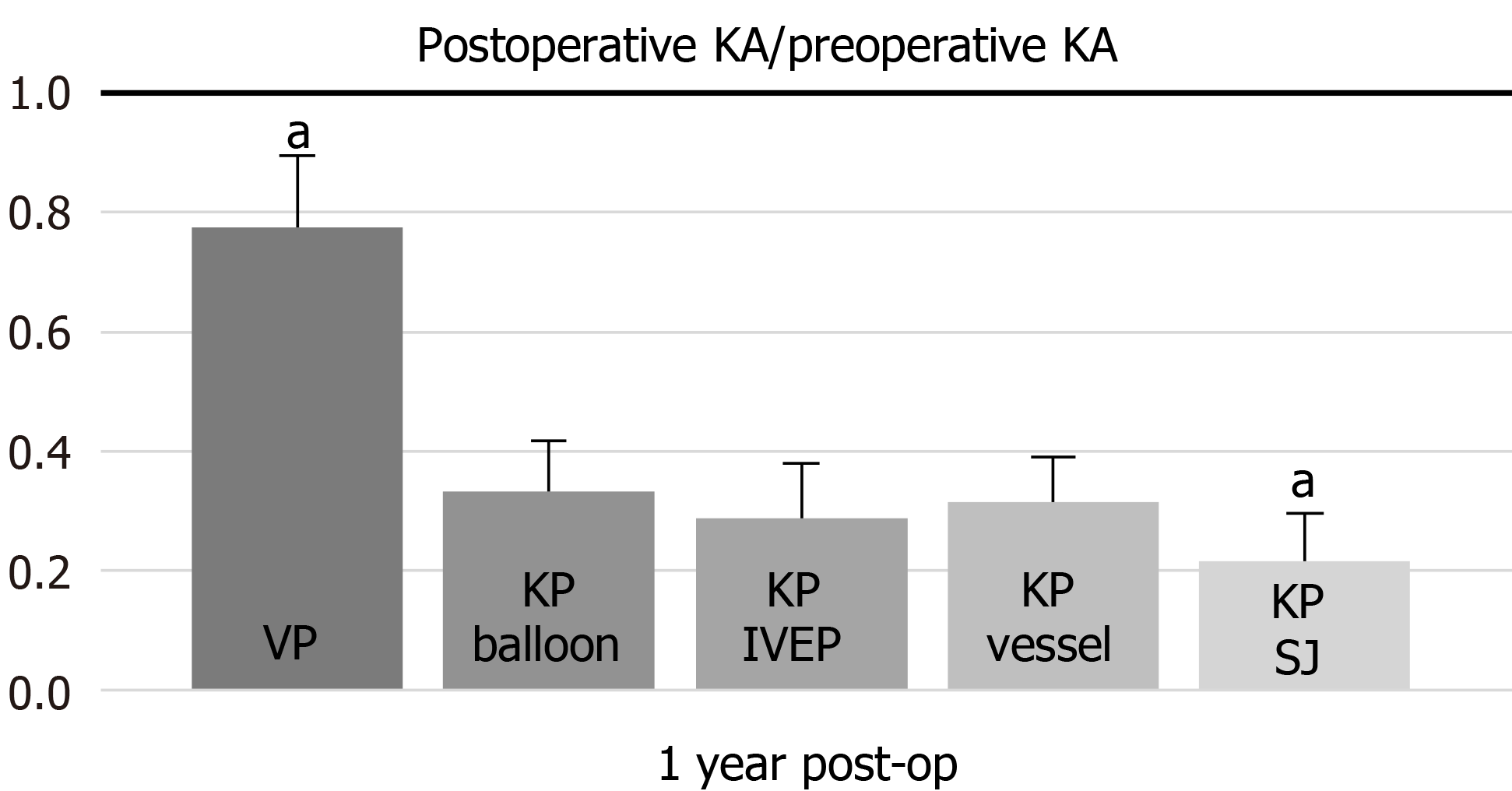
One-way ANOVA of the KA showed that the VP and KP with SpineJack groups showed a significant difference in comparison with the other groups in the post hoc Bonferroni analysis (P < 0.05) (Figure 3).
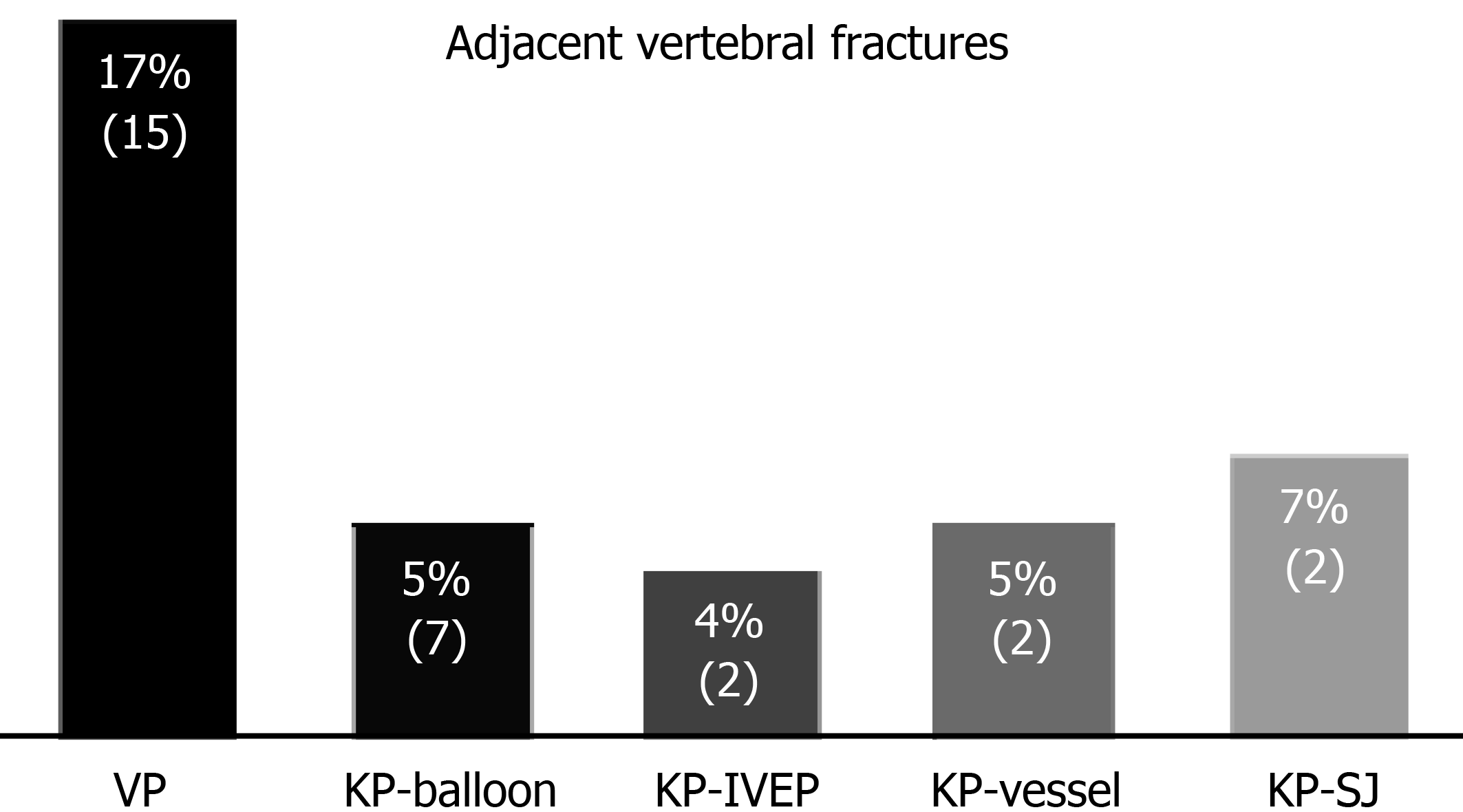
Within 1 year, the numbers of adjacent VCFs were 15 (15%), 4 (6.7%), 7 (5%), 2 (5%), and 2 (4%) in the VP, KP with SpineJack, balloon KP, KP with vesselplasty, and KP groups, respectively (Figure 4). According to the Fisher exact probability test, the P-value was < 0.050 (P = 0.020); thus, the null hypothesis was rejected. Nevertheless, after we excluded the value of the VP group, the Fisher exact probability test was performed again. The P-value was 0.93, which is substantially greater than 0.05.
Cement leakage is the most common complication among surgical treatments for VCF. In the VP group, there were 24 cases of cement leakage, with symptomatic problems occurring in eight patients. In the balloon KP group, cement leakage occurred in 30 cases, with seven symptomatic cases. One (1.7%) patient who underwent KP with SpineJack treatment suffered from non-symptomatic cement leakage. Failure of symptom relief occurred in only one (2%) patient who underwent KP with an IVEP due to implant migration. Similarly, one patient who underwent KP with vesselplasty suffered from symptomatic problems due to cement leakage.
VCFs cause debilitating back pain, and as a result of the aging population, the incidence of these fractures is increasing[9]. Conservative treatment for compression fractures has not been suggested, as it fails to restore the vertebral body to its origin height. Additionally, pain and spinal deformity are not improved or corrected, and the domino effect follows rapidly after the first VCF[10]. This effect is a direct consequence of the mechanical variations that affect the spine when physiological curves are modified[11]. Treatment of VCFs by VP or KP maintains the integrity of adjacent structures and reduces the likelihood of a domino effect[10]; thus, the role of surgical treatment has increased.
Currently, percutaneous cement augmentation procedures are gaining popularity[12]. VP or KP can provide immediate effective pain relief for patients with VCFs[13]. To date, several papers regarding minimally invasive cement augmentation procedures have been published. Nevertheless, no study has compared the efficacy and safety of these procedures; therefore, we compared VP, balloon KP, KP with an IVEP, KP with vesselplasty, and KP with SpineJack, performed via a bipedicular transpedicular approach by the same surgeon in five similar groups of patients.
The VAS is the most common pain measurement method. Several studies have found a significant improvement in pain after VP or KP[14,15]. These results are in line with those of our studies; we found that both VP and KP were effective in reducing pain, as measured by the VAS score at 12 mo postoperatively. The VAS score was not significantly different between the KP and VP groups at 12 mo postoperatively. The results showed that once polymethylmethacrylate (PMMA) was injected into the vertebral body to stabilize the fracture, pain relief was significantly increased, regardless of the type of surgical intervention.
KP with SpineJack provides the best performance for AVBH restoration 1 year postoperatively. SpineJack is a permanent titanium implant designed to restore vertebral height through an endplate distraction device by expanding like a small jack; it is equipped with a mechanical opening, ensuring a gradual and controlled vertebral fracture reduction. This technique allows good reconstruction of the anterior column and restores vertebral height[16].
The KA evaluates the reduction in kyphosis. In our study, VP was unfavorable for kyphotic reduction due to the lack of effective strength acting on the upper and lower endplates. VP requires cement injection at higher pressures, and hence, incurs a higher risk of cement extravasation than KP and merely eliminates the deformity, without reducing kyphosis[14,17].
Berlemann et al[18] reported a mean kyphotic reduction of approximately 8° in a group of 27 patients after KP, whereas Theodorou et al[14] revealed an average KA correction of 9°. Weisskopf et al[19] showed that the reduction in kyphotic deformity was achieved in only four cases (average, 8.5°) in a 22-patient trial. In contrast, the corrections of KAs in our study were 14.2°, 16.0°, 15.8°, and 14.2° in balloon KP, KP with an IVEP, KP with vesselplasty, and KP with SpineJack, respectively, 1 year after surgical treatment; our kyphotic correction was substantially higher than those reported in previous studies. We were unable to discriminate between the acute VCFs from old VCFs using only conventional two-plane radiography with the patient in an erect position instead of MRI. In the acute or subacute phase of VCF, edema was noted during replacement of the normal vertebral body bone marrow in a benign compression fracture, resulting in hypointensity on T1-weighted images and hyperintensity on T2-weighted images. The nonunion VCF presented a confined high intensity or a diffuse low-intensity area on T2-weighted MRI, which was found to be of significant importance[20,21]. The more recent the fracture, the better the kyphotic reduction after KP[22]. In contrast to previous studies, patients with a diagnosis of acute, subacute, or nonunion VCFs underwent surgical treatment in our study. Therefore, we achieved better satisfaction from patients and better postoperative correction of the kyphotic deformity.
Among the five groups, the VP group had the most adjacent VCF episodes. KP requires a larger injection pressure, creating a cancellous fill effect with PMMA, finally leading to better spinal biomechanical stability; as a result, the risk of secondary vertebral fractures is decreased[5].
To date, it is not clear how new vertebral fractures occur in adjacent vertebrae. Some studies have shown that the pressure on adjacent vertebrae was increased as a result of injected cement[23]. Both VP and KP reduced swelling of the vertebral endplate and vertebral joint mobility, causing swelling of the adjacent vertebrae and increasing the risk of adjacent vertebral fracture[24]. In the present study, we found that the highest rate (17%) of adjacent VCFs occurred in the VP group.
In the KP group, although the null hypothesis was not rejected, the difference may be related to the small amount of data. The prevalence of adjacent VCFs within 1 year was lowest in the KP with an IVEP group. Generally, balloon KP, KP with vesselplasty, or KP with SpineJack, as well as VP, are completed by filling the vertebral body with PMMA; only KP with an IVEP is associated with bone healing. The healed vertebrae following IVEP treatment have similar bone mineral densities to the adjacent vertebrae. The fractured vertebrae filled with PMMA were stiffer than the adjacent level vertebrae, explaining the lower adjacent VCF rate in the KP with an IVEP group.
Complications of percutaneous VP or KP are fairly common, and in most cases, they are asymptomatic[25,26]. The most common complications are cement leakage due to the fact that osteoporosis increases the vulnerability of the vertebral bone structure; extraction of the endplate during KP may further displace the fractured fragment[27,28], which can migrate into veins, paravertebral soft tissue, intervertebral discs, or the spinal canal, affecting the foraminal area or epidural space. In the VP and balloon KP groups, there was a higher percentage of cement leakage: 24 cases (16 asymptomatic, and 8 symptomatic) and 30 cases (23 asymptomatic, 7 symptomatic), respectively. Cement leakage or implant migration occurred less frequently in the KP with an IVEP, KP with vesselplasty, and SpineJack groups.
With VP, the cement was injected into the vertebral body while the PMMA flowed to fill the gaps between the fracture fragments. During this step, the less viscous the cement, the more easily it filled defects in the cortex. Additionally, impaction of the trabecular bone against the surrounding cortical bone reduced the risk of cement penetrating the cortex. Another factor related to the leakage of cement in both VP and KP is the fracture pattern. When the endplates and anterior or posterior wall are not intact, cement leakage occurs more often[29]. Although the number of cement leakages was small and the results inconclusive, the rate of cement leakage was lowest in the KP with SpineJack group. After expanding the implants, we stabilized the fractured vertebra via an injection of PMMA bone cement (Cohesion, Vexim), which is characterized by its high viscosity; this prevented the occurrence of cement leakage.
It was previously thought that vesselplasty was a safe surgical treatment because of the absence of potentially fatal cement leakage from the vertebral body and into the spinal canal[30]; however, one of our patients who underwent vesselplasty exper
Some studies reported rare complications of VP or KP including infections, epidural hemorrhage, fat embolism, cardiac damage, and arterial or renal embolism[31-35]. Fortunately, we did not observe these complications during our patients’ clinical courses.
Although we present promising results, this study has limitations owing to the rapid pace of innovations regarding spinal implants for VCFs. There are some published studies on innovative spinal implants, including the Vertebral Body Stent, KIVA Vertebral Compression Fractures Treatment System, and Osseofix. Although these implants are useful and reported to be effective for body height restoration[36], these have not yet been introduced to our hospital. Therefore, we could not include these data in our study.
Some researchers might question our choice of implants; however, the patients’ economic conditions were considered as well. The treatments used in this study, listed from the most to least expensive, were KP with SpineJack, KP with an IVEP, KP with vesselplasty, balloon KP, and VP. In clinical practice, we emphasized self-decision-making. Thus, we allowed patients to choose among these treatment methods. In addition, SpineJack implants had not been introduced to our hospital until 5 years prior to this report; therefore, all SpineJack data were collected within 5 years. Moreover, we experienced a tragic episode from cement leakage during vesselplasty; since then, we have not had the confidence to perform this procedure again[4]. For this reason, there was no bias of treatment choice based on disease severity; this was a fully randomized retrospective observational study.
We enrolled 354 patients, including 107 men and 247 women, in our study. Some researchers might doubt about the effect of sex. Actually, as we mentioned previously, we provided all treatment choices to patients. Female gender was also one of the risk factors for VCF[37]. Population studies have shown that the annual incidence of VCFs is 10.7 per 1000 women and 5.7 per 1000 men[38]. Dr. Lin surveyed the VCF in another study with 600 patients, which enrolled 186 men and 404 women[39]. The ratio of men and women was similar to that in our study, including each treatment subgroup. There was no existing gender selection bias in our study. Besides, according to the previous study, no significant difference was observed in the pain outcomes of cement augmentation among male and female patients[3].
Despite enrolling 354 patients in our study, which is a relatively large patient sample size for a single-doctor series observational study, our sample size was insufficient to demonstrate the safety of each intervention. We restricted the study to a single surgeon to minimize inter-operator variability. The safety and effectiveness of surgery depend on the surgeon’s technique; nevertheless, although we collected the data from a single surgeon over 10 years, the sample size remained insufficient. Thus, given the small sample size, the result for the least amount of cement leakage remains inconclusive. In further studies, we will enroll more patients to evaluate cement leakage rates in KP more accurately with SpineJack, vesselplasty, and an IVEP. Besides, larger sample size will also allow us to discuss more factors and postoperative outcomes.
In summary, our results suggest that VP or KP provides excellent treatment for VCF, with no difference in terms of pain relief. KP with SpineJack has the lower risk of cement extravasation and results in greater vertebral body height restoration, kyphotic reduction, and safety than the other procedures, whereas VP demonstrates the highest occurrence of adjacent compression fractures. Given the small sample size, the lowest results for cement leakage rate are not conclusive; nevertheless, we recommend that KP with SpineJack, vesselplasty, and an IVEP are safe, minimally invasive procedures. Our study will serve as a reference to surgeons when choosing a safe and effective procedure for treating VCFs.
Cement augmentation is an effective surgical treatment for osteoporotic compression fractures. There are several cement augmentation types. However, no studies have compared the safety and efficacy of different cement augmentation types for the treatment of compression fractures.
We performed this study to understand the efficacy of different cement augmentation methods on osteoporotic compression fractures.
The purpose of this study was to investigate the visual analog scale scores for pain, kyphotic angle, average body height, rate of cement leakage, and occurrence of adjacent vertebral compression fractures for each different cement augmentation type.
We retrospectively analyzed 354 patients with acute vertebral compression fractures. Visual analog scale scores for pain, kyphotic angle, average body height, rate of cement leakage, and occurrence of adjacent vertebral compression fractures were followed for 1 year and compared among different cement augmentation types.
In these 354 patients, all cement augmentation types showed significant improvement on pain scores, and no significant difference was noted between the groups (P = 0.325). Kyphoplasty with SpineJack performed best with regard to kyphotic angle reduction (P = 0.028) and vertebral body height restoration (P = 0.02). The rate of adjacent compression fractures was highest in the vertebroplasty group, with a statistically significant difference according to the Fisher’s exact probability test (P = 0.02). Although kyphoplasty with SpineJack, kyphoplasty with an intravertebral expandable pillar (IVEP), and vesselplasty resulted in lower rates of cement leakage than balloon kyphoplasty and vertebroplasty, the results might not be conclusive due to the small sample size.
Kyphoplasty with SpineJack has good outcomes in sagittal alignment correction. Vertebroplasty has the highest cement leakage rate and adjacent compression fracture occurrence.
In follow-up studies, more patients will be enrolled to validate and evaluate cement leakage rates in balloon kyphoplasty more accurately with SpineJack, vesselplasty, and an IVEP, and discuss more factors and postoperative outcomes.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hu L S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Dong R, Chen L, Tang T, Gu Y, Luo Z, Shi Q, Li X, Zhou Q, Yang H. Pain reduction following vertebroplasty and kyphoplasty. Int Orthop. 2013;37:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Cannada LK, Hill BW. Osteoporotic Hip and Spine Fractures: A Current Review. Geriatr Orthop Surg Rehabil. 2014;5:207-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Schlaich C, Minne HW, Bruckner T, Wagner G, Gebest HJ, Grunze M, Ziegler R, Leidig-Bruckner G. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int. 1998;8:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 310] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Yeh KL, Wu SH, Wu SS, Chang SH. Rare Episode of Cement Leakage During Vesselplasty in a Case of Vertebral Compression Fracture. World Neurosurg. 2020;137:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Xiao H, Yang J, Feng X, Chen P, Li Y, Huang C, Liang Y, Chen H. Comparing complications of vertebroplasty and kyphoplasty for treating osteoporotic vertebral compression fractures: a meta-analysis of the randomized and non-randomized controlled studies. Eur J Orthop Surg Traumatol. 2015;25 Suppl 1:S77-S85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Suzuki A, Sekiguchi S, Asano S, Itoh M. Pharmacological topics of bone metabolism: recent advances in pharmacological management of osteoporosis. J Pharmacol Sci. 2008;106:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Eastell R, Rosen CJ. Response to Letter to the Editor: "Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Clinical Practice Guideline". J Clin Endocrinol Metab. 2019;104:3537-3538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Garfin SR, Buckley RA, Ledlie J; Balloon Kyphoplasty Outcomes Group. Balloon kyphoplasty for symptomatic vertebral body compression fractures results in rapid, significant, and sustained improvements in back pain, function, and quality of life for elderly patients. Spine (Phila Pa 1976). 2006;31:2213-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Liu JT, Liao WJ, Tan WC, Lee JK, Liu CH, Chen YH, Lin TB. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int. 2010;21:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Hsieh JY, Wu CD, Wang TM, Chen HY, Farn CJ, Chen PQ. Reduction of the domino effect in osteoporotic vertebral compression fractures through short-segment fixation with intravertebral expandable pillars compared to percutaneous kyphoplasty: a case control study. BMC Musculoskelet Disord. 2013;14:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Nardi A, Tarantino U, Ventura L, Armotti P, Resmini G, Cozzi L, Tonini G, Ramazzina E, Rossini M. Domino Effect: mechanic factors role. Clin Cases Miner Bone Metab. 2011;8:38-42. [PubMed] |
| 12. | Bae JS, Park JH, Kim KJ, Kim HS, Jang IT. Analysis of Risk Factors for Secondary New Vertebral Compression Fracture Following Percutaneous Vertebroplasty in Patients with Osteoporosis. World Neurosurg. 2017;99:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Firanescu CE, de Vries J, Lodder P, Venmans A, Schoemaker MC, Smeets AJ, Donga E, Juttmann JR, Klazen CAH, Elgersma OEH, Jansen FH, Tielbeek AV, Boukrab I, Schonenberg K, van Rooij WJJ, Hirsch JA, Lohle PNM. Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): randomised sham controlled clinical trial. BMJ. 2018;361:k1551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 14. | Theodorou DJ, Theodorou SJ, Duncan TD, Garfin SR, Wong WH. Percutaneous balloon kyphoplasty for the correction of spinal deformity in painful vertebral body compression fractures. Clin Imaging. 2002;26:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Bouza C, López-Cuadrado T, Cediel P, Saz-Parkinson Z, Amate JM. Balloon kyphoplasty in malignant spinal fractures: a systematic review and meta-analysis. BMC Palliat Care. 2009;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Vanni D, Pantalone A, Bigossi F, Pineto F, Lucantoni D, Salini V. New perspective for third generation percutaneous vertebral augmentation procedures: Preliminary results at 12 months. J Craniovertebr Junction Spine. 2012;3:47-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Mousavi P, Roth S, Finkelstein J, Cheung G, Whyne C. Volumetric quantification of cement leakage following percutaneous vertebroplasty in metastatic and osteoporotic vertebrae. J Neurosurg. 2003;99:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Berlemann U, Franz T, Orler R, Heini PF. Kyphoplasty for treatment of osteoporotic vertebral fractures: a prospective non-randomized study. Eur Spine J. 2004;13:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Weisskopf M, Herlein S, Birnbaum K, Siebert C, Stanzel S, Wirtz DC. [Kyphoplasty - a new minimally invasive treatment for repositioning and stabilising vertebral bodies]. Z Orthop Ihre Grenzgeb. 2003;141:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Adler D, Tschoeke SK, von der Hoeh N, Gulow J, von Salis-Soglio G, Heyde CE. Non-union of osteoporotic vertebral fractures - identification and treatment of an underestimated pathology in elderly patients with persistent back pain. Acta Orthop Belg. 2014;80:444-450. [PubMed] |
| 21. | Tsujio T, Nakamura H, Terai H, Hoshino M, Namikawa T, Matsumura A, Kato M, Suzuki A, Takayama K, Fukushima W, Kondo K, Hirota Y, Takaoka K. Characteristic radiographic or magnetic resonance images of fresh osteoporotic vertebral fractures predicting potential risk for nonunion: a prospective multicenter study. Spine (Phila Pa 1976). 2011;36:1229-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Krauss M, Hirschfelder H, Tomandl B, Lichti G, Bär I. Kyphosis reduction and the rate of cement leaks after vertebroplasty of intravertebral clefts. Eur Radiol. 2006;16:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Kim MH, Lee AS, Min SH, Yoon SH. Risk factors of new compression fractures in adjacent vertebrae after percutaneous vertebroplasty. Asian Spine J. 2011;5:180-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Baroud G, Nemes J, Heini P, Steffen T. Load shift of the intervertebral disc after a vertebroplasty: a finite-element study. Eur Spine J. 2003;12:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Lee IJ, Choi AL, Yie MY, Yoon JY, Jeon EY, Koh SH, Yoon DY, Lim KJ, Im HJ. CT evaluation of local leakage of bone cement after percutaneous kyphoplasty and vertebroplasty. Acta Radiol. 2010;51:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Hao J, Hu Z. Percutaneous cement vertebroplasty in the treatment of symptomatic vertebral hemangiomas. Pain Physician. 2012;15:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Nieuwenhuijse MJ, Van Erkel AR, Dijkstra PD. Cement leakage in percutaneous vertebroplasty for osteoporotic vertebral compression fractures: identification of risk factors. Spine J. 2011;11:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Corcos G, Dbjay J, Mastier C, Leon S, Auperin A, De Baere T, Deschamps F. Cement leakage in percutaneous vertebroplasty for spinal metastases: a retrospective evaluation of incidence and risk factors. Spine (Phila Pa 1976). 2014;39:E332-E338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Hsieh MK, Chen LH, Chen WJ. Current concepts of percutaneous balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures: evidence-based review. Biomed J. 2013;36:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Flors L, Lonjedo E, Leiva-Salinas C, Martí-Bonmatí L, Martínez-Rodrigo JJ, López-Pérez E, Figueres G, Raoli I. Vesselplasty: a new technical approach to treat symptomatic vertebral compression fractures. AJR Am J Roentgenol. 2009;193:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Iliopoulos P, Korovessis P, Vitsas V. PMMA embolization to the left dorsal foot artery during percutaneous vertebroplasty for spinal metastases. Eur Spine J. 2014;23 Suppl 2:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Prokop A, Hägele M, Pfeilsticker U, Koll S, Chmielnicki M. [Pericardial perforation 2.5 years after kyphoplasty. A rare complication after cement extravasation]. Unfallchirurg. 2013;116:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Ahmadzai H, Campbell S, Archis C, Clark WA. Fat embolism syndrome following percutaneous vertebroplasty: a case report. Spine J. 2014;14:e1-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Hirata H, Hiwatashi A, Yoshiura T, Togao O, Yamashita K, Kamano H, Kikuchi K, Honda H. Resolution of epidural hematoma related to osteoporotic fracture after percutaneous vertebroplasty. World J Radiol. 2013;5:325-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Chung SE, Lee SH, Kim TH, Yoo KH, Jo BJ. Renal cement embolism during percutaneous vertebroplasty. Eur Spine J. 2006;15 Suppl 5:590-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Cornelis FH, Joly Q, Nouri-Neuville M, Ben-Ammar M, Kastler B, Kastler A, Amoretti N, Hauger O. Innovative Spine Implants for Improved Augmentation and Stability in Neoplastic Vertebral Compression Fracture. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Old JL, Calvert M. Vertebral compression fractures in the elderly. Am Fam Physician. 2004;69:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Alexandru D, So W. Evaluation and management of vertebral compression fractures. Perm J. 2012;16:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 39. | Lin YC, Juan YH, Chan WP, Yeh KY, Wong AMK, Sung CM, Lin YJ, Chang SC, Chen FP. Integrating Muscle Health in Predicting the Risk of Asymptomatic Vertebral Fracture in Older Adults. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |