Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9198
Peer-review started: April 18, 2021
First decision: May 24, 2021
Revised: May 28, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: October 26, 2021
Processing time: 185 Days and 21.3 Hours
Pancreaticoduodenectomy (PD) has been increasingly performed as a safe treatment option for periampullary malignant and benign disorders. However, the operation may result in significant postoperative complications. Here, we present a case that recurrent pyogenic liver abscess after PD is caused by common hepatic artery injury in atypical celiac axis anatomy.
A 56-year-old man with a 1-d history of fever and shivering was diagnosed with hepatic abscess. One year and five months ago, he underwent PD at a local hospital to treat chronic pancreatitis. After the operation, the patient had recurrent intrahepatic abscesses for 4 times, and the symptoms were relieved after percutaneous transhepatic cholangial drainage combining with anti-inflammatory therapy in the local hospital. Further examination showed that the recurrent liver abscess after PD was caused by common hepatic artery injury due to abnormal abdominal vascular anatomy. The patient underwent percutaneous drainage but continued to have recurrent episodes. His condition was eventually cured by right hepatectomy. In this case, preoperative examination of the patient’s anatomical variations with computed tomography would have played a pivotal role in avoiding arterial injuries.
A careful computed tomography analysis should be considered mandatory not only to define the operability (with radical intent) of PD candidates but also to identify atypical arterial patterns and plan the optimal surgical strategy.
Core Tip: The classic trifurcation of the celiac trunk contains common hepatic artery (CHA), left gastric artery, and splenic artery. CHA injury or interruption may lead to chronic biliary ischemia in the related hepatic territory or abscess. We presented a rare case of a 56-year-old man with recurrent pyogenic liver abscess and his common hepatic artery was injured by pancreatoduodenectomy. This case highlights a careful computed tomography analysis should be considered mandatory not only to define the operability (with radical intent) of pancreaticoduodenectomy candidates but also to identify atypical arterial patterns and plan the optimal surgical strategy.
- Citation: Xie F, Wang J, Yang Q. Recurrent pyogenic liver abscess after pancreatoduodenectomy caused by common hepatic artery injury: A case report. World J Clin Cases 2021; 9(30): 9198-9204
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9198.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9198
During the last few decades, pancreaticoduodenectomy (PD) has been increasingly performed as a safe treatment option for periampullary malignant and benign disorders. Several high-volume centers have reported a mortality rate of < 4%[1]. However, the operation may result in significant postoperative complications, including pancreatic leakage or fistula formation, abdominal abscess formation, bile leakage, delayed gastric emptying, and even postoperative bleeding requiring a blood transfusion or reoperation. The trauma of open pancreaticoduodenal surgery may cause the surgical site to become infected, which may delay healing and even lead to wound tearing[2]. Ischemic complications, such as mesenteric infarction, hepatic ischemia, and hepatic abscess formation, have been reported but never discussed in detail[3].
We herein present a rare case that recurrent pyogenic liver abscess after PD is caused by common hepatic artery injury in atypical celiac axis anatomy. Eventually, the patient is successfully cured through right hepatectomy.
A 56-year-old man presented to the emergency room with a 1-d history of fever and shivering.
One year and five months ago, the patient had experienced recurrent upper abdominal pain and distension with the cause of chronic pancreatitis, which were relieved after PD (Whipple procedure). Subsequently, postoperative liver abscess had recurred for 4 times, and the symptoms had been relieved after percutaneous transhepatic cholangial drainage (PTCD) combining with anti-inflammatory treatment at the local hospital. However, 1 d ago, the patient's fever and shivers resumed and the symptoms gradually deteriorated.
The patient had no medical history of any diseases with the exception of chronic pancreatitis.
The patient had no especial personal or family history.
The patient’s temperature was 39.4 ℃, heart rate was 121 bpm, respiratory rate was 24 breaths/min, and his blood pressures was 146/86 mmHg. A physical examination of the abdomen revealed that patient’s right abdominal muscle was tense with persistent tenderness and mild rebound pain at the right hypochondrium. And the oozing pus was around the PCTD tube.
Laboratory results on admission were as follows: leukocyte count, 11.28 × 109/L; hemoglobin level, 82 g/L; platelet count, 176 × 109/L; aspartate aminotransferase level, 52 IU/L; alanine aminotransferase level, 40 IU/L; alkaline phosphatase level, 260 IU/L; total protein level, 70.1 g/L; total bilirubin level, 40.5 µmol/L; C-reactive protein level, 15.6 mg/dL; and procalcitonin level, 75.5 ng/mL. The levels of tumor markers, including alpha-fetoprotein, carbohydrate antigen 19-9, and carcinoembryonic antigen, were within the reference ranges. Pus cultures suggested E. coli infection and antibiotic sensitivity results of the abscess indicated Amikacin, Cefotetan, Ertapenem, Imipenem and Piperacillin/Tazobactam were sensitive.
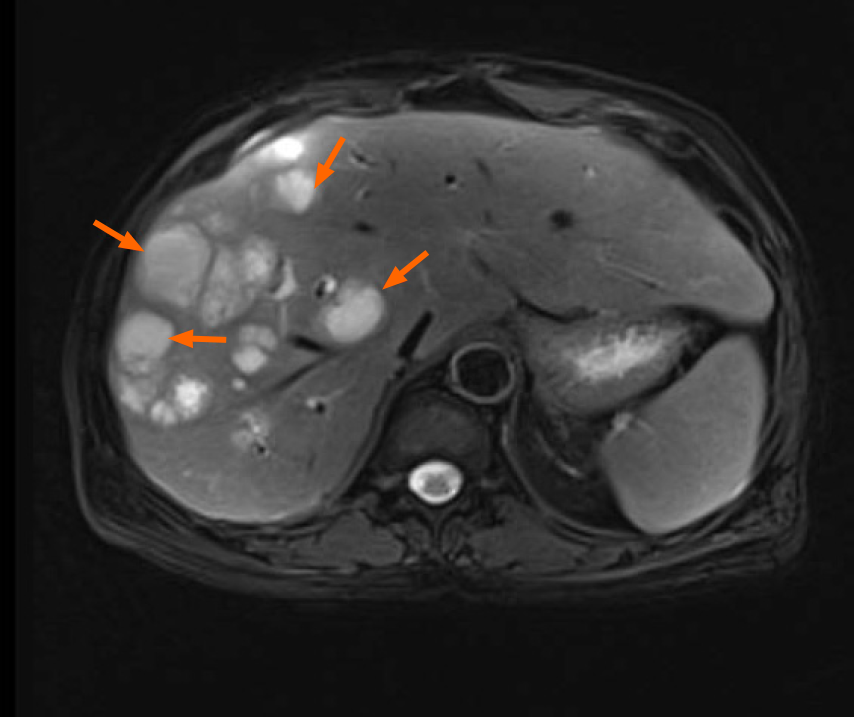
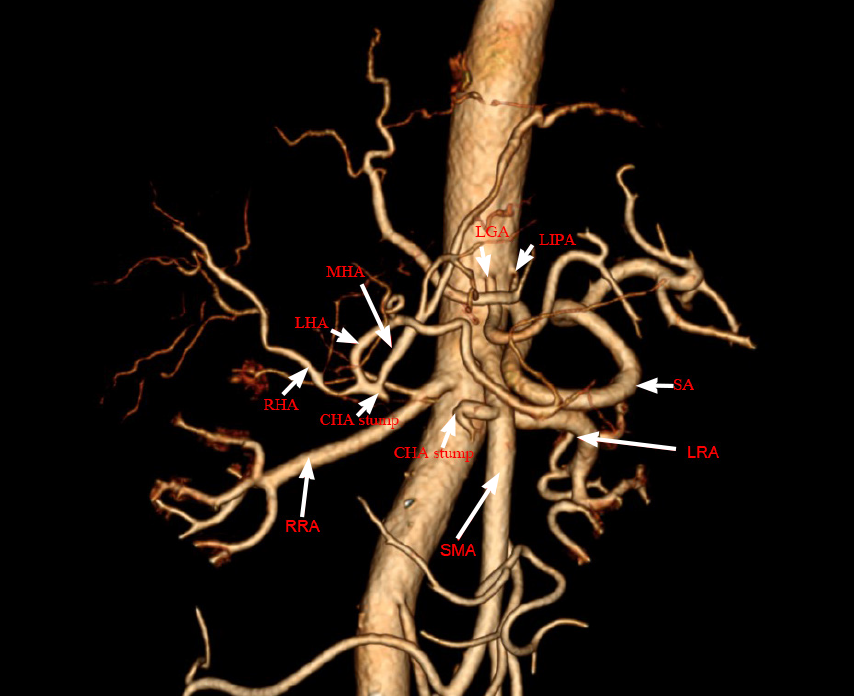
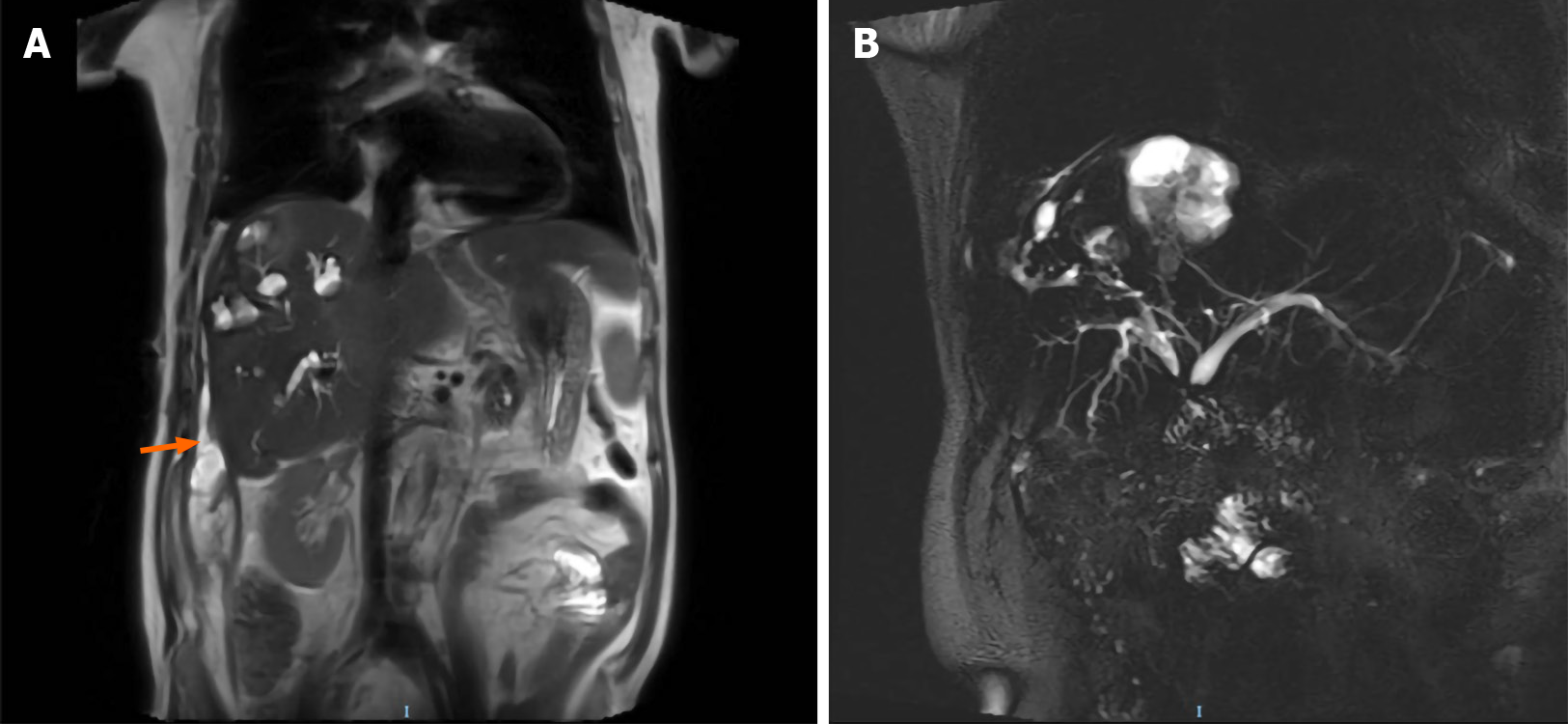
Magnetic resonance imaging (MRI) of the abdomen revealed lesions with long T1 and long T2 signals and multiple liver abscesses were located in the right liver and perihepatic space (Figure 1). An anomalous origin of the celiac axis and a dilated left inferior phrenic artery and CHA were also revealed (Figure 2). MRI in coronal section showed that the abscesses were interlinked with the intrahepatic bile duct and that the bile and pus flowed into the perihepatic space (Figure 3A). MR cholangiogram picture illustrated anatomical changes of bile duct after PD (Figure 3B).
The final diagnosis was right multiple intrahepatic abscesses that manifested as recurrent pyogenic cholangitis and celiac abscess, due to the CHA transection injury in atypical anatomy of celiac axis after PD.
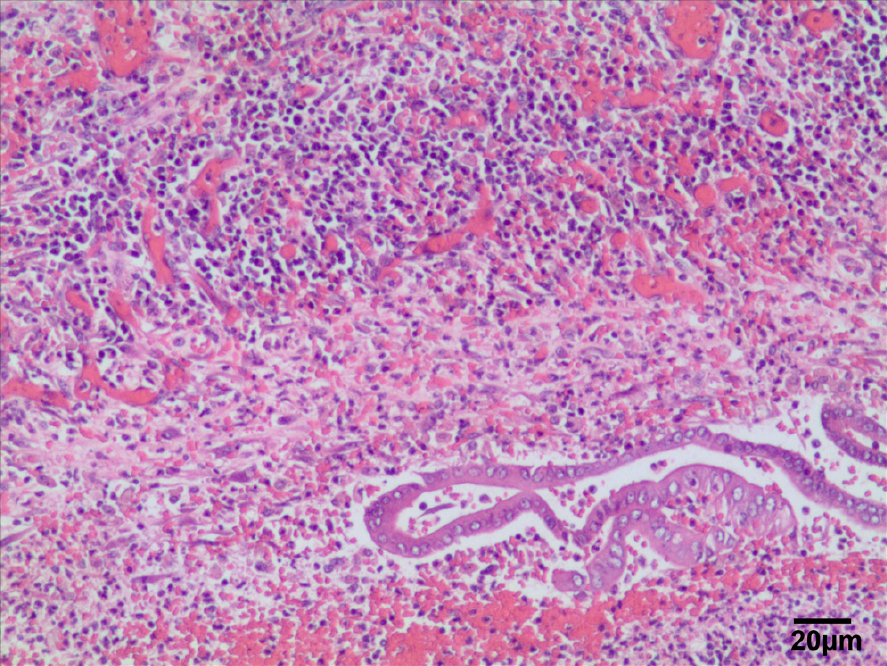
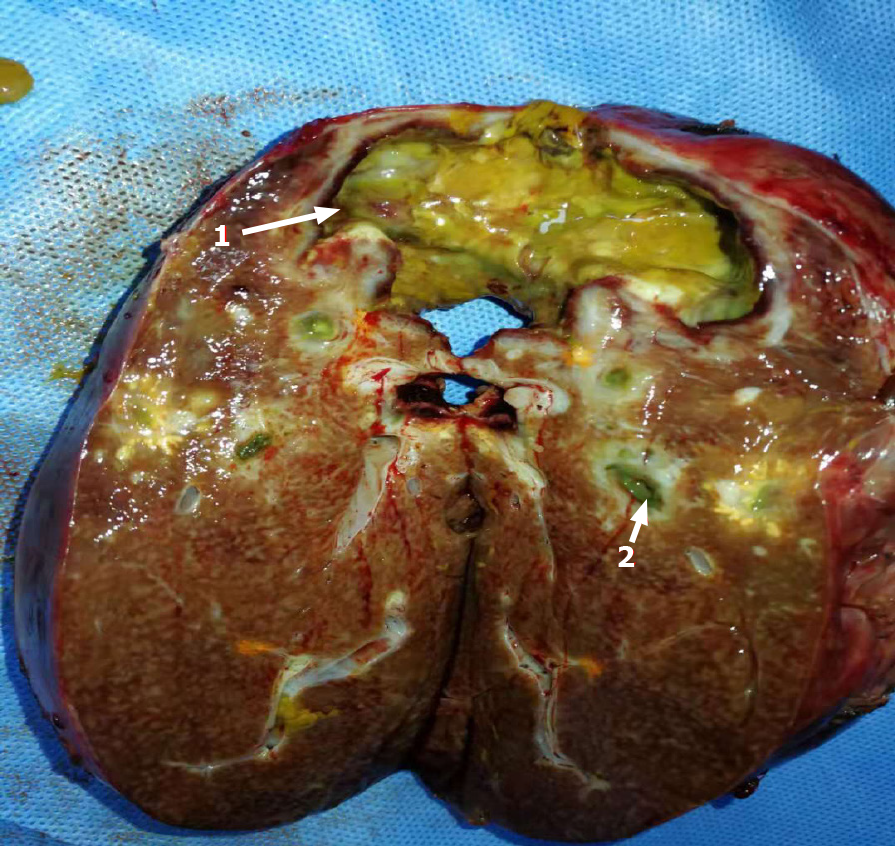
After admission to the hospital, the patient gradually recovered with antibiotic treatment and then underwent right hepatectomy. Dense adhesions were noted in the portal area during the operation; adhesiolysis was difficult, and the abscess was cleared from the perihepatic space. A histopathological section of the liver and a postoperative image of the resected right lobe of the liver are shown in Figures 4 and 5.
The patient recovered quickly after surgery and was discharged on postoperative day 9. At the 12-mo follow-up visit, the patient appeared healthy and had developed no additional episodes of fever.
Funamizu et al[4] reported for the first time that the CHA, left gastric artery and splenic artery mainly originated from the trifurcation of celiac trunk, accounting for more than 70% of the population. Additionally, the average incidence of celiac absence is reported to be only 0.4%[5]. Anatomical variation of the CHA originating as a branch of the superior mesenteric artery (SMA) is rare but not insignificant, occurring at an incidence of 1.5% to 4.0%[6].
In this case, there is no trifurcation of the celiac trunk, CHA and SMA originate from a common trunk called the “hepatomesenteric trunk”. However, the left gastric artery and splenic artery originate directly from the anterior wall of the abdominal aorta. If the anatomical variation is not recognized during PD, and the CHA injury or interruption may easily occur, leading to chronic biliary ischemia in the related hepatic territory[7]. Such ischemia may affect merely the supplied region or, in the most severe cases (usually involving the CHA or proper hepatic artery), resulting in acute necrosis of the entire liver[8].
No reports have described hepatic infarction affecting the entire liver. Such cases are attributable to the recruitment of other collateral pathways, including the inferior phrenic arteries, intercostal arteries, and gastric arteries, that were presumably not ligated during the initial surgery. In the present case, we detected compensatory dilatation of the left inferior phrenic artery and common hepatic artery. Furthermore, we discovered superinfection of the liver's infarcted sections, which was most likely induced by biliary tree contamination through the hepaticojejunostomy site. In one study, 12 of 13 patients (92%) with infected hepatic infarctions responded to percutaneous drainage and survived to hospital discharge[9]. In our case, however, the patient underwent percutaneous drainage but continued to have recurrent episodes, and his condition was eventually cured by right hepatectomy. To our knowledge, unclassified hepatic artery variants with celiac trunk deletions have been rarely reported to date. Indeed, the anatomical variation of the CHA originating as a branch of the SMA is rare but not insignificant, occurring at an incidence of 1.5% to 4.0%[9].
Hepatic abscess is the most common type of liver disease, with a mortality rate of 5% to 30%[10]. However, hepatic abscess is a rare complication of PD, to our knowledge, there are only 46 patients involved in hepatic abscess in isolated reports after PD[11-14]. Virgilio et al[15] reported that postoperative biliary fistula and reoperation were the most common risk factors for hepatic abscess after PD. Portal vein and biliary tract are the two main pathways for microbes to enter the liver and cause hepatic abscess. Reflux of intestinal contents following cholangiojejunostomy can cause cholangitis and raise the risk of liver abscess[16]. In addition, the number of bacteria in the circulating blood or the factors of the patient's immunity are also potential risk factors for hepatic abscess after PD. Moreover, patients with diabetes will increase the prevalence of liver abscess due to impaired immunity and intestinal reflux as indicated previously[17]. Intrusive examinations before PD, such as endoscopic ultrasound or ERCP, increase the risk of bile contamination which may increase the risk of hepatic abscess after PD[18-20]. Hepatic arterial flow is paramount in preserving biliary integrity[21]. Biliary stricture and vascular injury may cause liver atrophy or abscess[22]. In our case, we found the common hepatic artery was damaged after PD which might induce local ischemia necrosis of liver tissue, resulting in recurrent pyogenic hepatic abscess. Although PTCD was performed to drain pus and bile for improving patient's symptoms, prolonged drainage might expose the probability of bile contact with external bacteria, leading to delayed healing of hepatic abscess.
In this case, preoperative examination of the patient’s anatomical variations with computed tomography would have played a pivotal role in avoiding arterial injuries. Therefore, careful CT analysis should be considered mandatory not only to define the operability (with radical intent) of PD candidates but also to identify atypical arterial patterns and plan the optimal surgical strategy.
We want to acknowledge Mrs. Yang for preparing and editing CT or MR images.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Cerwenka H, Kumar R, Shelat VG S-Editor: Liu M L-Editor: A P-Editor: Ma YJ
| 1. | Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 969] [Article Influence: 51.0] [Reference Citation Analysis (34)] |
| 2. | Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic pancreaticoduodenectomy vs open pancreaticoduodenectomy--a comparative study. Int J Surg. 2012;10:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Khachfe HH, Chahrour MA, Habib JR, Yu J, Jamali FR. A Quality Assessment of the Information Accessible to Patients on the Internet About the Whipple Procedure. World J Surg. 2021;45:1853-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Funamizu N, Omura K, Ozaki T, Honda M, Mishima K, Igarashi K, Takada Y, Wakabayashi G. Geriatric nutritional risk index serves as risk factor of surgical site infection after pancreatoduodenectomy: a validation cohort Ageo study. Gland Surg. 2020;9:1982-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Ugurel MS, Battal B, Bozlar U, Nural MS, Tasar M, Ors F, Saglam M, Karademir I. Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography. Br J Radiol. 2010;83:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Cirocchi R, D'Andrea V, Amato B, Renzi C, Henry BM, Tomaszewski KA, Gioia S, Lancia M, Artico M, Randolph J. Aberrant left hepatic arteries arising from left gastric arteries and their clinical importance. Surgeon. 2020;18:100-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 7. | Prakash, Rajini T, Mokhasi V, Geethanjali BS, Sivacharan PV, Shashirekha M. Coeliac trunk and its branches: anatomical variations and clinical implications. Singapore Med J. 2012;53:329-331. [PubMed] |
| 8. | Cho SK, Kim SS, Do YS, Park KB, Shin SW, Park HS, Choo SW, Choo IW. Ischemic liver injuries after hepatic artery embolization in patients with delayed postoperative hemorrhage following hepatobiliary pancreatic surgery. Acta Radiol. 2011;52:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Stewart BG, Gervais DA, O'Neill MJ, Boland GW, Hahn PF, Mueller PR. Imaging and percutaneous treatment of secondarily infected hepatic infarctions. AJR Am J Roentgenol. 2008;190:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Chen SC, Huang CC, Tsai SJ, Yen CH, Lin DB, Wang PH, Chen CC, Lee MC. Severity of disease as main predictor for mortality in patients with pyogenic liver abscess. Am J Surg. 2009;198:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Chen W, Ma T, Bai X, Zhang X, Shen Y, Lao M, Li G, Liang T. Pyogenic Liver Abscess After Pancreaticoduodenectomy: A Single-Center Experience. J Surg Res. 2019;239:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Njoku VC, Howard TJ, Shen C, Zyromski NJ, Schmidt CM, Pitt HA, Nakeeb A, Lillemoe KD. Pyogenic liver abscess following pancreaticoduodenectomy: risk factors, treatment, and long-term outcome. J Gastrointest Surg. 2014;18:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Yamaguchi K, Tanaka M, Chijiiwa K, Nagakawa T, Imamura M, Takada T. Early and late complications of pylorus-preserving pancreatoduodenectomy in Japan 1998. J Hepatobiliary Pancreat Surg. 1999;6:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Sanada Y, Yamada N, Taguchi M, Morishima K, Kasahara N, Kaneda Y, Miki A, Ishiguro Y, Kurogochi A, Endo K, Koizumi M, Sasanuma H, Fujiwara T, Sakuma Y, Shimizu A, Hyodo M, Sata N, Yasuda Y. Recurrent cholangitis by biliary stasis due to non-obstructive afferent loop syndrome after pylorus-preserving pancreatoduodenectomy: report of a case. Int Surg. 2014;99:426-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Virgilio E, Mercantini P, Ferri M, Cavallini M. Pyogenic liver abscess: A very late and rare complication after pancreaticoduodenectomy. Microbiol Immunol. 2016;60:568-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Limongelli P, Pai M, Bansi D, Thiallinagram A, Tait P, Jackson J, Habib NA, Williamson RC, Jiao LR. Correlation between preoperative biliary drainage, bile duct contamination, and postoperative outcomes for pancreatic surgery. Surgery. 2007;142:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Lin YT, Wang FD, Wu PF, Fung CP. Klebsiella pneumoniae liver abscess in diabetic patients: association of glycemic control with the clinical characteristics. BMC Infect Dis. 2013;13:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Jagannath P, Dhir V, Shrikhande S, Shah RC, Mullerpatan P, Mohandas KM. Effect of preoperative biliary stenting on immediate outcome after pancreaticoduodenectomy. Br J Surg. 2005;92:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Krutsri C, Kida M, Yamauchi H, Iwai T, Imaizumi H, Koizumi W. Current status of endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy. World J Gastroenterol. 2019;25:3313-3333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (3)] |
| 20. | Katanuma A, Hayashi T, Kin T, Toyonaga H, Honta S, Chikugo K, Ueki H, Ishii T, Takahashi K. Interventional endoscopic ultrasonography in patients with surgically altered anatomy: Techniques and literature review. Dig Endosc. 2020;32:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Castaing D. Surgical anatomy of the biliary tract. HPB (Oxford). 2008;10:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Perini MV, Herman P, Montagnini AL, Jukemura J, Coelho FF, Kruger JA, Bacchella T, Cecconello I. Liver resection for the treatment of post-cholecystectomy biliary stricture with vascular injury. World J Gastroenterol. 2015;21:2102-2107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |