Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9406
Peer-review started: May 18, 2021
First decision: June 12, 2021
Revised: June 13, 2021
Accepted: September 23, 2021
Article in press: September 23, 2021
Published online: November 6, 2021
Processing time: 164 Days and 9.1 Hours
The artificial liver support system (ALSS) is an effective treatment method for liver failure, but it requires deep venous intubation and long-term indwelling catheterization. However, the coagulation mechanism disorder of basic liver failure diseases, and deep venous thrombosis (DVT) often occur.
To evaluate the risk factors for DVT following use of an ALSS and establish a risk assessment score.
This study was divided into three stages. In the first stage, the risk factors for DVT were screened and the patient data were collected, including ALSS treatment information; biochemical indices; coagulation and hematology indices; complications; procoagulant use therapy status; and a total of 24 indicators. In the second stage, a risk assessment score for DVT after ALSS treatment was developed. In the third stage, the DVT risk assessment score was validated.
A total of 232 patients with liver failure treated with ALSS were enrolled in the first stage, including 12 with lower limb DVT. Logistic regression analysis showed that age [odds ratio (OR), 1.734; P = 0.01], successful catheterization time (OR, 1.667; P = 0.005), activity status (strict bed rest) (OR, 3.049; P = 0.005), and D-dimer level (≥ 500 ng/mL) (OR, 5.532; P < 0.001) were independent risk factors for DVT. We then established a scoring system for risk factors. In the validation group, a total of 213 patients with liver failure were treated with ALSS, including 14 with lower limb DVT. When the cutoff value of risk assessment was 3, the specificity and sensitivity of the risk assessment score were 88.9% and 85.7%, respectively.
A simple risk assessment scoring system was established for DVT patients with liver failure treated with ALSS and was verified to have good sensitivity and specificity.
Core Tip: The risk factors for deep vein thrombosis (DVT) after the use of an artificial liver support system (ALSS) and a risk assessment score were established. A total of 232 patients with liver failure treated with ALSS were enrolled in the first stage, including 12 with lower limb DVT. Logistic regression analysis showed that age, successful catheterization time, activity status (strict bed rest), and D-dimer level (≥ 500 ng/mL) were independent risk factors for DVT. In the risk assessment scoring system validation group, when the cutoff value of risk assessment was 3, the specificity and sensitivity of the risk assessment score were 88.9% and 85.7%, respectively.
- Citation: Ye Y, Li X, Zhu L, Yang C, Tan YW. Establishment of a risk assessment score for deep vein thrombosis after artificial liver support system treatment. World J Clin Cases 2021; 9(31): 9406-9416
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9406.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9406
Liver failure is a common but serious liver disease with high mortality rates[1,2]. The artificial liver support system (ALSS) is an effective treatment method for liver failure[3,4], but it requires deep venous intubation and long-term indwelling catheterization. However, the coagulation mechanism disorder of basic liver failure diseases, and complications such as bleeding, infection, and deep venous thrombosis (DVT) often occur[5,6]. The risk of DVT is significantly increased in cases of severe liver disease[7], mainly lower limb DVT and pulmonary embolism[8,9].
The commonly used DVT risk assessment systems are the Wells scoring system[10], Autar DVT risk assessment scale[11], and Caprini risk assessment model[12]. However, these evaluation systems are not suitable for the treatment of liver failure using ALSS. The development of new risk assessment indices for DVT is beneficial for patients with liver failure treated using ALSS therapy.
This study was divided into three stages. In the first stage, the risk factors for DVT were screened. A retrospective survey was conducted of consecutive hospitalized patients with liver failure who had received ALSS treatment in our hospital between January 2014 and December 2017. Patient data were collected, including demographic information (sex, age, weight, height, body mass index, blood pressure, smoking, past diseases, family history); ALSS treatment information (catheterization method, successful catheterization time, whether heparin was used in catheterization, ALSS treatment duration, etc.); biochemical indices (blood glucose, serum total bilirubin, and alanine aminotransferase levels); blood coagulation and hematological indices (platelet count, international normalized ratio, D-dimer level); complications (ascites, hepatic encephalopathy, hemorrhage, comorbid serious diseases); whether procoagulant therapy was used; and 24 other indicators. A total of 232 patients with liver failure treated with ALSS were evaluated, including 14 with lower limb DVT and 218 without DVT. The incidence of DVT was 6.03%, and the median occurrence duration was 14 d (range, 7-21 d) after deep venous indwelling. The second stage involved the establishment of a risk assessment for DVT after ALSS treatment. The variable parameters screened out in the first stage were thrombosis and non-thrombosis as dichotomous variables for logistic analysis. The independent risk factors were scored, and a risk assessment score was established. The third stage involved verification of the DVT risk assessment score. Patients were hospitalized in the Liver Disease Department of our hospital between January 1, 2018 and October 31, 2020. A total of 213 patients with liver failure were included and treated with ALSS, including 14 cases with DVT (6.57%) in the lower extremities and 199 cases without DVT The median time to occurrence was 16 d (range, 7-28 d) after deep vein indwelling.
The liver failure diagnosis and ALSS treatment of all patients were delivered according to China’s 2012 Guidelines for the Diagnosis and Treatment of Liver Failure[13]. Lower extremity DVT was diagnosed using B-ultrasound (JE Philips L9 model) for venous examination of the bilateral lower extremities. The diagnostic criteria included the following[14]: (1) After the probe was pressurized, the lumen was not compressed; (2) A strong echo or low echo was detected in the lumen, the distal lumen of the obstruction was dilated, the pathological vein wall was thickened, the wall structure was unclear, and lumen stenosis or obstruction was noted; and (3) When the vein was completely obstructed, the proximal color Doppler ultrasound could not detect the blood flow signal.
Plasma exchange (PE) or continuous hemofiltration therapy was administered to patients treated with ALSS. Jugular or femoral vein catheterization was performed with a single-needle and a double-lumen catheter (B. Braun GmbH, Model 12F, Germany). The PE involved a plasma volume of 100 mL/min, plasma separation rate of 30%, and plasma exchange volume of 1-1.3 times the patient’s plasma volume. Patient plasma volume = patient weight (kg) × 70 mL × [(1.0 hematocrit) × 0.91] × 1.15. In hemofiltration, filtration after PE lasted for 24-72 h. The catheter remained indwelling until the end of the ALSS treatment or complications occurred.
Measurement data are expressed as mean ± SD, and all data were processed using SPSS22.0 (IBM, Chicago, IL, United States). Measurement data were tested by independent sample t test, count data by the χ2 test, and logistic regression by binary classification and the full entry method. The risk assessment form was validated by the area under the receiver operating characteristic curve (AUC) using MedCalc software (version 10.4.7.0; Medcalc, Mariakerke, Belgium). Values of P < 0.05 were considered statistically significant. The AUC was used to evaluate the diagnostic value of the DVT risk assessment score after ALSS treatment.
The demographic and clinical characteristics of patients treated with ALSS in Stage I were compared. Age (χ2 = 7.17, P = 0.027), catheterization method (χ2 = 4.99, P = 0.025), successful catheterization time (χ2 = 10.856, P = 0.004), last ALSS (χ2 = 67.481, P < 0.001), activity status (χ2 = 9.607, P = 0.02) 2), D-dimer level (χ2 = 12.318, P = 0.002), and infection status (χ2 = 17.231, P = 0.001) were significantly different between the thrombus and no-thrombus groups (Table 1).
| Parameters | DVT (n = 12) | No-DVT (n = 220) | Statistics | P value |
| Gender, n (%) | ||||
| Man | 7 (58.3) | 165 (75) | 1.649 | 0.306 |
| Woman | 5 (41.7) | 55 (25) | ||
| Age (yrs), n (%) | ||||
| < 40 | 2 (18.2) | 51 (28.7) | 7.17 | 0.027 |
| < 60, ≥ 40 | 4 (36.4) | 101 (56.7) | ||
| ≥ 60 | 5 (45.5) | 26 (14.6) | ||
| Mean arterial pressure (mmHg), mean ± SD | 83.45 ± 14.47 | 84.11 ± 13.26 | 0.768 | 0.707 |
| Fasting plasma glucose (mmol/L), mean ± SD | 5.43 ± 1.51 | 5.41 ± 1.65 | 0.332 | 0.542 |
| Smoke, n (%) | ||||
| No | 7 (58.3) | 142 (64.5) | 0.197 | 0.759 |
| Yes | 5 (41.7) | 78 (35.5) | ||
| Catheterization mode, n (%) | ||||
| Jugular vein | 0 (0) | 72 (30) | 5.09 | 0.022 |
| Femoral vein | 12 (100) | 168 (70) | ||
| Times of successful catheterization, n (%) | ||||
| One | 3 (25) | 151 (71.9) | 16.669 | 0 |
| Two | 4 (33.3) | 41 (19.5) | ||
| Three and more | 5 (21.7) | 18 (8.6) | ||
| Heparin therapy, n (%) | ||||
| No | 3 (25) | 56 (25.5) | 0.01 | 0.971 |
| Yes | 9 (75) | 164 (74.5) | ||
| Procoagulant therapy, n (%) | ||||
| No | 8 (67.7) | 164 (74.5) | 0.368 | 0.513 |
| Yes | 4 (33.3) | 56 (125.5) | ||
| Etiology, n (%) | ||||
| Virus hepatitis | 9 (75) | 133 (60.5) | 1.028 | 0.795 |
| Drug injury | 1 (8.3) | 29 (13.2) | ||
| Autoimmune liver disease | 1 (8.3) | 24 (11.8) | ||
| Others | 1 (8.3) | 32 (14.5) | ||
| ALSS times, n (%) | ||||
| < 3 | 3 (25) | 61 (27.7) | 7.344 | 0.025 |
| ≥ 3, < 5 | 5 (21.7) | 139 (63.2) | ||
| ≥ 5 | 4 (33.3) | 20 (9.1) | ||
| Activity status, n (%) | ||||
| Free activities | 0 (0) | 23 (10.5) | 10.773 | 0.013 |
| Less than 4 h/d | 2 (16.7) | 89 (40.5) | ||
| Less than 1 h/d | 4 (33.3) | 74 (33.6) | ||
| Strict bed rest | 6 (50.0) | 32 (15.5) | ||
| Ascites status, n (%) | ||||
| No | 3 (25) | 67 (28.1) | 0.923 | 0.82 |
| Mild | 4 (33.3) | 79 (37.6) | ||
| Moderate | 3 (25) | 56 (25.7) | ||
| Severe | 2 (16.7) | 19 (8.6) | ||
| Bleeding, n (%) | ||||
| No | 8 (67.7) | 153 (69.5) | 0.436 | 0.833 |
| Yes | 4 (33.3) | 67 (30.5) | ||
| Hepatic encephalopathy, n (%) | ||||
| No | 6 (54.5) | 148 (67.3) | 1.933 | 0.385 |
| I-II | 4 (33.3) | 55 (25.0) | ||
| III-IV | 2 (17.5) | 17 (7.7) | ||
| Infection, n (%) | ||||
| No | 4 (33.3) | 144 (65.5) | 5.083 | 0.031 |
| Yes | 8 (67.7) | 76 (34.5) | ||
| Complicated by other serious diseases, n (%) | ||||
| No | 8 (67.7) | 174 (79.1) | 1.039 | 0.294 |
| Yes | 4 (33.3) | 46 (20.9) | ||
| Body mass index (kg/m2), n (%) | ||||
| < 23 | 2 (20.0) | 77 (35) | 2.262 | 0.332 |
| ≥ 23, < 25 | 4(40.0) | 97 (44.1) | ||
| ≥ 25 | 4 (40.0) | 46 (20.9) | ||
| Platelet count (109/L), n (%) | ||||
| < 100 | 3 (25) | 99 (45) | 2.258 | 0.277 |
| ≥ 100, < 300 | 8 (66.7) | 95 (43.2) | ||
| ≥ 300 | 1 (8.3) | 16 (11.8) | ||
| Total bilirubin (μmol/L), mean ± SD | 234.45 ± 155.12 | 231.65 ± 132.86 | 1.476 | 0.583 |
| Alanine aminotransferase (U/L), mean ± SD | 256.82 ± 243.455 | 294.65 ± 341.36 | 1.20· | 0.578 |
| D-dimer (ng/mL), n (%) | ||||
| < 200 | 1 (8.3) | 88 (40) | 12.232 | 0.002 |
| ≥ 200, < 500 | 3 (25.9) | 82 (37.3) | ||
| ≥ 500 | 8 (66.7) | 50 (22.7) | ||
| Fibrinogen (g/L), mean ± SD | 1.25 ± 0.43 | 1.33 ± 1.12 | 2.554 | 0.212 |
| International normalized ratio, n (%) | ||||
| < 1.5 | 0 (0) | 0 (0) | 0.215 | 0.767 |
| ≥ 1.5, < 2 | 6 (54.5) | 125 (56.8) | ||
| ≥ 2 | 6 (45.5) | 85 (43.2) | ||
Taking DVT and non-DVT as dichotomous variables, all entry methods were adopted and conditional parameter variables were included (P < 0.1). The dichotomous logistic regression analysis showed that age [odds ratio (OR), 1.734; P = 0.01], successful catheterization time (OR, 1.667; P = 0.005), activity status (strict bed rest) (OR, 3.049; P = 0.005), D-dimer level (≥ 500 ng/mL) (OR, 5.532; P < 0.001), and infection status (OR, 2.426; P = 0.008) were independent risk factors for DVT (Table 2).
| Parameters | β | Wald χ2 | OR | 95%CI | P value |
| Age (yrs) | 0.454 | 5.776 | 1.734 | 1.034-2.543 | 0.01 |
| < 40 | 1 | ||||
| < 60, ≥ 40 | 0.034 | 2.765 | 1.114 | 0.324-3.654 | 0.95 |
| ≥ 60 | 3.354 | 8.234 | 1.886 | 1.154-4.853 | 0.005 |
| Catheterization mode | |||||
| Jugular vein | 1 | ||||
| Femoral vein | 0.068 | 0.976 | 0.645 | 0.550-1.655 | 0.121 |
| ALSS times | 1.63 | 1.424 | 0.257-1.667 | 0.223 | |
| < 3 | 1 | ||||
| ≥ 3, < 5 | 0.354 | 0.564 | 0.454 | 0.416-2.534 | 0.531 |
| ≥ 5 | 0.243 | 0.486 | 1.321 | 1.013-6.534 | 0.615 |
| Times of successful catheterization | |||||
| One | 1 | ||||
| Two | 0.672 | 1.534 | 0.674 | 0.056-1.132 | 0.242 |
| Three and more | 1.246 | 6.435 | 1.667 | 1.005-3.235 | 0.005 |
| Activity status | |||||
| Free activities | 1 | ||||
| Less than 4 h/d | 0.54 | 1.004 | 1.028 | 0.062-1.002 | 0.972 |
| Less than 1 h/d | 0.764 | 0.172 | 1.565 | 0.999-1.504 | 0.128 |
| Strict bed rest | 1.547 | 11.074 | 3.049 | 1.744-8.414 | < 0.001 |
| D-dimer (ng/mL) | |||||
| < 200 | 1 | ||||
| ≥ 200, < 500 | 1.322 | 1.653 | 1.674 | 1.056-2.232 | 0.064 |
| ≥ 500 | 2.115 | 12.231 | 5.532 | 1.404-12.133 | < 0.001 |
| Infection | |||||
| No | 1 | ||||
| Yes | 2.431 | 16.236 | 2.426 | 1.003-89.342 | 0.008 |
According to the results of the multivariate logistic regression analysis, the proposed scores are listed in Table 3.
| Indicators | Score |
| D-dimer | 3 |
| Infection | 2 |
| Strict bed rest | 2 |
| Age (≥ 60 yrs) | 2 |
| Times of successful catheterization (≥ 3) | 1 |
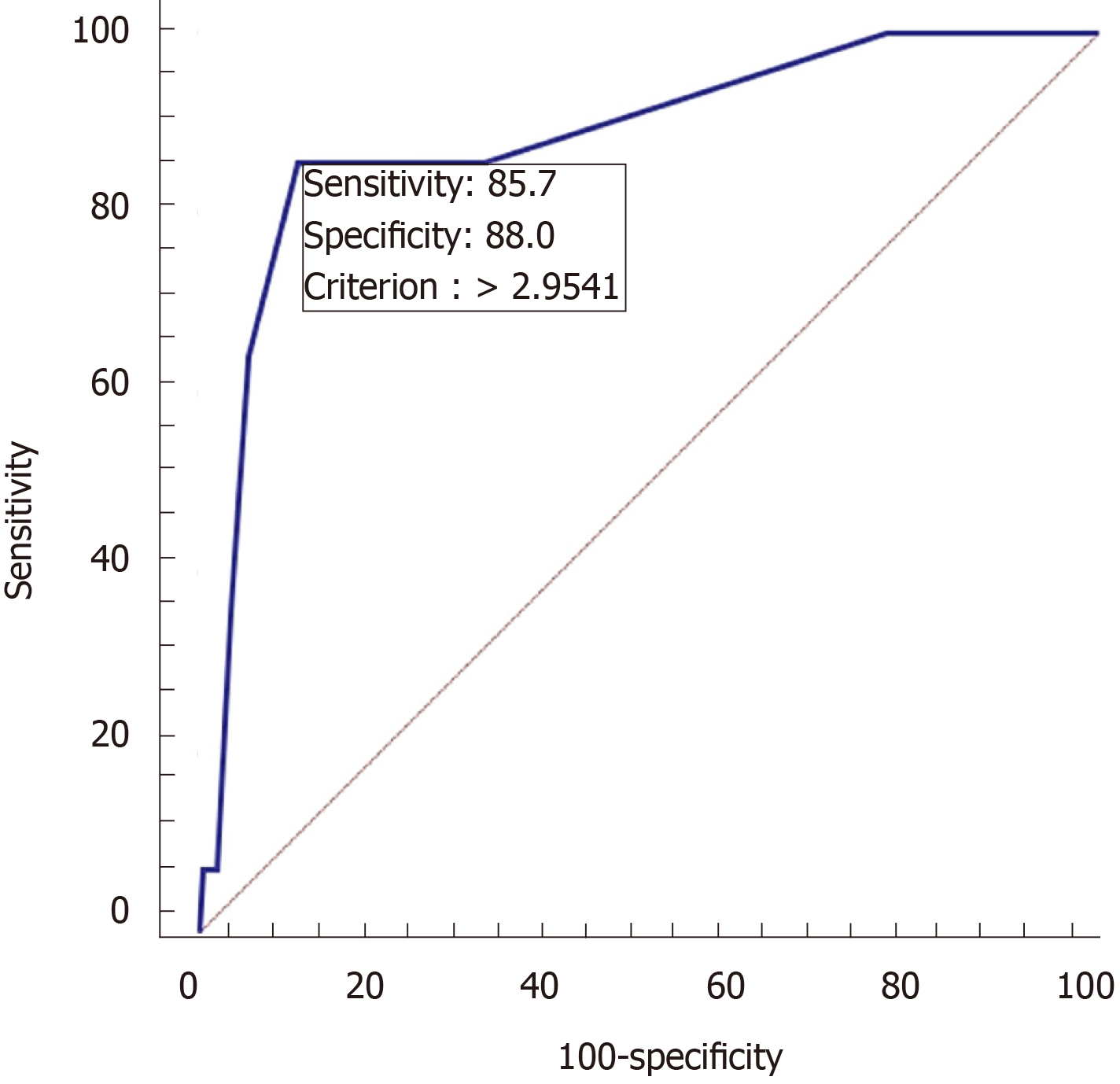
Analysis of the validation group data revealed significant differences in age, successful catheterization time, activity status, D-dimer level, and infection between patients with and without DVT as well as differences in the ALSS treatment duration (Table 4). AUC was used to evaluate the diagnostic value of the DVT risk assessment score after ALSS treatment. The DVT and no DVT after ALSS groups were considered classification variables, while the DVT risk assessment score was considered a variable. When the cutoff value of the risk assessment score was 3, the specificity and sensitivity of the risk assessment score for DVT were 88.9% and 85.7%, respectively (Figure 1).
| Parameters | DVT (n = 14) | No-DVT (n = 199) | Statistics | P value |
| Gender | ||||
| Man | 9 (64.3) | 165 (82.9) | 3.035 | 0.082 |
| Woman | 5 (35.7) | 34 (17.1) | ||
| Age (yrs) | ||||
| < 40 | 2 (16.7) | 51 (25.6) | 11.004 | 0.004 |
| < 60, ≥ 40 | 2 (16.7) | 101 (50.8) | ||
| ≥ 60 | 8 (66.7) | 47 (23.6) | ||
| Catheterization mode | ||||
| Jugular vein | 2 (14.3) | 51 (25.6) | 0.903 | 0.343 |
| Femoral vein | 12 (85.7) | 148 (74.4) | ||
| Times of successful catheterization | ||||
| One | 3 (21.4) | 120 (60.3) | 13.742 | 0.001 |
| Two | 4 (28.6) | 51 (25.6) | ||
| Three and more | 7 (50.0) | 28 (14.1) | ||
| ALSS times | ||||
| < 3 | 4 (28.6) | 61 (30.7) | 9.207 | 0.01 |
| ≥ 3, < 5 | 5 (35.7) | 119 (50.8) | ||
| ≥ 5 | 5 (35.7) | 19 (9.5) | ||
| Activity status | ||||
| Free activities | 0 (0) | 23 (11.6) | 11.761 | 0.008 |
| Less than 4 h/d | 4 (28.6) | 69 (34.7) | ||
| Less than 1 h/d | 4 (28.6) | 84 (42.6) | ||
| Strict bed rest | 6 (42.9) | 23 (11.6) | ||
| Infection | ||||
| No | 4 (28.6) | 114 (57.6) | 4.365 | 0.037 |
| Yes | 10 (71.4) | 85 (42.4) | ||
| D-dimer (ng/mL) | ||||
| < 200 | 1 (7.1) | 65 (32.7) | 14.84 | 0.001 |
| ≥ 200, < 500 | 4 (28.6) | 94 (47.2) | ||
| ≥ 500 | 9 (64.3) | 40 (20.1) | ||
The mechanism of ALSS is based on the strong regenerative ability of liver cells through mechanical, physical, chemical, and biological equipment in vitro, removing all types of harmful substances, supplying necessary materials, improving the internal environment, temporarily replacing the partial failure of liver function, creating a good condition for liver cell regeneration and liver function recovery, or waiting for an opportunity for liver transplantation[15,16].
Virchow proposed three causes of DVT[17]: Vascular endothelial injury, hemody
Liver failure leads to a decrease in hepatic synthetic coagulation factors, hyperfibrinolysis, and thrombocytopenia caused by hypersplenism[27,28]. Therefore, patients with liver failure have always been considered to be in a low coagulation state [29,30]. However, in severe liver disease, the risk of DVT is also significantly increased and mainly manifests as lower extremity DVT and pulmonary embolism[7,31]. During the catheter indwelling period, to prevent bleeding at the puncture site and keep the catheter unobstructed, the patient requires absolute bed rest and limb braking at the puncture side. Indwelling central venous catheter, intraoperative heparin anticoagulation, postoperative bed rest, and other related factors increased the incidence of DVT in patients with liver failure treated with ALSS.
In this study, older age, more catheterization time, absolute bed rest, increased D-dimer level, and infection were all high-risk factors for DVT. Older age has always been a risk factor in various DVT risk assessment forms, such as the Autoar Thrombosis Risk Assessment Scale, the Caprini risk assessment model, and the JFK Medical Center Thrombosis Assessment Scale[32]. ALSS requires more treatment times. To avoid repeated puncture, puncture and indwelling are adopted, and the jugular vein, subclavian vein, and femoral vein are most commonly used. Deep vein puncture and catheterization itself will inevitably damage the blood vessels, and the literature shows that the proficiency of the puncture technique is negatively correlated with the incidence of DVT[33]. DVT caused by deep vein catheterization occurs primarily in the lower extremities, and use of the femoral vein indwelling method in all of our centers was also a risk factor for DVT formation. Liver failure is often complicated by infection, commonly in the abdominal cavity or lungs[34], and is a risk factor for the development of DVT. DVT and inflammatory responses are closely related from the beginning to the end[35]. Infection promotes the release of inflammatory factors, leading to an increase in immunoglobulin levels that can lead to an increase in blood viscosity and the formation of thrombosis[36,37].
The tool currently used for the risk assessment of DVT is the Autoar Thrombosis Risk Assessment Scale, which includes seven submodules: age, body size, activity, special risk category, trauma, surgery, and high-risk disease. Studies using the Autoar scale showed that it was the most effective at warning the risk of perioperative DVT in patients with bone trauma and could reduce the incidence of VTE in patients with perioperative trauma[38]. The Caprini risk assessment model[39], originally released in 1991 for use in all inpatients as a weighted risk stratification tool, contains 39 indicators and is subject to constant updating. The Wells scoring system is a prediction system based on D-dimer level that can be better used for the early diagnosis of DVT[40] rather than the risk assessment of DVT. However, the setting of these indicators does not fully consider the risk of thrombus formation in this special group of patients with liver failure after treatment using ALSS.
In this study, through the risk assessment of DVT in patients with liver failure, the advantages of other DVT risk assessment systems were fully used and combined with the special clotting state of liver failure to establish a simple scoring system that has good sensitivity and specificity. The shortcoming of this study is that it was a single-center retrospective study. Multi-center, prospective, and large-sample validation is still needed.
The artificial liver support system (ALSS) is an effective treatment method for liver failure, but it requires deep venous intubation and long-term indwelling catheterization. However, the coagulation mechanism disorder of basic liver failure diseases, and deep venous thrombosis (DVT) often occur.
The commonly used DVT risk assessment systems are not suitable for the treatment of liver failure using ALSS. The development of new risk assessment indices for DVT is beneficial for patients with liver failure treated using ALSS therapy.
To evaluate the risk factors for DVT following the use of an ALSS and establish a risk assessment score.
This study was divided into three stages. In the first stage, the risk factors for DVT were screened and the patient data were collected, including ALSS treatment information; biochemical indices; coagulation and hematology indices; complications; procoagulant use therapy status; and a total of 24 other indicators. In the second stage, a risk assessment score for DVT after ALSS treatment was developed. In the third stage, the DVT risk assessment score was validated.
A total of 232 patients with liver failure treated with ALSS were enrolled in the first stage, including 12 with lower limb DVT. We then established a scoring system for risk factors. In the validation group, a total of 213 patients with liver failure were treated with ALSS, including 14 with lower limb DVT. When the cutoff value of risk assessment was 3, the specificity and sensitivity of the risk assessment score were 88.9% and 85.7%, respectively.
A simple risk assessment scoring system was established for DVT patients with liver failure treated with ALSS and was verified to have good sensitivity and specificity.
In this study, through the risk assessment of DVT in patients with liver failure, the advantages of other DVT risk assessment systems were fully used and combined with the special clotting state of liver failure to establish a simple scoring system that has good sensitivity and specificity.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang Y S-Editor: Wu YXJ L-Editor: Webster JR P-Editor: Yu HG
| 1. | Squires JE, McKiernan P, Squires RH. Acute Liver Failure: An Update. Clin Liver Dis. 2018;22:773-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 263] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 3. | Tandon R, Froghi S. Artificial liver support systems. J Gastroenterol Hepatol. 2021;36:1164-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Larsen FS. Artificial liver support in acute and acute-on-chronic liver failure. Curr Opin Crit Care. 2019;25:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Huang K, Ji F, Xie Z, Wu D, Xu X, Gao H, Ouyang X, Xiao L, Zhou M, Zhu D, Li L. Artificial liver support system therapy in acute-on-chronic hepatitis B liver failure: Classification and regression tree analysis. Sci Rep. 2019;9:16462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Bachli EB, Schuepbach RA, Maggiorini M, Stocker R, Müllhaupt B, Renner EL. Artificial liver support with the molecular adsorbent recirculating system: activation of coagulation and bleeding complications. Liver Int. 2007;27:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Dhar A, Mullish BH, Thursz MR. Anticoagulation in chronic liver disease. J Hepatol. 2017;66:1313-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Forkin KT, Colquhoun DA, Nemergut EC, Huffmyer JL. The Coagulation Profile of End-Stage Liver Disease and Considerations for Intraoperative Management. Anesth Analg. 2018;126:46-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Saner FH, Bezinover D. Assessment and management of coagulopathy in critically-ill patients with liver failure. Curr Opin Crit Care. 2019;25:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Modi S, Deisler R, Gozel K, Reicks P, Irwin E, Brunsvold M, Banton K, Beilman GJ. Wells criteria for DVT is a reliable clinical tool to assess the risk of deep venous thrombosis in trauma patients. World J Emerg Surg. 2016;11:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Autar R. Nursing assessment of clients at risk of deep vein thrombosis (DVT): the Autar DVT scale. J Adv Nurs. 1996;23:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Golemi I, Salazar Adum JP, Tafur A, Caprini J. Venous thromboembolism prophylaxis using the Caprini score. Dis Mon. 2019;65:249-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Liver Failure and Artificial Liver Group; Chinese Society of Infectious Diseases; Chinese Medical Association. ; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. [Diagnostic and treatment guidelines for liver failure (2012 version)]. Zhonghua Gan Zang Bing Za Zhi. 2013;21:177-183. [PubMed] |
| 14. | Quinn KL, Vandeman FN. Thrombosis of a duplicated superficial femoral vein. Potential error in compression ultrasound diagnosis of lower extremity deep venous thrombosis. J Ultrasound Med. 1990;9:235-238. [PubMed] [DOI] [Full Text] |
| 15. | Takikawa Y, Kakisaka K, Suzuki Y, Ido A, Shimamura T, Nishida O, Oda S, Shimosegawa T. Multicenter study on the consciousness-regaining effect of a newly developed artificial liver support system in acute liver failure: An on-line continuous hemodiafiltration system. Hepatol Res. 2021;51:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Liu H, Zhang Q, Liu L, Cao Y, Ye Q, Liu F, Liang J, Wen J, Li Y, Han T. Effect of artificial liver support system on short-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure. Artif Organs. 2020;44:E434-E447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Stone J, Hangge P, Albadawi H, Wallace A, Shamoun F, Knuttien MG, Naidu S, Oklu R. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7:S276-S284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 18. | Xue YL, Zhao SF, Luo Y, Li XJ, Duan ZP, Chen XP, Li WG, Huang XQ, Li YL, Cui X, Zhong DG, Zhang ZY, Huang ZQ. TECA hybrid artificial liver support system in treatment of acute liver failure. World J Gastroenterol. 2001;7:826-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Wu Y, Su SA, Xie Y, Shen J, Zhu W, Xiang M. Murine models of vascular endothelial injury: Techniques and pathophysiology. Thromb Res. 2018;169:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | He L, Huang X, Kanisicak O, Li Y, Wang Y, Pu W, Liu Q, Zhang H, Tian X, Zhao H, Liu X, Zhang S, Nie Y, Hu S, Miao X, Wang QD, Wang F, Chen T, Xu Q, Lui KO, Molkentin JD, Zhou B. Preexisting endothelial cells mediate cardiac neovascularization after injury. J Clin Invest. 2017;127:2968-2981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 21. | Mezger M, Nording H, Sauter R, Graf T, Heim C, von Bubnoff N, Ensminger SM, Langer HF. Platelets and Immune Responses During Thromboinflammation. Front Immunol. 2019;10:1731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 22. | Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109:2832-2839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Takahashi A, Takahashi S, Tsujino T, Isobe K, Watanabe T, Kitamura Y, Nakata K, Kawase T. Platelet adhesion on commercially pure titanium plates in vitro I: effects of plasma components and involvement of the von Willebrand factor and fibronectin. Int J Implant Dent. 2019;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Deng L, Bremme K, Hansson LO, Blombäck M. Plasma levels of von Willebrand factor and fibronectin as markers of persisting endothelial damage in preeclampsia. Obstet Gynecol. 1994;84:941-945. [PubMed] |
| 25. | Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu Rev Pathol. 2020;15:493-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 535] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 26. | Evans J, Salamonsen LA. Inflammation, leukocytes and menstruation. Rev Endocr Metab Disord. 2012;13:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 27. | Bulut Y, Sapru A, Roach GD. Hemostatic Balance in Pediatric Acute Liver Failure: Epidemiology of Bleeding and Thrombosis, Physiology, and Current Strategies. Front Pediatr. 2020;8:618119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Caldwell SH, Chang C, Macik BG. Recombinant activated factor VII (rFVIIa) as a hemostatic agent in liver disease: a break from convention in need of controlled trials. Hepatology. 2004;39:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Northup PG, Caldwell SH. Coagulation in liver disease: a guide for the clinician. Clin Gastroenterol Hepatol. 2013;11:1064-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 30. | O'Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology. 2019;157:34-43.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 31. | Saner FH, Kirchner C. Monitoring and Treatment of Coagulation Disorders in End-Stage Liver Disease. Visc Med. 2016;32:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Wang MM, Qin XJ, He XX, Qiu MJ, Peng G, Yang SL. Comparison and screening of different risk assessment models for deep vein thrombosis in patients with solid tumors. J Thromb Thrombolysis. 2019;48:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Gelonch J, Alastrué A, Monreal M, Iglesias C, Rull M, Lafoz E, Casals A, Salvá JA. [Deep venous thrombosis of the upper limb. A prospective study of the central venous catheter as an etiologic factor and clinical and subclinical incidence of pulmonary thromboembolism]. Nutr Hosp. 1991;6:161-171. [PubMed] |
| 34. | Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 685] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 35. | Borgel D, Bianchini E, Lasne D, Pascreau T, Saller F. Inflammation in deep vein thrombosis: a therapeutic target? Hematology. 2019;24:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Yao X, Chen W, Liu J, Liu H, Zhan JY, Guan S, Lu Z, Tang P, Li P, Lin B. Deep Vein Thrombosis is Modulated by Inflammation Regulated via Sirtuin 1/NF-κB Signalling Pathway in a Rat Model. Thromb Haemost. 2019;119:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Mukhopadhyay S, Johnson TA, Duru N, Buzza MS, Pawar NR, Sarkar R, Antalis TM. Fibrinolysis and Inflammation in Venous Thrombus Resolution. Front Immunol. 2019;10:1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 38. | Aggarwal A, Puri K, Liangpunsakul S. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: systematic review. World J Gastroenterol. 2014;20:5737-5745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 39. | Caprini JA, Arcelus JI, Traverso CI, Hasty JH. Low molecular weight heparins and external pneumatic compression as options for venous thromboembolism prophylaxis: a surgeon's perspective. Semin Thromb Hemost. 1991;17:356-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells Score for Deep Vein Thrombosis in the Inpatient Setting. JAMA Intern Med. 2015;175:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |