Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9302
Peer-review started: June 7, 2021
First decision: June 25, 2021
Revised: July 9, 2021
Accepted: August 6, 2021
Article in press: August 6, 2021
Published online: October 26, 2021
Processing time: 135 Days and 19 Hours
The DYNC1H1 gene encodes a part of the dynamic protein, and the protein mutations may further affect the growth and development of neurons, resulting in degeneration of anterior horn cells of the spinal cord, and a variety of clinical phenotypes finally resulting in axonal Charcot-Marie-Tooth disease type 20 (CMT20), mental retardation 13 (MRD13) and spinal muscular atrophy with lower extremity predominant 1 (SMA-LED). The incidence of the disease is low, and it is difficult to diagnose, especially in children. Here, we report a case of DYNC1H1 gene mutation and review the related literature to improve the pediatrician’s understanding of DYNC1H1 gene-related disease to make an early correct diagnosis and provide better services for children.
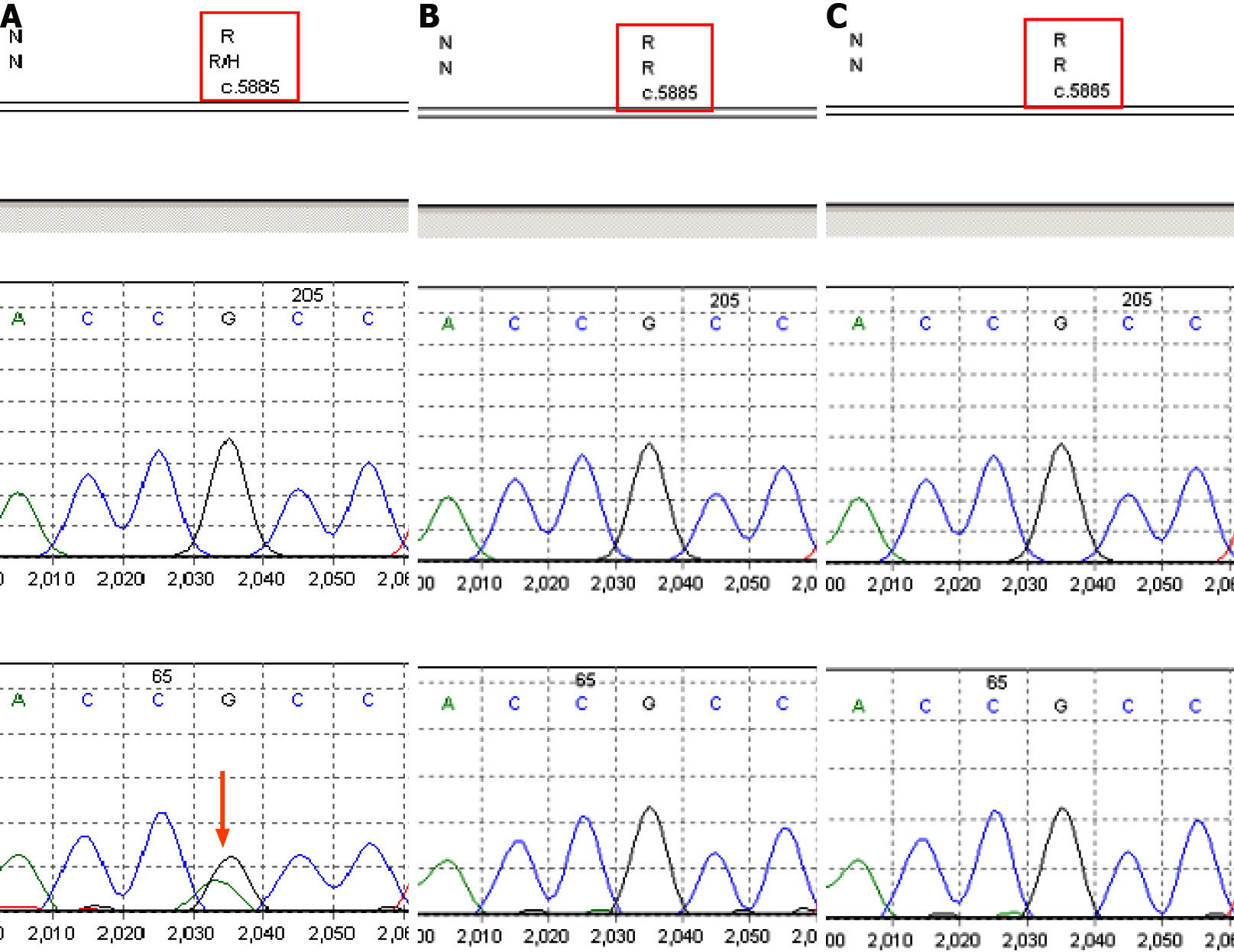
A 4-mo-old Chinese female child with adducted thumbs, high arch feet, and epileptic seizure presented slow response, delayed development, and low limb muscle strength. Electroencephalogram showed abnormal waves, a large number of multifocal sharp waves, sharp slow waves, and multiple spasms with a series of attacks. High-throughput sequencing and Sanger sequencing identified a heterozygous mutation, c.5885G>A (p.R1962H), in the DYNC1H1 gene (NM_001376) of the proband, which was not identified in her parents. Combined with the clinical manifestations and pedigree of this family, this mutation is likely pathogenic based on the American Academy of Medical Genetics and Genomics guidelines. The child was followed when she was 1 year and 2 mo old. The magnetic resonance imaging result was consistent with the findings of white matter myelinated dysplasia and congenital giant gyrus. The extensive neuro
We herein report a de novo variant of DYNC1H1 gene, c.5885G>A (p.R1962H), leading to overlapping phenotypes (seizure, general growth retardation, and muscle weakness) of CMT20, MRD13, and SMA-LED, but there is no effective treatment for such condition. Our case enriches the DYNC1H1 gene mutation spectrum and provides an important basis for clinical diagnosis and treatment and genetic counseling.
Core Tip: The dynein cytoplasmic1 heavy chain 1 gene-related diseases include Charcot-Marie-Tooth disease type 20, mental retardation 13, and spinal muscular atrophy with lower extremity predominant 1, all of which are inherited in an autosomal dominant manner. A novel mutation, c.5885G>A (p.R1962H) in the DYNC1H1 gene, led to overlapping phenotypes (seizure, general growth retardation, and muscle weak
- Citation: Ding FJ, Lyu GZ, Zhang VW, Jin H. Missense mutation in DYNC1H1 gene caused psychomotor developmental delay and muscle weakness: A case report. World J Clin Cases 2021; 9(30): 9302-9309
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9302.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9302
DYNC1H1-related diseases include axonal Charcot-Marie-Tooth disease type 20 (CMT20), mental retardation 13 (MRD13), and spinal muscular atrophy with lower extremity predominant 1 (SMA-LED), all of which are inherited in an autosomal dominant manner. The clinical symptoms of CMT20 are mainly peripheral neu
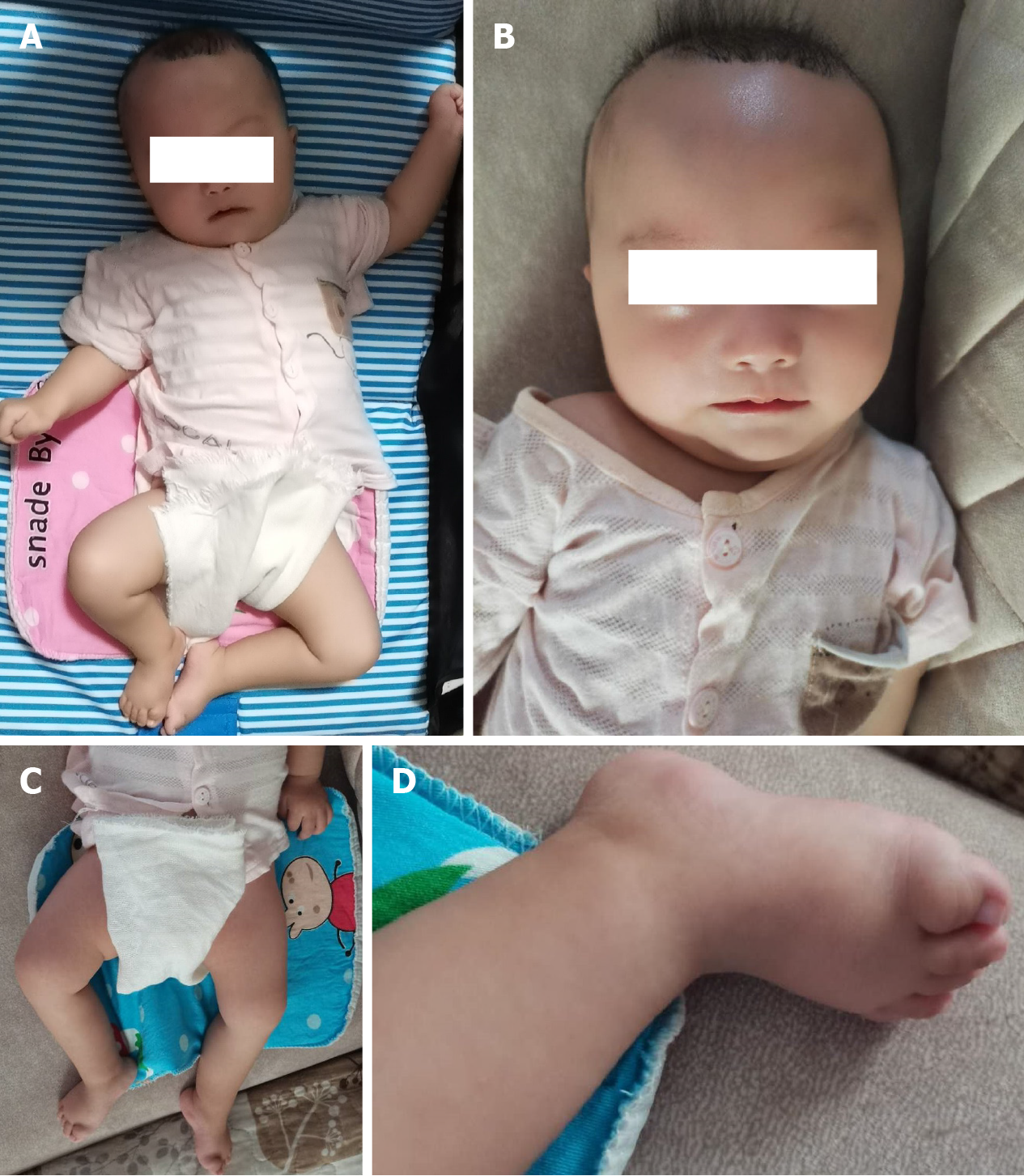
A female, 4 mo old, was admitted to the hospital due to hypoplasia (Figure 1).
Physical examination revealed a slow response, no obvious gaze with no follow-up, no eyesight, no smile, no recognition of the mother, head drooping, hands with clenched fists, adducted thumbs, symmetrical limbs, fully stretched limbs, high hips, and low head. She raised the head and back when laid on her back. She was unable to turn over, seated fully forward, seated upright, and pointed feet. Both of her lower limbs were unable to support her weight. The muscles of the limbs were tense. The internal adductor angle was 30°, the popliteal angle was 60°, the foot dorsiflexion angle was 20°, and the Vojta posture reflex showed abnormal reflexes due to poor head, neck, and trunk extension. The grip and embrace reflexes were present, and the bilateral knee tendon reflex was elicited. Normal children raise their heads at 4 mo of age, and they can stand up independently, will turn their heads and look for it when they hear the sound, can be amused, and will also make a first babble. However, the patient in our case had attention deficit disorder and delayed motor performance.
The baby was delivered at 41 wk of gestation, amniotic fluid was turbid, birth weight was 3250 g, and she had weak crying and vomiting, with a weak hugging reflex, and was transferred to the neonatal department for hospitalization.
Both parents were healthy (G1P1) without a family history of the disease and consanguinity was denied.
Physical examination revealed a slow response, no obvious gaze with no follow-up, no eyesight, no smile, no recognition of the mother, head drooping, hands with clenched fists, adducted thumbs, symmetrical limbs, fully stretched limbs, high hips, and low head. She raised the head and back when laid on her back. She was unable to turn over, seated fully forward, seated upright, and pointed feet. Both of her lower limbs were unable to support her weight. The muscles of the limbs were tense. The internal adductor angle was 30°, the popliteal angle was 60°, the foot dorsiflexion angle was 20°, and the Vojta posture reflex showed abnormal reflexes due to poor head, neck, and trunk extension. The grip and embrace reflexes were present, and the bilateral knee tendon reflex was elicited (Figure 1).
Peripheral blood samples (2 mL) were obtained from the proband and her parents. DNA was extracted using a standard phenol-chloroform protocol. Medical exome sequencing (MES) was performed using the lllumina NovaSeq 6000 system with an average sequencing depth of 200 × as previously described[5]. Sanger sequencing was performed to verify the mutation. The pathogenicity of the variant was classified according to the guidelines of the American Academy of Medical Genetics and Genomics (ACMG)[6].
Magnetic resonance imaging (MRI, Phiilips, 1.5 T, Achieva) of the brain showed that the cortex of the cerebral hemispheres was thickened, and the sulcus gyrus was reduced. The corpus callosum was short and widened on both sides of the ventricle, the shape was not natural, the transparent septum was shown, and the subarachnoid space of the frontotemporal area was slightly wider on both sides.
In this case, MES analysis, which included 5177 disease-associated genes, was per
The current treatment for patients with DYNC1H1 gene related disease is mainly supportive, aiming to provide nutritional and respiratory support as needed, and to treat or prevent the complications of muscle weakness. The prognosis of the disease is poor.
We plan to continue to follow the child’s disease progression. This diagnosis permitted proper genetic counseling with associated risk assessment.
The heterozygous variant c.5885G>A (p.R1962H) in the DYNC1H1 gene detected in this case is a novel de novo variant. The DYNC1H1 gene is located at 14q32.31, which encodes a large key subunit of the cytoplasmic dynein complex[2]. DYNC1H1 mu
| Patient | Age (yr) | Sex | Main clinical manifestations | Mutation | Ref. |
| A four-generation family with 23 members affected | - | - | Pes cavus at birth; delayed motor milestones; lower limb weakness; speech delay; learning difficulties | Heterozygous; c.917A>G, p.His306Arg | [9] |
| I 2 | 82 | Female | Lower limb muscle wasting and weakness; walked with a waddling gait | Heterozygous; c.1809A>T, p.glu603Asp | [11] |
| II 1 | 60 | Male | Lower limb muscle wasting and weakness; had learning difficulties and epilepsy | ||
| II 2 | 59 | Male | Bilateral talipes equinovarus, congenital hip dislocation, and scoliosis; lower limb weakness and difficulty walking | ||
| II 3 | 58 | Male | Bilateral talipes equinovarus and congenital hip dislocation requiring surgeries; bilateral pes cavus, wasting of muscles (particularly quadriceps), reduced reflexes, but normal sensation in the lower limbs and walked very slowly with a waddling gait | ||
| III 1 | 32 | Male | Similar features as #II 3 | ||
| III 2 | 30 | Male | Similar features as #II 3 | ||
| Patient 1 | 15 | Female | Middle East. Twenty months: Delayed walking and early predominant weakness in the lower extremities. Investigations showed normal creatine kinase levels and nerve conduction study findings, and needle electromyography suggested neuronal degeneration. Brain MRI: Mild ventricular dilatation. Muscle biopsy from right vastus lateralis: Neurogenic atrophy with pathological fibre-type grouping and fatty infiltration in the muscle fascicles. At 15 yr old, walked independently with waddling and needed support to rise from floor. Longer distance travel: Wheelchair. At present, mild proximal upper limbs weakness; mild intellectual disabilities | De novo heterozygous; c.751C>T, p.Arg251Cys | [12] |
| Patient 2 | 16 | Female | Chinese. At birth: Clubfeet; 2 years old: Delayed walking; 7 years old: Pes cavus, significant lower limb muscle wasting and weakness, absent knee jerks but preserved ankle jerks, positive Gower sign, mild proximal muscle weakness in the upper extremities with preserved reflexes. Mildly elevated creatine kinase level (255 U/L, normal reference: < 154 U/L). Muscle biopsy from the right deltoid: Type 2 fibre atrophy. Needle electromyography: Chronic denervation. 12 years old: Mild scoliosis with Cobb’s angle of 14 degrees from T12 to L5. Attention deficit and hyperactivity disorder with dyslexia. 14 years old: Walk independently but required to use a walking stick for long distance travel. Leg muscle MRI: Selective muscle involvement and no deterioration when repeated 1.5 yr later. 18 years old: Knee tightness increase; scoliosis and motor performance stable. Brain MRI: Mild ventricular dilatation | ||
| Patient 3 | 8 | Male | Chinese. At birth: Club feet. 2 years old: Started walking and fell easily. Normal to mildly elevated creatine kinase levels (CK148 - 216 IU/L: normal reference: < 163 IU/L). Muscle biopsy from left quadriceps reported predominant type 1 fibres with rare scattered atrophic fibres. Brain MRI: Mildly dilated lateral ventricles and a left posterior fossa arachnoid cyst. 7 years old: Positive Gower sign and predominant lower limbs weakness and atrophy with absent knee jerks and decreased ankle jerks. Mild shoulder girdle weakness with preserved reflexes. Needle electromyography: Chronic denervation. 8 years old: Selective muscles involvement. 11 years old: Attention deficit disorder and motor performance remained stable with knee and tendoachilles tightness | ||
| Patient 4 | 21 | Male | Caucasian. At birth: Left clubfoot. 27 mo old: Walked led by hand. Right quadricep muscle biopsy: Predominant type 1 fibres surrounded by fat and fibrosis. Needle electromyography: Neurogenic pattern. Urinary and faecal incontinence problem. 6 years old: Predominant weakness and atrophy of both legs, more pronounced on the left side. His knee jerks were absent but the ankle jerks were preserved. He could walk up to a 100-m distance. 8 years old: Muscle ultrasound: Abnormal echogenicity of the quadriceps and bicep brachii. Mild grade intellectual disabilities and autism with hyperactive behaviour. Over the next few years: Lower limb weakness increased and upper extremities proximal weakness. Twelve years old: Walk with walking stick. 21 years old: Walk up to several meters and required a wheelchair for long distance travel. Pronounced muscle atrophy of the legs and marked contractures at both knees. Repeated brain MRI: Small right-sided posterior fossa arachnoid cyst | ||
| Family 10 | 4 | Male | Parental nonconsanguinity; Seizure onset; 3 mo: Focaltonic/opisthotonic posturing. IS (6 mo) to multiple types. EEG: MEA + AB, Severe DD, ASD, focal pachygyria | Heterozygous; c.5884C>T, p.Arg1962Cys | [15] |
| Case 4 | 7 | Female | Prenatally believed to have isolated mild ventriculomegaly but with additional postnatal findings; ventricular width: 12.0 mm; MRI: Sinuous malformation; intellectual disability, impaired psychomotor development; follow-up sonograms: Regression to normal | Heterozygous; c.5884c>T; p.Arg1962Cys | [16] |
Moreover, the clinical features observed for the DYNC1H1 gene mutation c.5885G>A (p.R1962H) were similar to those previously described for the same amino acid site variation (c.5884C>T, p.R1962C). Heterozygous DYNC1H1 c.5884C>T (p.Arg1962Cys) variants have been reported in three unrelated individuals with neurodevelopmental disorders (Table 1). It was first reported that a 19-year-old boy with normal head intellectual disabilities had focal seizures from 2 mo to 8 years old, mainly in the posterior gyrus[2]. A 4-year-old boy with epileptic encephalopathy with a de novo heterozygous mutation had seizures, growth retardation, autism spectrum disorders, and focal brain hyperfunction 3 mo later[15]. A 7-year-old woman with mental disability, psychomotor development impairment, prenatal history of ventricular enlargement, and sinus malformation[16]. The in vitro motility assays showed that DYNC1H1 mutation (p. R1962C) inhibited dynein activity, dynein’s core mechanochemical properties, and did not produce any movement of microtubules along the glass surface[4] (Table 1). In our case, considering the children’s growth and de
However, for this de novo mutation, Sanger sequencing could not rule out the possibility of low-level mosaicism in the parents of the proband. Therefore, it is recommended to select a high overage next generation sequencing method to evaluate the source of variation and the risk of offspring reoccurrence. At the same time, in this case, the mother of the child had no clear indications for prenatal diagnosis during pregnancy, but for couples who have given birth to such children, genetic counseling and prenatal diagnosis are required for the next pregnancy to avoid the birth of such children.
We herein report a de novo novel variant of DYNC1H1 gene, c.5885G>A (p.R1962H), leading to overlapping phenotypes (seizure, general growth retardation, and muscle weakness) of CMT20, MRD13, and SMA-LED. And there is no effective treatment for this disease. Our case enriches the DYNC1H1 gene mutation spectrum and provides an important basis for clinical diagnosis and treatment and genetic counseling.
The authors would like to thank the patient and her family for their collaboration. The DYNC1H1 gene analysis was conducted in Prenatal Diagnosis Center (Jinan Maternal and Child Health Hospital, Jinan, Shandong Province), and Amcare Genomics Labo
Manuscript source: Unsolicited manuscript
Specialty type: Genetics and heredity
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oley MH, Strainiene S S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Bird TD, Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, Amemiya A. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2021. 1998 Sep 28 [updated 2021 May 20]. [PubMed] |
| 2. | Poirier K, Lebrun N, Broix L, Tian G, Saillour Y, Boscheron C, Parrini E, Valence S, Pierre BS, Oger M, Lacombe D, Geneviève D, Fontana E, Darra F, Cances C, Barth M, Bonneau D, Bernadina BD, N'guyen S, Gitiaux C, Parent P, des Portes V, Pedespan JM, Legrez V, Castelnau-Ptakine L, Nitschke P, Hieu T, Masson C, Zelenika D, Andrieux A, Francis F, Guerrini R, Cowan NJ, Bahi-Buisson N, Chelly J. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat Genet. 2013;45:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 3. | Tsurusaki Y, Saitoh S, Tomizawa K, Sudo A, Asahina N, Shiraishi H, Ito J, Tanaka H, Doi H, Saitsu H, Miyake N, Matsumoto N. A DYNC1H1 mutation causes a dominant spinal muscular atrophy with lower extremity predominance. Neurogenetics. 2012;13:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Scoto M, Rossor AM, Harms MB, Cirak S, Calissano M, Robb S, Manzur AY, Martínez Arroyo A, Rodriguez Sanz A, Mansour S, Fallon P, Hadjikoumi I, Klein A, Yang M, De Visser M, Overweg-Plandsoen WC, Baas F, Taylor JP, Benatar M, Connolly AM, Al-Lozi MT, Nixon J, de Goede CG, Foley AR, Mcwilliam C, Pitt M, Sewry C, Phadke R, Hafezparast M, Chong WK, Mercuri E, Baloh RH, Reilly MM, Muntoni F. Novel mutations expand the clinical spectrum of DYNC1H1-associated spinal muscular atrophy. Neurology. 2015;84:668-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Wang Z, Lin J, Qiao K, Cai S, Zhang VW, Zhao C, Lu J. Novel mutations in HINT1 gene cause the autosomal recessive axonal neuropathy with neuromyotonia. Eur J Med Genet. 2019;62:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22547] [Article Influence: 2254.7] [Reference Citation Analysis (0)] |
| 7. | Han J, Yang YD, He Y, Liu WJ, Zhen L, Pan M, Yang X, Zhang VW, Liao C, Li DZ. Rapid prenatal diagnosis of skeletal dysplasia using medical trio exome sequencing: Benefit for prenatal counseling and pregnancy management. Prenat Diagn. 2020;40:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Punetha J, Monges S, Franchi ME, Hoffman EP, Cirak S, Tesi-Rocha C. Exome Sequencing Identifies DYNC1H1 Variant Associated With Vertebral Abnormality and Spinal Muscular Atrophy With Lower Extremity Predominance. Pediatr Neurol. 2015;52:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Weedon MN, Hastings R, Caswell R, Xie W, Paszkiewicz K, Antoniadi T, Williams M, King C, Greenhalgh L, Newbury-Ecob R, Ellard S. Exome sequencing identifies a DYNC1H1 mutation in a large pedigree with dominant axonal Charcot-Marie-Tooth disease. Am J Hum Genet. 2011;89:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Harms MB, Allred P, Gardner R Jr, Fernandes Filho JA, Florence J, Pestronk A, Al-Lozi M, Baloh RH. Dominant spinal muscular atrophy with lower extremity predominance: linkage to 14q32. Neurology. 2010;75:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Das J, Lilleker JB, Jabbal K, Ealing J. A missense mutation in DYNC1H1 gene causing spinal muscular atrophy - Lower extremity, dominant. Neurol Neurochir Pol. 2018;52:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Chan SHS, van Alfen N, Thuestad IJ, Ip J, Chan AO, Mak C, Chung BH, Verrips A, Kamsteeg EJ. A recurrent de novo DYNC1H1 tail domain mutation causes spinal muscular atrophy with lower extremity predominance, learning difficulties and mild brain abnormality. Neuromuscul Disord. 2018;28:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Yang ML, Shin J, Kearns CA, Langworthy MM, Snell H, Walker MB, Appel B. CNS myelination requires cytoplasmic dynein function. Dev Dyn. 2015;244:134-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Hoang HT, Schlager MA, Carter AP, Bullock SL. DYNC1H1 mutations associated with neurological diseases compromise processivity of dynein-dynactin-cargo adaptor complexes. Proc Natl Acad Sci U S A. 2017;114:E1597-E1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Palmer EE, Schofield D, Shrestha R, Kandula T, Macintosh R, Lawson JA, Andrews I, Sampaio H, Johnson AM, Farrar MA, Cardamone M, Mowat D, Elakis G, Lo W, Zhu Y, Ying K, Morris P, Tao J, Dias KR, Buckley M, Dinger ME, Cowley MJ, Roscioli T, Kirk EP, Bye A, Sachdev RK. Integrating exome sequencing into a diagnostic pathway for epileptic encephalopathy: Evidence of clinical utility and cost effectiveness. Mol Genet Genomic Med. 2018;6:186-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Thorup E, Jensen LN, Bak GS, Ekelund CK, Greisen G, Jørgensen DS, Hellmuth SG, Wulff C, Petersen OB, Pedersen LH, Tabor A. Neurodevelopmental disorder in children believed to have isolated mild ventriculomegaly prenatally. Ultrasound Obstet Gynecol. 2019;54:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |