Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9368
Peer-review started: February 20, 2021
First decision: May 13, 2021
Revised: May 27, 2021
Accepted: August 17, 2021
Article in press: August 17, 2021
Published online: November 6, 2021
Processing time: 251 Days and 0.6 Hours
Major depressive disorder (MDD) is a multifactorial disorder, where multiple susceptibility genes interact with environmental factors, predisposing individuals to the development of the illness. In this article, we reviewed different gene × environment interaction (G×E) studies shifting from a candidate gene to a genome-wide approach. Among environmental factors, childhood adversities and stressful life events have been suggested to exert crucial impacts on MDD. Importantly, the diathesis-stress conceptualization of G×E has been challenged by the differential susceptibility theory. Finally, we summarized several limitations of G×E studies and suggested how future G×E studies might reveal complex interactions between genes and environments in MDD.
Core Tip: The effects of environmental factors on the risk of developing major depressive disorder are likely to be moderated by genetic variants which confer a sensitivity to both positive and negative environmental factors.
- Citation: Zhao MZ, Song XS, Ma JS. Gene × environment interaction in major depressive disorder. World J Clin Cases 2021; 9(31): 9368-9375
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9368.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9368
Major depressive disorder (MDD) is a debilitating illness which severely restricts psychosocial function and diminishes quality of life. MDD is characterized by alterations in mood, interests, pleasure, neurovegetative function and cognition. The average 12-mo prevalence of MDD was estimated to be approximately 6%[1]. Lifetime MDD risk is typically threefold higher than the 12-mo prevalence, meaning MDD is common, with almost one in every six adults experiencing one episode at some point in their lifetime[2]. The World Health Organization ranked MDD as the third cause of burden of disease in 2008 and predicted that, by 2030, MDD will rank first and account for 13% of the total global burden of disease replacing cardiovascular disorders[3].
Although the pathophysiology of MDD is not yet fully understood, the presence of a genetic component to MDD has been established by family, twins, and adoption studies[4]. It has been known for more than a century that MDD shows family aggregation. There is a threefold increased risk of MDD among first-degree relatives, with a heritability risk that is quantified as approximately 35%[5]. Furthermore, genetic overlaps between MDD and other psychiatric disorders have been identified[6,7].
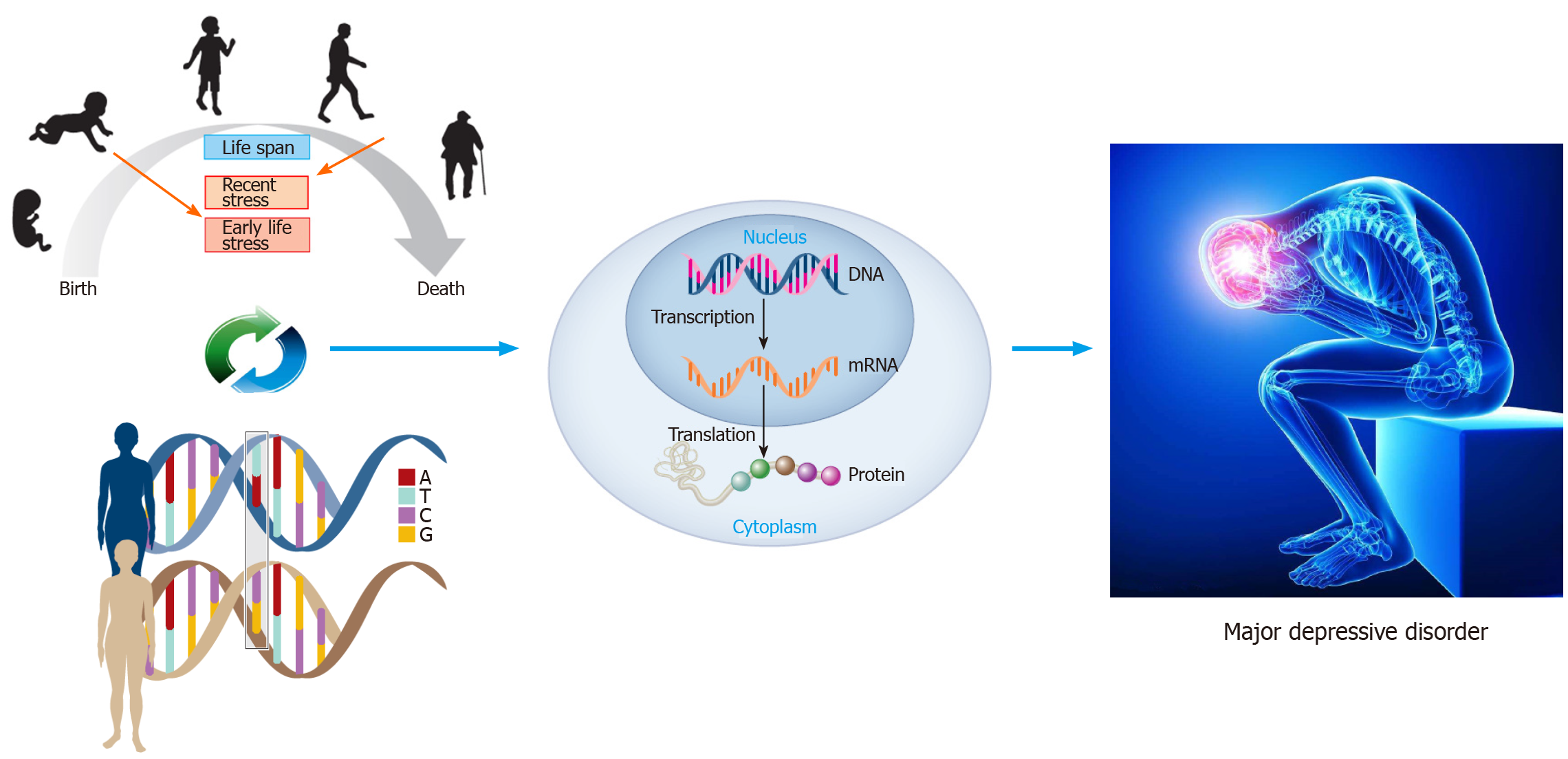
However, identification of the main genetic effects in MDD so far has not revealed replicated significant findings[8]. One of the potential reasons for this weak genetic effect is the fact that an individual gene is likely to exert only a modest effect and individual genotypic variations may increase the risk of MDD only in the presence of exposure to adverse environmental circumstances, a phenomenon known as “gene×environment interaction (G×E)”[9] (Figure 1). This review will focus on studies that aimed to assess the joint contribution of gene and environment in the development of MDD.
To date, as a part of a concerted effort to investigate the genetic contribution to MDD, many G×E studies have been based on candidate genes. The serotonin transporter promoter short/Long polymorphism (5-HTTLPR) and a functional single nucleotide polymorphism (SNP) within the FK506 binding protein 51 (FKBP5) gene were two of the most investigated examples of candidate G×E linking environments to MDD. Caspi and colleagues were the first to estimate the moderating effects of 5-HTTLPR on MDD within a G×E framework, showing that individuals with a short allele of 5-HTTLPR were at higher risk of MDD and suicidality as compared with those with homozygous or long alleles[10]. Since then, more than fifty studies have been conducted to replicate this finding, however, not all of them have achieved their aims[11]. G×E has been suggested to predict MDD in individuals exposed to negative environmental factors[12,13]. Similarly, many studies have found that a functional SNP within FKBP5 interacted with environments to predict MDD[14-17].
The 5-HTTLPR or FKBP5 interacting with environments were based on hypothesis-driven studies which aimed to identify not only genetic variants that increased the risk of MDD, but also potential biological and molecular mechanisms underlying this increased risk. It was extensively discussed elsewhere that the hypothesis-driven approach could only discover a fraction of potential existing genetic variants. Genome-wide by environment interaction studies (GWEIS) using a hypothesis-free approach to identify genes associated with MDD are emerging, but enormous datasets with both environments and phenotype data are required. So far, three GWEIS have been performed in this regard. The first was conducted by Dunn and colleagues[18] who used data from the SNP Health Association Resource (SHARe) cohort of the Women’s Health Initiative and investigated both genetic main effects and G×E effects in the development of depressive symptoms. The second was a pilot study and conducted in 320 subjects with no interactions reaching genome-wide significance[19]. The third used an omics-based approach to identify genetic variants with G×E effects in GWAS datasets and found three candidate genes associated with MDD[20].
Environmental factors are associated with a dramatic increase in the risk of developing MDD. A number of environmental factors contributing to MDD vulnerability have been identified, including lack of nutrients, social disadvantage, childhood adversities, maternal stress, and stressful life events[21-24]. Of these environmental factors, stressful life events and childhood adversities have been shown to exert crucial impacts on MDD[25,26]. Stressful life events are described as circumstances that have negative effects on an individual occurring close to the onset of MDD[27]. Childhood adversities are defined as stressful experiences which occur early in life. It is very important to differentiate distal environmental factors (childhood adversities) and proximal environmental factors (stressful life events) in G×E studies. Distal environmental factors are critical because they can increase the likelihood of the occurrence of proximal environmental factors. Proximal environmental factors are more relevant than distal environmental factors to the G×E effects on MDD.
Environmental influences on MDD also exhibit a cumulative effect[28,29]. The effect of a single environmental factor may be small, cumulative effects of multiple factors may be quite large, which can be described as a dose-response relationship between environments and phenotype[30]. Many powerful effects involved chains of environmental factors rather than a single factor at just one point in time. It was shown that a developmental cascade of negative experiences was a leading cause of MDD in women[31].
Traditionally, the Diathesis-Stress model was the leading conceptual framework for G×E studies, because most investigators usually focused on genetic influences moderated by environments[32]. Specifically, the Diathesis-Stress model hypothesizes that individuals with specific biological (e.g., genetic) and/or psychological (temperament) traits are more vulnerable to adverse environmental influences, but that this inherent vulnerability is not sufficient to lead to psychopathology if such adverse environmental influences are absent. Furthermore, individuals who do not succumb to the negative effects of environmental factors, either as the function of not carrying genetic vulnerabilities or due to the presence of other protective factors, are deemed resilient.
However, the Diathesis-Stress model has been challenged by Stress Generation[33] and Differential Susceptibility Theory[34,35]. Stress Generation suggested that the Diathesis-Stress model of MDD was woefully inadequate because the model was unidirectional, with the environment predicting MDD in those with the diathesis. Stress Generation suggested that the reverse was also true: MDD patients caused stressful life events. Stressors might occur in an individual for random reasons, but many stressful life events are not random. It was shown that women with a history of MDD had higher levels of stressful life events compared with women without a history of MDD[36]. Differential Susceptibility Theory suggested that individuals differed in their general susceptibility to both negative and positive environmental influences. Specifically, individuals who were genetically more vulnerable to environmental influences might be more likely to develop MDD in response to stressful life events and childhood adversities. However, these individuals with higher genetic vulnerability might also be likely to benefit from positive and supportive environmental influences. In contrast to the Diathesis-Stress model, Differential Susceptibility Theory was an evolutionary-inspired developmental model taking into account both negative and positive effects on MDD. This evolutionary model may better account for the findings that most of candidate genes in G×E studies were “common” variants. Findings of these common variants indicated that they might have benefits that counteracted the negative influences of heightened vulnerability[37,38]. Hence, these variants might not merely infer negative effects, as proposed by the Diathesis-Stress model, but they also infer positive effects, as suggested by Differential Susceptibility Theory[39].
Although candidate G×E studies have made a critical contribution to the field of MDD, several limitations should also be acknowledged. First, a robust biological hypothesis is required to select appropriate candidate genes. Given the fact that knowledge regarding the specific biological mechanisms underlying MDD remain limited, there is a high risk of selecting inappropriate candidate genes and having publication bias[8,40]. Second, a recent discovery in MDD suggested that the genetic architecture of MDD was highly complex and polygenic[41]. In other words, MDD was influenced by many thousands of gene variants with very small effects rather than by several gene variants with large effects[13]. Third, it is difficult to replicate findings from G×E studies, which is a particular concern. Initial studies might use small sample sizes that were unable to provide statistical power required to detect G×E and, consequently, might increase the risk of false positive findings[42,43]. Finally, most G×E studies in MDD were based on the Diathesis-Stress model which ignored the positive effects of environmental influences[39,44]. The notion that selected candidate genes might be associated with heightened sensitivity to both environmental risk and protective factors, might also contribute to heterogeneous findings.
Genome-wide association studies have been conducted for decades to investigate genetic main effects and G×E studies should also be shifted from candidate genes to a genome-wide approach. In addition to collecting large samples required for GWEIS, high quality and more objective measures of environments are also important. The experience sampling method (ESM) may provide a novel approach to obtain more accurate self-report data on an individual’s experience of their environments. The ESM has also been used to investigate individual difference in response to both positive and negative environments[45]. Empirical studies indicated that there were substantial aetiological overlaps between MDD and other psychiatric disorders[7]. The Transdiagnostic phenotypes may be more suited as outcomes in G×E studies than categorical and narrowly defined clinical diagnoses for psychiatric disorders[46]. G×E effects may differ across life span, with effects being stronger in early development. Genetically sensitive children who experienced a poor early environment showed increased sensitivity to stressful life events. Applying a developmental perspective may fully understand the role of G×E in the progress of MDD.
Despite the importance of candidate gene studies for G×E in MDD, to date, only a few candidate vulnerability genes explain little of the variance. GWEIS are needed for a more complete understanding of G×E in the pathophysiology of MDD.
Among environmental factors, stressful life events and childhood adversities have been recognized to have prominent roles in MDD. Environmental influences on MDD have a cumulative effect, and a dose-response relationship or interaction between stressful life events and childhood adversities may exist in the develop
Most G×E studies in MDD were guided by the Diathesis-Stress model which did not consider that individuals who were genetically more sensitive to negative environmental factors might also be more sensitive to positive ones as proposed by Differential Susceptibility Theory. The Differential Susceptibility Theory as a solid evolutionary theory may advance knowledge on interactions between genes and environments in the development of MDD.
We thank Professor Sheng-Ying Qin for helpful comments and we are grateful to all researchers in this area for advancing our understanding of G×E in MDD.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koo BH, Kotzalidis GD S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Wang LYT
| 1. | Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1342] [Cited by in RCA: 1660] [Article Influence: 138.3] [Reference Citation Analysis (0)] |
| 2. | Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lépine JP, Levinson D, Matschinger H, Mora ME, Browne MO, Posada-Villa J, Viana MC, Williams DR, Kessler RC. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1172] [Cited by in RCA: 1355] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 3. | WHO. The global burden of disease: 2004 update. Geneva: World Health Organization 2008. |
| 4. | Lesch KP. Gene-environment interaction and the genetics of depression. J Psychiatry Neurosci. 2004;29:174-184. [PubMed] |
| 5. | Geschwind DH, Flint J. Genetics and genomics of psychiatric disease. Science. 2015;349:1489-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 273] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 6. | Cross-Disorder Group of the Psychiatric Genomics Consortium; Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, Mowry BJ, Thapar A, Goddard ME, Witte JS, Absher D, Agartz I, Akil H, Amin F, Andreassen OA, Anjorin A, Anney R, Anttila V, Arking DE, Asherson P, Azevedo MH, Backlund L, Badner JA, Bailey AJ, Banaschewski T, Barchas JD, Barnes MR, Barrett TB, Bass N, Battaglia A, Bauer M, Bayés M, Bellivier F, Bergen SE, Berrettini W, Betancur C, Bettecken T, Biederman J, Binder EB, Black DW, Blackwood DH, Bloss CS, Boehnke M, Boomsma DI, Breen G, Breuer R, Bruggeman R, Cormican P, Buccola NG, Buitelaar JK, Bunney WE, Buxbaum JD, Byerley WF, Byrne EM, Caesar S, Cahn W, Cantor RM, Casas M, Chakravarti A, Chambert K, Choudhury K, Cichon S, Cloninger CR, Collier DA, Cook EH, Coon H, Cormand B, Corvin A, Coryell WH, Craig DW, Craig IW, Crosbie J, Cuccaro ML, Curtis D, Czamara D, Datta S, Dawson G, Day R, De Geus EJ, Degenhardt F, Djurovic S, Donohoe GJ, Doyle AE, Duan J, Dudbridge F, Duketis E, Ebstein RP, Edenberg HJ, Elia J, Ennis S, Etain B, Fanous A, Farmer AE, Ferrier IN, Flickinger M, Fombonne E, Foroud T, Frank J, Franke B, Fraser C, Freedman R, Freimer NB, Freitag CM, Friedl M, Frisén L, Gallagher L, Gejman PV, Georgieva L, Gershon ES, Geschwind DH, Giegling I, Gill M, Gordon SD, Gordon-Smith K, Green EK, Greenwood TA, Grice DE, Gross M, Grozeva D, Guan W, Gurling H, De Haan L, Haines JL, Hakonarson H, Hallmayer J, Hamilton SP, Hamshere ML, Hansen TF, Hartmann AM, Hautzinger M, Heath AC, Henders AK, Herms S, Hickie IB, Hipolito M, Hoefels S, Holmans PA, Holsboer F, Hoogendijk WJ, Hottenga JJ, Hultman CM, Hus V, Ingason A, Ising M, Jamain S, Jones EG, Jones I, Jones L, Tzeng JY, Kähler AK, Kahn RS, Kandaswamy R, Keller MC, Kennedy JL, Kenny E, Kent L, Kim Y, Kirov GK, Klauck SM, Klei L, Knowles JA, Kohli MA, Koller DL, Konte B, Korszun A, Krabbendam L, Krasucki R, Kuntsi J, Kwan P, Landén M, Långström N, Lathrop M, Lawrence J, Lawson WB, Leboyer M, Ledbetter DH, Lee PH, Lencz T, Lesch KP, Levinson DF, Lewis CM, Li J, Lichtenstein P, Lieberman JA, Lin DY, Linszen DH, Liu C, Lohoff FW, Loo SK, Lord C, Lowe JK, Lucae S, MacIntyre DJ, Madden PA, Maestrini E, Magnusson PK, Mahon PB, Maier W, Malhotra AK, Mane SM, Martin CL, Martin NG, Mattheisen M, Matthews K, Mattingsdal M, McCarroll SA, McGhee KA, McGough JJ, McGrath PJ, McGuffin P, McInnis MG, McIntosh A, McKinney R, McLean AW, McMahon FJ, McMahon WM, McQuillin A, Medeiros H, Medland SE, Meier S, Melle I, Meng F, Meyer J, Middeldorp CM, Middleton L, Milanova V, Miranda A, Monaco AP, Montgomery GW, Moran JL, Moreno-De-Luca D, Morken G, Morris DW, Morrow EM, Moskvina V, Muglia P, Mühleisen TW, Muir WJ, Müller-Myhsok B, Murtha M, Myers RM, Myin-Germeys I, Neale MC, Nelson SF, Nievergelt CM, Nikolov I, Nimgaonkar V, Nolen WA, Nöthen MM, Nurnberger JI, Nwulia EA, Nyholt DR, O'Dushlaine C, Oades RD, Olincy A, Oliveira G, Olsen L, Ophoff RA, Osby U, Owen MJ, Palotie A, Parr JR, Paterson AD, Pato CN, Pato MT, Penninx BW, Pergadia ML, Pericak-Vance MA, Pickard BS, Pimm J, Piven J, Posthuma D, Potash JB, Poustka F, Propping P, Puri V, Quested DJ, Quinn EM, Ramos-Quiroga JA, Rasmussen HB, Raychaudhuri S, Rehnström K, Reif A, Ribasés M, Rice JP, Rietschel M, Roeder K, Roeyers H, Rossin L, Rothenberger A, Rouleau G, Ruderfer D, Rujescu D, Sanders AR, Sanders SJ, Santangelo SL, Sergeant JA, Schachar R, Schalling M, Schatzberg AF, Scheftner WA, Schellenberg GD, Scherer SW, Schork NJ, Schulze TG, Schumacher J, Schwarz M, Scolnick E, Scott LJ, Shi J, Shilling PD, Shyn SI, Silverman JM, Slager SL, Smalley SL, Smit JH, Smith EN, Sonuga-Barke EJ, St Clair D, State M, Steffens M, Steinhausen HC, Strauss JS, Strohmaier J, Stroup TS, Sutcliffe JS, Szatmari P, Szelinger S, Thirumalai S, Thompson RC, Todorov AA, Tozzi F, Treutlein J, Uhr M, van den Oord EJ, Van Grootheest G, Van Os J, Vicente AM, Vieland VJ, Vincent JB, Visscher PM, Walsh CA, Wassink TH, Watson SJ, Weissman MM, Werge T, Wienker TF, Wijsman EM, Willemsen G, Williams N, Willsey AJ, Witt SH, Xu W, Young AH, Yu TW, Zammit S, Zandi PP, Zhang P, Zitman FG, Zöllner S, Devlin B, Kelsoe JR, Sklar P, Daly MJ, O'Donovan MC, Craddock N, Sullivan PF, Smoller JW, Kendler KS, Wray NR; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC). Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1903] [Cited by in RCA: 1636] [Article Influence: 136.3] [Reference Citation Analysis (1)] |
| 7. | Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2173] [Cited by in RCA: 2154] [Article Influence: 179.5] [Reference Citation Analysis (0)] |
| 8. | Bosker FJ, Hartman CA, Nolte IM, Prins BP, Terpstra P, Posthuma D, van Veen T, Willemsen G, DeRijk RH, de Geus EJ, Hoogendijk WJ, Sullivan PF, Penninx BW, Boomsma DI, Snieder H, Nolen WA. Poor replication of candidate genes for major depressive disorder using genome-wide association data. Mol Psychiatry. 2011;16:516-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 664] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 10. | Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5478] [Cited by in RCA: 4741] [Article Influence: 215.5] [Reference Citation Analysis (0)] |
| 11. | Munafò MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 371] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 12. | Russotti J, Warmingham JM, Duprey EB, Handley ED, Manly JT, Rogosch FA, Cicchetti D. Child maltreatment and the development of psychopathology: The role of developmental timing and chronicity. Child Abuse Negl. 2021;120:105215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 13. | Culverhouse RC, Saccone NL, Horton AC, Ma Y, Anstey KJ, Banaschewski T, Burmeister M, Cohen-Woods S, Etain B, Fisher HL, Goldman N, Guillaume S, Horwood J, Juhasz G, Lester KJ, Mandelli L, Middeldorp CM, Olié E, Villafuerte S, Air TM, Araya R, Bowes L, Burns R, Byrne EM, Coffey C, Coventry WL, Gawronski KAB, Glei D, Hatzimanolis A, Hottenga JJ, Jaussent I, Jawahar C, Jennen-Steinmetz C, Kramer JR, Lajnef M, Little K, Zu Schwabedissen HM, Nauck M, Nederhof E, Petschner P, Peyrot WJ, Schwahn C, Sinnamon G, Stacey D, Tian Y, Toben C, Van der Auwera S, Wainwright N, Wang JC, Willemsen G, Anderson IM, Arolt V, Åslund C, Bagdy G, Baune BT, Bellivier F, Boomsma DI, Courtet P, Dannlowski U, de Geus EJC, Deakin JFW, Easteal S, Eley T, Fergusson DM, Goate AM, Gonda X, Grabe HJ, Holzman C, Johnson EO, Kennedy M, Laucht M, Martin NG, Munafò MR, Nilsson KW, Oldehinkel AJ, Olsson CA, Ormel J, Otte C, Patton GC, Penninx BWJH, Ritchie K, Sarchiapone M, Scheid JM, Serretti A, Smit JH, Stefanis NC, Surtees PG, Völzke H, Weinstein M, Whooley M, Nurnberger JI Jr, Breslau N, Bierut LJ. Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression. Mol Psychiatry. 2018;23:133-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 14. | Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene-Stress-Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacology. 2016;41:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 410] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 15. | Comasco E, Gustafsson PA, Sydsjö G, Agnafors S, Aho N, Svedin CG. Psychiatric symptoms in adolescents: FKBP5 genotype--early life adversity interaction effects. Eur Child Adolesc Psychiatry. 2015;24:1473-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 16. | Kohrt BA, Worthman CM, Ressler KJ, Mercer KB, Upadhaya N, Koirala S, Nepal MK, Sharma VD, Binder EB. Cross-cultural gene- environment interactions in depression, post-traumatic stress disorder, and the cortisol awakening response: FKBP5 polymorphisms and childhood trauma in South Asia. Int Rev Psychiatry. 2015;27:180-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Tozzi L, Carballedo A, Wetterling F, McCarthy H, O'Keane V, Gill M, Morris D, Fahey C, Meaney J, Frodl T. Single-Nucleotide Polymorphism of the FKBP5 Gene and Childhood Maltreatment as Predictors of Structural Changes in Brain Areas Involved in Emotional Processing in Depression. Neuropsychopharmacology. 2016;41:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Dunn EC, Wiste A, Radmanesh F, Almli LM, Gogarten SM, Sofer T, Faul JD, Kardia SL, Smith JA, Weir DR, Zhao W, Soare TW, Mirza SS, Hek K, Tiemeier H, Goveas JS, Sarto GE, Snively BM, Cornelis M, Koenen KC, Kraft P, Purcell S, Ressler KJ, Rosand J, Wassertheil-Smoller S, Smoller JW. Genome-Wide Association Study (Gwas) and Genome-Wide by Environment Interaction Study (Gweis) of Depressive Symptoms in African American and Hispanic/Latina Women. Depress Anxiety. 2016;33:265-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Otowa T, Kawamura Y, Tsutsumi A, Kawakami N, Kan C, Shimada T, Umekage T, Kasai K, Tokunaga K, Sasaki T. The First Pilot Genome-Wide Gene-Environment Study of Depression in the Japanese Population. PLoS One. 2016;11:e0160823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Cattaneo A, Cattane N, Malpighi C, Czamara D, Suarez A, Mariani N, Kajantie E, Luoni A, Eriksson JG, Lahti J, Mondelli V, Dazzan P, Räikkönen K, Binder EB, Riva MA, Pariante CM. FoxO1, A2M, and TGF-β1: three novel genes predicting depression in gene X environment interactions are identified using cross-species and cross-tissues transcriptomic and miRNomic analyses. Mol Psychiatry. 2018;23:2192-2208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Lin YL, Wang S. Prenatal lipopolysaccharide exposure increases depression-like behaviors and reduces hippocampal neurogenesis in adult rats. Behav Brain Res. 2014;259:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Gámez-Guadix M, Orue I, Smith PK, Calvete E. Longitudinal and reciprocal relations of cyberbullying with depression, substance use, and problematic internet use among adolescents. J Adolesc Health. 2013;53:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 23. | Gullander M, Hogh A, Hansen ÅM, Persson R, Rugulies R, Kolstad HA, Thomsen JF, Willert MV, Grynderup M, Mors O, Bonde JP. Exposure to workplace bullying and risk of depression. J Occup Environ Med. 2014;56:1258-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Cummings CM, Caporino NE, Kendall PC. Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychol Bull. 2014;140:816-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 573] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 25. | Ho TC. Stress and Neurodevelopment in Adolescent Depression. Biol Psychiatry. 2019;86:e33-e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Harmer B, Lee S, Duong TVH, Saadabadi A. 2021 Aug 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. |
| 27. | Ezquiaga E, Ayuso Gutierrez JL, García López A. Psychosocial factors and episode number in depression. J Affect Disord. 1987;12:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 987] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 29. | Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9396] [Cited by in RCA: 9318] [Article Influence: 345.1] [Reference Citation Analysis (0)] |
| 30. | Evans GW. The environment of childhood poverty. Am Psychol. 2004;59:77-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1279] [Cited by in RCA: 909] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 31. | Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in men. Am J Psychiatry. 2006;163:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 32. | Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull. 1991;110:406-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1125] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 33. | Hammen C. Risk Factors for Depression: An Autobiographical Review. Annu Rev Clin Psychol. 2018;14:1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 274] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 34. | Belsky J, Pasco Fearon RM, Bell B. Parenting, attention and externalizing problems: testing mediation longitudinally, repeatedly and reciprocally. J Child Psychol Psychiatry. 2007;48:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary--neurodevelopmental theory. Dev Psychopathol. 2011;23:7-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 895] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 36. | Hammen C. Generation of stress in the course of unipolar depression. J Abnorm Psychol. 1991;100:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 99] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Pluess M, Belsky J. Vantage sensitivity: individual differences in response to positive experiences. Psychol Bull. 2013;139:901-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 286] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 38. | Pluess M. Vantage Sensitivity: Environmental Sensitivity to Positive Experiences as a Function of Genetic Differences. J Pers. 2017;85:38-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1722] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 40. | Collins AL, Kim Y, Sklar P; International Schizophrenia Consortium, O'Donovan MC, Sullivan PF. Hypothesis-driven candidate genes for schizophrenia compared to genome-wide association results. Psychol Med. 2012;42:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Donnelly P. Progress and challenges in genome-wide association studies in humans. Nature. 2008;456:728-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 42. | Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 681] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 43. | Munafò MR, Flint J. Replication and heterogeneity in gene x environment interaction studies. Int J Neuropsychopharmacol. 2009;12:727-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 745] [Cited by in RCA: 677] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 45. | Menne-Lothmann C, Jacobs N, Derom C, Thiery E, van Os J, Wichers M. Genetic and environmental causes of individual differences in daily life positive affect and reward experience and its overlap with stress-sensitivity. Behav Genet. 2012;42:778-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Ofrat S, Krueger RF. How research on the meta-structure of psychopathology aids in understanding biological correlates of mood and anxiety disorders. Biol Mood Anxiety Disord. 2012;2:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |