Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9431
Peer-review started: March 26, 2021
First decision: April 28, 2021
Revised: May 6, 2021
Accepted: September 27, 2021
Article in press: September 27, 2021
Published online: November 6, 2021
Processing time: 216 Days and 22.1 Hours
Atrophic gastritis is a precancerous lesion of the stomach. It has been reported that pepsinogen (PG) can reflect the morphology and function of the gastric mucosa, and it is therefore used as a marker for the early diagnosis of atrophic gastritis.
To evaluate the diagnostic value of serum PG for degree of gastric mucosal atrophy in asymptomatic Chinese upon physical examination.
Medical data were collected from subjects who underwent transnasal gastroscopy between October 2016 and October 2018. For each study subject, serum PG levels and presence of Helicobacter pylori (H. pylori) infection were investigated. Pathology was evaluated using the Operative Link for Gastritis Assessment (OLGA) classification and Operative Link on Gastric Intestinal Metaplasia Assessment (OLGIM) systems. All statistical analyses were carried out using SPSS statistical software.
A total of 2256 subjects were enrolled and 1922 cases were finally included in the study. Based on the OLGA grading system, the levels of PGI were slightly decreased, while those of PGII were slightly increased. The PGI/PGII ratio (PGR) was reduced with increasing atrophy. The association between PG and OLGA grading was higher compared with that between PG and the OLGIM grading system. Compared with the OLGA-0 group, a statistically significant difference was observed in the mean age of OLGA-I, III, and IV groups (P < 0.05). In the H. pylori-positive subjects, the PGR levels were notably lower in the OLGA-I, II, and III groups compared with the OLGA-0 group (P < 0.05). H. pylori-positive subjects exhibited significantly higher PGI and PGII serum levels and a significantly lower PGR compared with H. pylori-negative patients in different OLGA groups (P < 0.05).
Serum PG levels may represent a non-invasive screening marker for gastric mucosal atrophy in asymptomatic subjects.
Core Tip: The current study evaluated the diagnostic value of serum pepsinogen (PG) as a screening marker for atrophic gastritis in asymptomatic healthy check-up populations in different regions of China. Serum PG levels were closely associated with Operative Link for Gastritis Assessment grading and could be used as an effective non-invasive screening tool for atrophic gastritis in asymptomatic subjects. In addition, better results could be obtained in Helicobacter pylori-positive individuals. Screening is more necessary in the elderly, and the application of the aforementioned screening tool may be beneficial for this population.
- Citation: Cai HL, Tong YL. Association of serum pepsinogen with degree of gastric mucosal atrophy in an asymptomatic population. World J Clin Cases 2021; 9(31): 9431-9439
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9431.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9431
In 2014, approximately 410400 new cases of gastric cancer (GC) were diagnosed in China, accounting for 10.79% of all cancer cases[1]. The prognosis of GC is closely associated with the times of diagnosis and treatment. The 5-year survival rate of advanced GC is less than 30%, while early GC, with a 5-year survival rate of over 90%, can be treated by endoscopy[2]. Therefore, improving the diagnostic rate of early GC is an efficient and feasible method to improve the survival rate of patients with GC.
GC follows the previously described Correa cascade of active gastritis-atrophic gastritis-intestinalization-intraepithelial neoplasia-GC, where atrophic gastritis is considered as the turning point and is referred to as a gastric precancerous lesion. Therefore, effective screening of subjects with gastric precancerous lesions and appropriate intervention can reduce the incidence of GC and increase the diagnostic rate of early GC.
In 2005, the International Atrophy Study Group proposed the Operative Link for Gastritis Assessment (OLGA) grading and staging system for chronic gastritis[3]. This staging system represents the extent and degree of gastric mucosal atrophy to link the histopathology of chronic gastritis to the risk of GC. In terms of pathological diagnosis, the consistency of gastric mucosal atrophy diagnosis among different physicians is low, while that of intestinal metaplasia is high. Therefore, in 2010, the standard Operative Link on Gastric Intestinal Metaplasia Assessment (OLGIM) staging system put forth the proposal to replace the term “atrophy” with “intestinal metaplasia”[4]. Several studies have now confirmed that the OLGA/OLGIM staging system, reflecting the severity of atrophic gastritis and the risk of GC, facilitates the identification of patients with a high risk of developing GC (OLGA/OLGIM stages III and IV), thus promoting the early diagnosis and prevention of the disease[5,6].
Endoscopy is an invasive diagnostic approach that requires a large number of samples, and its diagnostic accuracy is affected by the practicing pathologists themselves. Therefore, endoscopy is not considered as an ideal method in clinical practice, particularly for the physical examination of healthy individuals. Never
Serum levels of pepsinogen (PG) can reflect the morphology and function of the gastric mucosa. PGI reflects the function of gastric acid secretion of the gastric gland. Therefore, it has been reported that the levels of PGI are reduced when gastric mucosal glands are atrophied. Additionally, the PGII serum levels are mainly associated with the extent of lesions in the gastric mucosa of the gastric fundus. Increased PGII serum levels are associated with gastric fundus atrophy, intestinal metaplasia, or pseudopyloric metaplasia and dysplasia. Several studies have demonstrated that the PGI/PGII ratio (PGR) was associated with progressive gastric mucosal atrophy and GC[7,8].
The current study aimed to evaluate the diagnostic value of the serum PG levels and the presence of Helicobacter pylori (H. pylori) infection as a screening tool for atrophic gastritis in asymptomatic Chinese who attend standard health check-up in China.
A total of nine health management centers from different areas of China participated in the study, which was conducted between October 2016 and October 2018. The centers were the No. 924 Hospital of the People's Liberation Army of China (southern China), the Second Affiliated Hospital of Zhejiang University College of Medicine, the Zhongshan Affiliated Hospital of Xiamen University, the Kunshan Hospital of Traditional Chinese Medicine (all in eastern China), the Sichuan Provincial People’s Hospital, the Southwest Affiliated Hospital of the Third Military Medical University (both in southwest China), the Jilin City People’s Hospital (northeast China), the PLA General Hospital, and the Jingzhou Hospital of Traditional Chinese Medicine (both in central-northern China). More specifically, subjects between 20-80 years of age, without gastrointestinal symptoms, and who presented for asymptomatic health check-up that included transnasal endoscopy were enrolled in the present study.
Participants with one of the following symptoms or clinical findings were excluded from the present study: (1) Subjects with contraindications to transnasal gastroscopy; (2) Previous history of definitive benign or malignant diseases of the upper gastrointestinal tract, including peptic ulcers, gastric polyps, esophageal cancer, and GC, or history of surgery; (3) Treatment with acid suppressants within the past month; (4) Serious organic diseases, including heart, liver, and kidney diseases; (5) Severe mental illness precluding an ability to cooperate; or (6) Current pregnancy or lactation.
All participants received serum PG testing, H. pylori testing, and transnasal endoscopy in a single-day hospital visit. Approximately 5 mL of fasting blood was collected from each subject. Serum PG levels were measured by particle-based chemiluminescence immunoassay using the PGI and PGII kits (Abbott Laboratories Inc., Chicago, IL, United States). H. pylori infection was evaluated using a 13C-urea breath test (Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd., Shenzhen, China) or serum H. pylori antibodies (MP Biomedicals, Santa Ana, CA, United States) in combination with pathological H. pylori findings. Individuals with a positive result for any of the aforementioned three tests were categorized into a H. pylori-positive group, while those with a negative result for all tests were categorized into a H. pylori-negative group. Endoscopy was performed by experienced endoscopists who were blinded to the H. pylori and PG test results. Pathological samples were obtained from the gastric body, angulus, and antrum according to the Chinese Consensus on Screening and Endoscopic Diagnosis and Treatment of Early GC (2014 version)[9]. Additional biopsies were performed at the sites where lesions were detected.
The data are expressed as the mean ± SD for normally distributed data and as the median ± interquartile range for non-normally distributed data. Statistical analyses were performed using SPSS statistical software (version 20; IBM Corp., Armonk, NY, United States). Age, PGI and PGII levels, and PGR were tested for normal distribution by the Kolmogorov-Smirnov test. After confirming a normal distribution, ANOVA with post hoc Scheffe’s test was used for further assessment. Bonferroni’s correction and Pearson’s chi-square test were used to evaluate the differences between H. pylori-negative and H. pylori-positive patients. Receiver operating characteristic (ROC) curve analysis was used to estimate the cutoff values for PGI and PGR. P values less than 0.05 were considered statistically significant.
Among the total 2256 subjects included in the current study, 14 were diagnosed with GC (0.6%), including six cases of early GC. In addition, one subject was diagnosed with pharyngeal cancer. There were 326 cases (15.9%) of atrophic gastritis and 391 cases (19.0%) of intestinal metaplasia, while the total H. pylori infection rate was 41.7%. A total of 1922 subjects, with an average age of 52.3 ± 9.8 years and male to female ratio of 1.2:1 (1065/857), underwent serological, gastroscopic, and pathological examinations (Table 1).
| Age | 52.3 ± 9.8 |
| Gender (male) | 1065 (55.41) |
| Helicobacter pylori | 757(39.39) |
| Gastric cancer | 10 (0.52) |
Based on OLGA grading, the PGI serum levels were slightly decreased, while those of PGII were slightly increased. In addition, the PGR was reduced in conjunction with increasing degrees of atrophy. Compared with the OLGA-0 group, the PGII levels were slightly increased and PGR was slightly decreased in the OLGA-I to IV groups (P < 0.05). Additionally, compared with the OLGA-I and II groups, PGR levels in the OLGA-III and IV groups were significantly reduced (P < 0.05). A statistically significant difference was also observed in the PGR between the OLGA-II and OLGA-III/IV groups (P < 0.05). A notable difference in PGR was also identified between the GC group and both OLGA-0 and I groups (P < 0.05; Table 2).
Furthermore, as shown in Table 2, compared with the OLGIM-0 group, the PGI levels were significantly lower in the OLGIM-II, III, and IV groups (P < 0.05), and PGR was reduced in the OLGIM-II and III groups (P < 0.05). The PGI serum levels were significantly lower in the OLGIM-III/IV groups, and PGR was also markedly decreased in the OLGIM- II/ III groups compared with the OLGIM-I group (P < 0.05). Finally, compared with the OLGIM-II group, the OLGIM-III group exhibited significantly reduced PGR (P < 0.05; Table 3).
The aforementioned results indicated that the PG serum levels were more relevant to OLGA grade compared with the OLGIM grade. Therefore, the OLGA grading system was applied for the subsequent analyses.
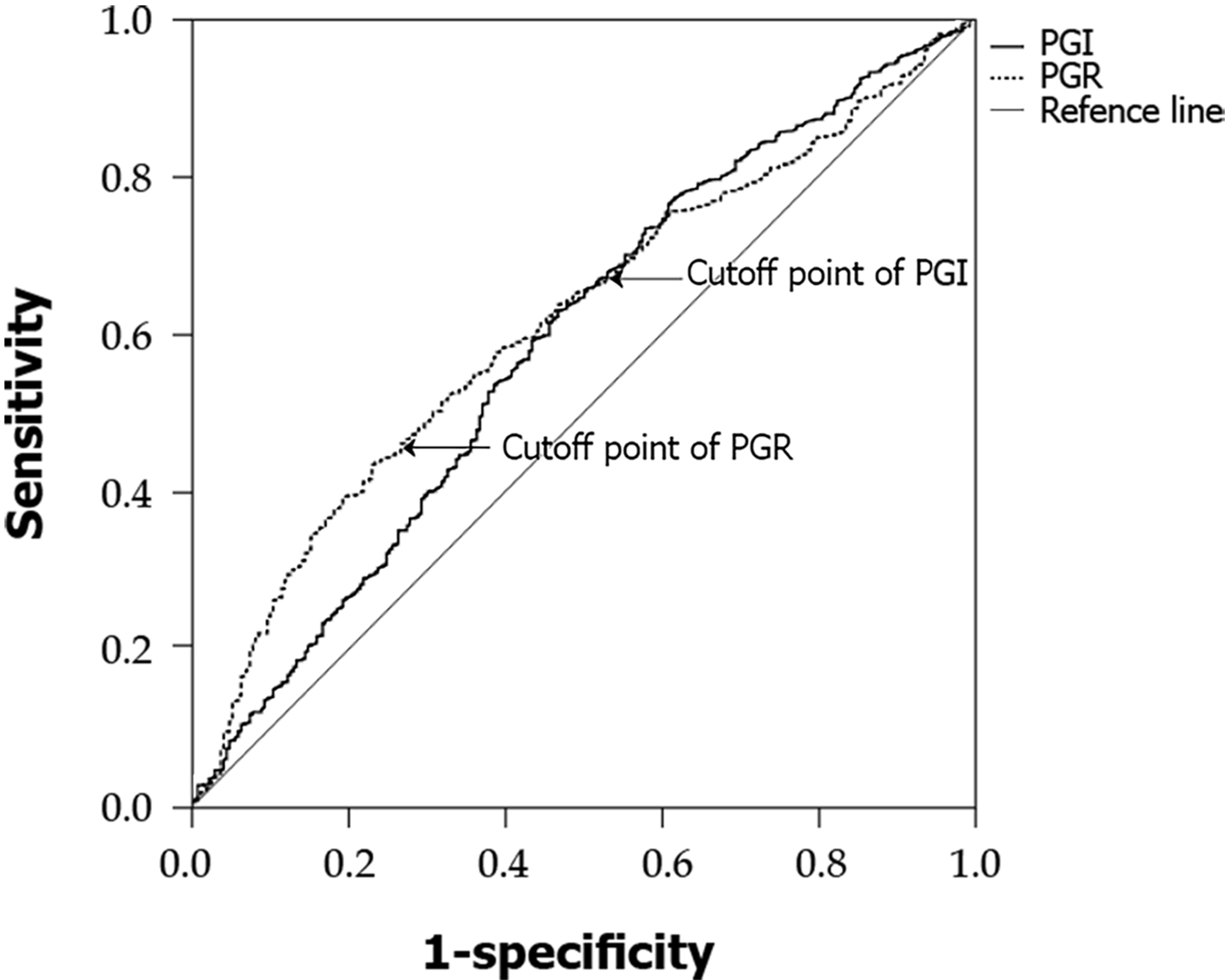
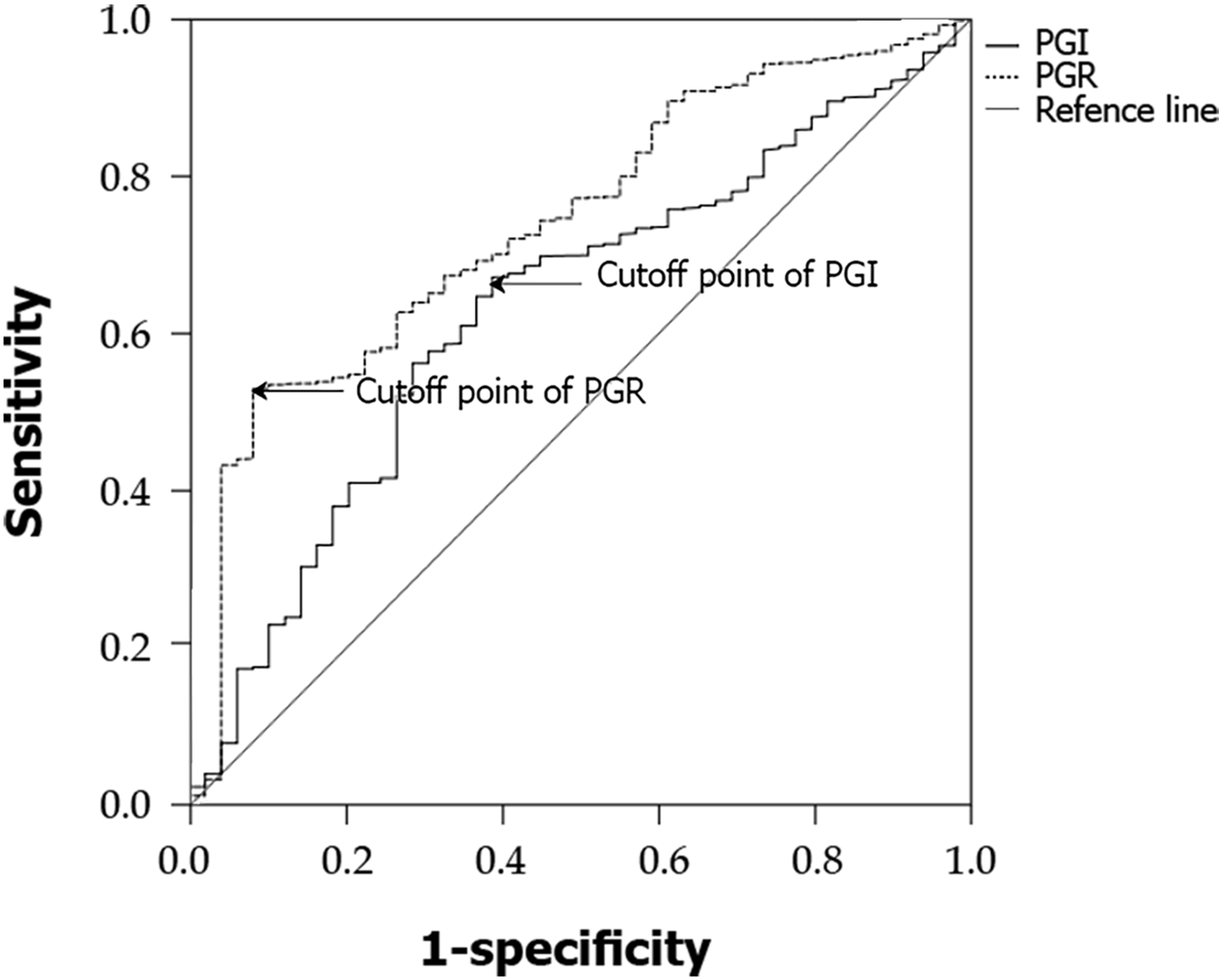
Rugge et al’s[10] prospective study confirmed that the high-risk stage (defined as stage III or IV by the OLGA classification) is closely associated with high risk of GC. Accordingly, OLGA I and II groups were then combined in a low-risk group and compared with the OLGA-0 group. The best cutoff for low risk was estimated at PGI 73.14 ng/mL [area under the curve (AUC) = 0.585, sensitivity = 62.1%, specificity = 53.8%] and PGR 11.54 (AUC = 0.611, sensitivity = 43.2%, specificity = 77.7%) (Figure 1). The OLGA III and IV groups were combined in a high-risk group and compared with the remaining three groups; the best cutoff for high risk was estimated at PGI 64.00 ng/mL (AUC = 0.631, sensitivity = 67.2%, specificity = 61.2%) and PGR 9.11 (AUC = 0.740, sensitivity = 53.0%, specificity = 91.8%) (Figure 2).
Compared with the OLGA-0 group, the mean ages in the OLGA-I, III, and IV groups were significantly higher (P < 0.05) and increased with an increasing OLGA grade. In addition, a statistically significant difference was observed in the H. pylori positivity rate between the OLGA-I/II/III/IV groups and the OLGA-0 group (P < 0.05; Table 4).
Further analyses were performed with the H. pylori-negative or positive groups. In the OLGA-0 group, H. pylori positive subjects exhibited significantly higher PGI and PGII serum levels and a significantly lower PGR compared with H. pylori-negative patients (P < 0.05). The same trend was observed among H. pylori-positive/H. pylori-negative subjects in the OLGA-I group (P < 0.05). Additionally, H. pylori-positive subjects in the OLGA-II group had significantly elevated PGI and PGII levels compared with the H. pylori-negative subjects (P < 0.05). In the OLGA-III group, significantly increased serum levels of PGII were observed in the H. pylori-positive group compared with the H. pylori-negative group, while the ratio PGI/PGII was notably reduced. By contrast, no statistically significant differences were obtained in the PG levels between the H. pylori-positive/H. pylori-negative subjects in the OLGA-IV and GC groups (P > 0.05).
In the H. pylori-positive population, compared with the OLGA-0 group, both PGI levels and PGR were significantly lower in the OLGA-I and III groups (P < 0.05), and PGR was also notably reduced lower in the OLGA-II group (P < 0.05). In the H. pylori-negative population, the serum levels of PGI and PGR were markedly decreased in the OLGA-I/II (P < 0.05) and GC groups (P < 0.05; Table 5).
| OLGA-0 | I | II | III | IV | ||||||
| Helicobacter pylori-(n = 1007) | Helicobacter pylori + (583) | Helicobacter pylori - (n = 99 | Helicobacter pylori+ (n = 86) | Helicobacter pylori- (n = 37) | Helicobacter pylori+ (n = 51) | Helicobacter pylori- (n = 16 | Helicobacter pylori+ (n = 27) | Helicobacter pylori-(n = 1) | Helicobacter pylori+ (n = 5) | |
| PGI (ng/mL) | 82.18 ± 78.64 | 101.70 ± 89.011 | 56.71 ± 66.102 | 73.05 ± 78.201,2 | 55.40 ± 54.182 | 92.12 ± 73.511 | 60.00 ± 47.55 | 60.8 ± 54.302 | 34.3 | 63.50 ± 43.882 |
| PGII (ng/mL) | 7.35 ± 6.61 | 10.80 ± 11.361 | 6.60 ± 6.31 | 10.05 ± 10.231 | 7.10 ± 4.88 | 13.00 ± 10.491 | 7.00 ± 6.13 | 13.5 ± 10.641 | 3.8 | 16.40 ± 8.70 |
| PGR | 10.68 ± 8.76 | 8.82 ± 9.891 | 8.77 ± 5.072 | 7.24 ± 6.281,2 | 7.94 ± 3.542 | 7.18 ± 5.512 | 8.32 ± 3.25 | 4.50 ± 3.451,2 | 9.026316 | 3.90 ± 2.652 |
Atrophic gastritis is considered as one of the main precursor lesions in the Correa cascade, which may eventually result in GC. A study reported that the odds of developing GC 5 years after the diagnosis of atrophic gastritis, enterocolitis, mild to moderate atypical hyperplasia, and severe atypical hyperplasia were 0.1%, 0.25%, 0.6%, and 6%, respectively[1].
The current study found that approximately 15.9% of the asymptomatic individuals undergoing standard physical examination exhibited atrophic gastritis and 19.0% exhibited enterocolitis. Among these patients, 2.5% suffered from severe atrophic gastritis (OLGA grade III or IV). These patients have a higher risk of progression to GC after 5 years. Therefore, effective screening of such individuals in the asymptomatic population via endoscopic surveillance interventions are important for reducing the incidence of GC and improving the diagnostic rate of early GC.
Serum PG levels reflect the morphological and functional status of the gastric mucosa. It has been reported that the gradual decrease of PGR is associated with the progression of gastric mucosal atrophy and GC[2]. A meta-analysis of 31 studies, including 1520 patients with GC and 2265 with atrophic gastritis, suggested that serum PG levels could be considered as a potent, non-invasive, population-based screening tool for the diagnosis of GC and atrophic gastritis[11]. Additionally, a study by Zoalfaghari et al[12] indicated that the serum PGI levels and PGR could be potential serological markers for the diagnosis of atrophic gastritis, with a high sensitivity and specificity. The present study also demonstrated that PGI levels and PGR were significantly decreased with increasing atrophy, with PGR showing more significant diagnostic value. The association between PG levels and OLGA grade was more pronounced compared with the OLGIM grading system, suggesting that PG could be more closely associated with atrophy compared with intestinal chemosis. Furthermore, ROC curve analysis showed that serum PGI levels and PGR could be used to predict atrophy and intestinal metaplasia of the gastric mucosa in asymptomatic health check-up subjects in China.
Several regional studies have demonstrated that the H. pylori infection rate is approximately 40%-55% in China. The overall H. pylori infection rate in the study population was 39.39%. The results showed that the PGI and PGII serum levels were significantly increased, and PGR was markedly decreased in the H. pylori-infected group. H. pylori infection also affected the levels of PG in different pathological subgroups[13]. Al-Ezzy et al[14] reported that H. pylori infection can affect the expression of the Fas gene through inflammatory factors, thus regulating PG concentration.
Furthermore, the results of the current study also demonstrated that patients with high-grade lesions were older and had a higher rate of H. pylori infection. A positive association has been reported between the severity of gastric atrophy or intestinal metaplasia and the age of the subjects[15,16]. Additionally, a study showed that the prevalence of high-risk OLGA stage was increased with increasing age in H. pylori-negative subjects[17]. A Korean study demonstrated that advanced age, long-term smoking, and H. pylori infection were independent risk factors associated with advanced stages determined by the OLGA staging system[18]. The transition from H. pylori infection into GC is a multistep process, including the progression of chronic gastritis into precancerous lesions and ultimately to GC[19]. The presence or absence of H. pylori infection allows a preliminary prediction of whether a patient is at high risk by OLGA staging. Therefore, it has been suggested that routine endoscopy may not be necessary for all patients by OLGA staging or those at a low-risk by OLGIM stage, since H. pylori eradication can effectively prevent the development of GC. Effectively eradicating H. pylori before the age of 40 years could reduce the number of routine endoscopies in individuals requiring GC surveillance and substantially reduce the related healthcare burden.
The current study has some limitations in. First, the overall sample size in the study was not large enough; in particular, the number of patients with OLGA stage III/IV and GC was insufficient, which could cause statistical bias. Therefore, further studies with increased sample size are needed to verify the results of the present study. Second, there could be a selection bias in this study, since all enrolled subjects voluntarily underwent physical examination and gastroscopy, suggesting that their economic status was moderate.
The present study demonstrated that serum PG levels were closely associated with OLGA classification. The results suggested that PG levels could be used as an effective non-invasive screening tool for atrophic gastritis in the asymptomatic population undergoing standard physical examination, while the diagnostic value of PG levels could be more potent in patients with H. pylori infection. This screening tool could be valuable and represent an important screening method for the elderly population.
Atrophic gastritis is a precancerous lesion of the stomach. Pepsinogen (PG) has been reported to reflect the morphology and function of the gastric mucosa.
PG can be used for non-invasive screening of atrophic gastritis and even gastric cancer (GC). Effective screening of subjects with gastric precancerous lesions and appropriate intervention can reduce the incidence of GC and increase the diagnostic rate of early GC.
The main objective of this research was to evaluate the diagnostic value of serum PG in the degree of gastric mucosal atrophy in an asymptomatic Chinese population undergoing standard physical examination.
The study subjects underwent transnasal gastroscopy, and serum PG levels and the presence of Helicobacter pylori (H. pylori) infection were investigated to assess the diagnostic accuracy of PG for evaluating the degree of gastric mucosal atrophy. Pathology was evaluated using the Operative Link for Gastritis Assessment (OLGA) classification and Operative Link on Gastric Intestinal Metaplasia Assessment (OLGIM) systems. ANOVA with post hoc Scheffe’s test was used for further assessment of the differences in age, PGI and PGII levels, and PGI/PGII ratio (PGR). Bonferroni’s correction and Pearson’s chi-square test were used to evaluate the differences between H. pylori-negative and H. pylori-positive patients. Receiver operating characteristic curve analysis was used to estimate the cutoff values for PGI, PGII, and PGR.
The association between PG and OLGA grade was higher compared with that between PG and the OLGIM grading system. Based on the OLGA grading system, the levels of PGI were slightly decreased, while those of PGII were slightly increased. PGR was reduced with increasing atrophy (P < 0.05). A slightly increasing trend was observed in the mean age of different OLGA groups. H. pylori-positive subjects exhibited significantly higher PGI and PGII serum levels and a significantly lower PGR compared with H. pylori-negative patients in the different OLGA groups (P < 0.05).
Serum levels of PG are closely associated with OLGA stage and could be used as an effective non-invasive screening tool for evaluating the degree of gastric mucosal atrophy in asymptomatic subjects.
Future studies could focus on the cutoff values of PG for the diagnosis of precancerous lesions or early gastric cancer in different regions of China.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cho JH, Mohammadi M, Yücel O S-Editor: Liu M L-Editor: Wang TQ P-Editor: Liu M
| 1. | Yang L, Zheng R, Wang N, Yuan Y, Liu S, Li H, Zhang S, Zeng H, Chen W. Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res. 2018;30:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 2. | Fang WL, Huang KH, Chen MH, Liu CA, Hung YP, Chao Y, Tai LC, Lo SS, Li AF, Wu CW, Shyr YM. Comparative study of the 7th and 8th AJCC editions for gastric cancer patients after curative surgery. PLoS One. 2017;12:e0187626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Rugge M, Genta RM. Staging and grading of chronic gastritis. Hum Pathol. 2005;36:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Capelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, Bruno MJ, van Dekken H, Meijer J, van Grieken NC, Kuipers EJ. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 375] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 5. | Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ; European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1589] [Article Influence: 122.2] [Reference Citation Analysis (5)] |
| 6. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1184] [Article Influence: 118.4] [Reference Citation Analysis (0)] |
| 7. | Miki K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels - "ABC method". Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Yamaguchi Y, Nagata Y, Hiratsuka R, Kawase Y, Tominaga T, Takeuchi S, Sakagami S, Ishida S. Gastric Cancer Screening by Combined Assay for Serum Anti-Helicobacter pylori IgG Antibody and Serum Pepsinogen Levels--The ABC Method. Digestion. 2016;93:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Zou WB, Yang F, Li ZS. [How to improve the diagnosis rate of early gastric cancer in China]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015;44:9-14. [PubMed] |
| 10. | Rugge M, Meggio A, Pravadelli C, Barbareschi M, Fassan M, Gentilini M, Zorzi M, Pretis G, Graham DY, Genta RM. Gastritis staging in the endoscopic follow-up for the secondary prevention of gastric cancer: a 5-year prospective study of 1755 patients. Gut. 2019;68:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 11. | Tu H, Sun L, Dong X, Gong Y, Xu Q, Jing J, Bostick RM, Wu X, Yuan Y. A Serological Biopsy Using Five Stomach-Specific Circulating Biomarkers for Gastric Cancer Risk Assessment: A Multi-Phase Study. Am J Gastroenterol. 2017;112:704-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Zoalfaghari A, Aletaha N, Roushan N, Taslimi R, Foroutan H, Faridnia B. Accuracy of pepsinogens for early diagnosis of atrophic gastritis and gastric cancer in Iranian population. Med J Islam Repub Iran. 2014;28:150. [PubMed] |
| 13. | De Re V, Orzes E, Canzonieri V, Maiero S, Fornasarig M, Alessandrini L, Cervo S, Steffan A, Zanette G, Mazzon C, De Paoli P, Cannizzaro R. Pepsinogens to Distinguish Patients With Gastric Intestinal Metaplasia and Helicobacter pylori Infection Among Populations at Risk for Gastric Cancer. Clin Transl Gastroenterol. 2016;7:e183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Al-Ezzy AI. Immunomodulatory Effect of H. Pylori CagA Genotype and Gastric Hormones On Gastric Versus Inflammatory Cells Fas Gene Expression in Iraqi Patients with Gastroduodenal Disorders. Open Access Maced J Med Sci. 2016;4:364-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Graham DY, Nurgalieva ZZ, El-Zimaity HM, Opekun AR, Campos A, Guerrero L, Chavez A, Cardenas V. Noninvasive vs histologic detection of gastric atrophy in a Hispanic population in North America. Clin Gastroenterol Hepatol. 2006;4:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Rokkas T, Rokka A, Portincasa P. A systematic review and meta-analysis of the role of Helicobacter pylori eradication in preventing gastric cancer. Ann Gastroenterol. 2017;30:414-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Nam JH, Choi IJ, Kook MC, Lee JY, Cho SJ, Nam SY, Kim CG. OLGA and OLGIM stage distribution according to age and Helicobacter pylori status in the Korean population. Helicobacter. 2014;19:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. 2011;12:725-730. [PubMed] |
| 19. | Bornschein J, Kandulski A, Selgrad M, Malfertheiner P. From gastric inflammation to gastric cancer. Dig Dis. 2010;28:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |