Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9182
Peer-review started: April 14, 2021
First decision: June 25, 2021
Revised: June 29, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: October 26, 2021
Processing time: 189 Days and 21.4 Hours
Colorectal mucinous adenocarcinoma is a rare subtype of colorectal cancer and is characterized by an abundance of mucin in the tumor. In addition, the colorectal mucinous adenocarcinoma often demonstrates poor differentiation in the histology of tumor cells and poor prognosis compared with those with adenocarcinoma. Here, we present the case of a young woman with colonic mucinous adenocarcinoma showing significantly rapid progression within four months of immunosuppressant therapy for Henoch–Schönlein purpura.
Here we report a rare case of ascending colon mucinous adenocarcinoma with lymph node and liver metastases which developed and progressed rapidly within four months during the treatment of Henoch–Schönlein purpura using corticosteroids. The systemic screening examinations showed no tumors before the immunosuppressant therapy. Fortunately, the patient was successfully treated with chemotherapy.
While no direct evidence that the immunosuppressants accelerated the tumor development, the case presen
Core Tip: Here, we report a rare case of ascending colon mucinous adenocarcinoma with lymph node and liver metastases that developed within four months of immunosuppressant therapy. The information obtained from this case and from a review of the relevant literature highlights the importance of surveillance for malig
- Citation: Koseki Y, Kamimura K, Tanaka Y, Ohkoshi-Yamada M, Zhou Q, Matsumoto Y, Mizusawa T, Sato H, Sakamaki A, Umezu H, Yokoyama J, Terai S. Rapid progression of colonic mucinous adenocarcinoma with immunosuppressive condition: A case report and review of literature. World J Clin Cases 2021; 9(30): 9182-9191
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9182.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9182
Colorectal mucinous adenocarcinoma is a subtype of colorectal cancer that accounts for approximately 10% of colorectal cancers[1-6] and is characterized by an abundance of mucin in the tumor. It is often diagnosed in young women and in the right-sided colon at an advanced stage[1,4-12]. In addition, patients with colorectal mucinous adenocarcinoma often demonstrate rapid progression and poor differentiation in the histology of tumor cells compared with those with adenocarcinoma[4,13-15]. Here we present the case of a young woman with ascending colon mucinous adenocarcinoma diagnosed at stage IV, which developed and progressed rapidly within four months during the treatment of Henoch–Schönlein purpura using corticosteroids. The patient was successfully treated with chemotherapy. Although there was no direct evidence that the immunosuppressants accelerated the tumor development, the case presentation and review of the literature demonstrated that surveillance for malignancies before and during treatment with immunosuppressive agents is essential.
A 37-year-old woman was referred to our department in August 2019 due to an increase in the levels of hepatobiliary enzymes.
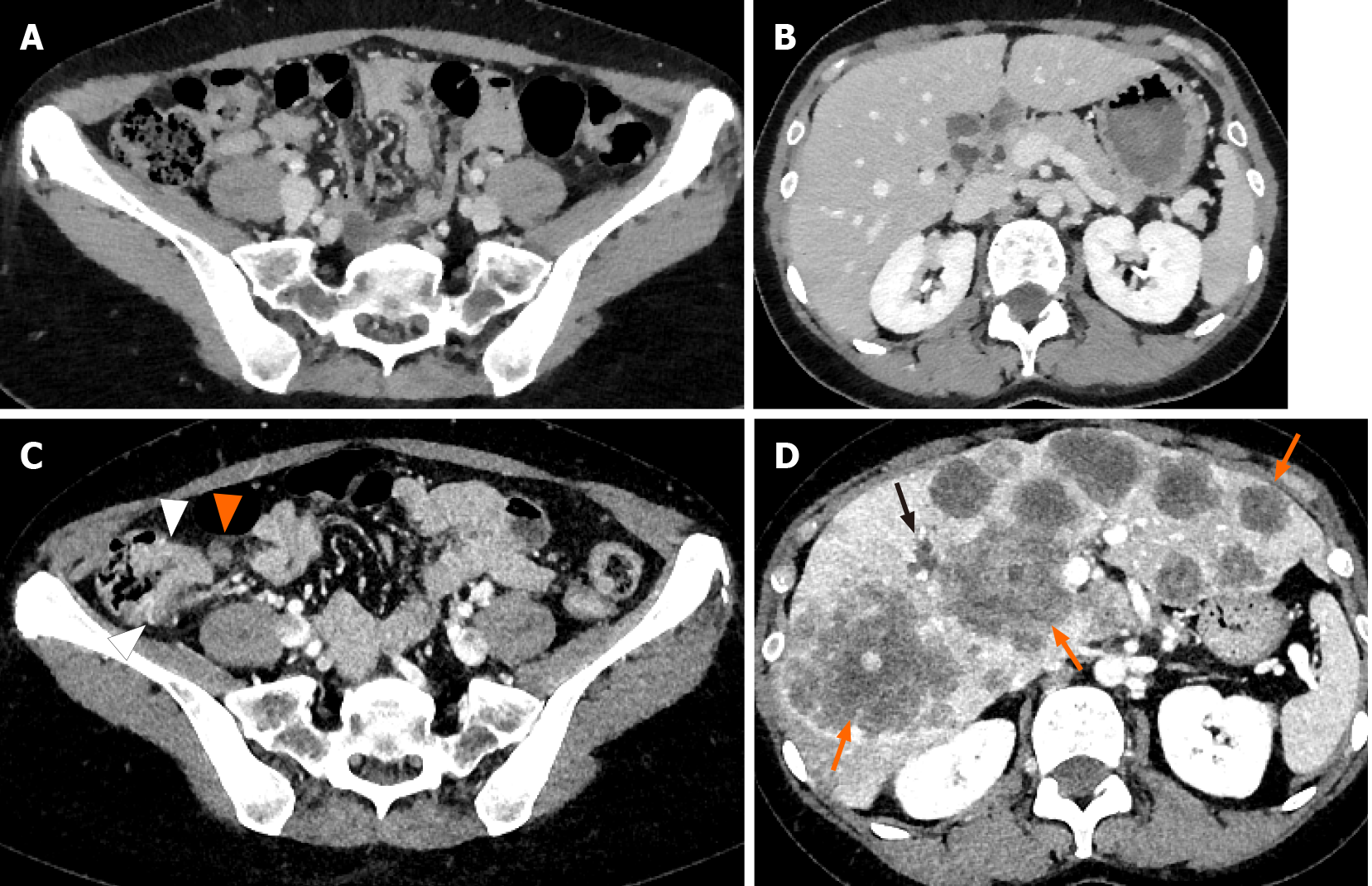
After four months of prednisolone (PSL) administration, the neurological symptoms improved; however, the levels of hepatobiliary enzymes, which were normal before the pulse therapy (Table 1), had increased (Table 2). Contrast-enhanced computed tomography (CT) performed in March 2019 as a screening examination to detect potential infectious lesions or malignancies before the pulse therapy showed no gross lesions in the gastrointestinal tract, lungs, liver, gall bladder, pancreas, spleen, kidneys, and adrenal glands and no swelling in the lymph nodes (Figure 1A and B).
| Hematology | Biochemistry | Marker | ||||||
| Values | Normal range | Values | Normal range | Values | Normal range | |||
| WBC | 6600 | 3300-8600/µL | TP | 7.4 | 6.6-8.1 g/dL | HBs Ag | - | |
| Neutro. | 64.3 | 38.0%-71.0% | Alb | 4.2 | 4.1-5.1 g/dL | Anti-HBs | - | |
| Lymph. | 29.7 | 21.0%-50.0% | BUN | 9 | 8-20 mg/dL | Anti-HBc | - | |
| Eos. | 0.8 | 7.3% | Cre | 0.62 | 0.46-0.79 mg/dL | Anti-HCV | - | |
| Bas. | 0.2 | 2.0% | AST | 18 | 13-30 IU/L | |||
| Mon. | 5.0 | 3.0%-8.0% | ALT | 18 | 7-23 IU/L | CEA | 1.1 | < 5.8 ng/mL |
| RBC | 493 | 386-492 ×104/μL | ALP | 179 | 106-322 IU/L | CA19-9 | 14 | < 37 IU/mL |
| Hb | 14.0 | 11.6-14.8 g/dL | LDH | 145 | 124-222 IU/L | CA125 | 8 | < 35 IU/mL |
| Ht. | 41.5 | 35.1%-44.4% | γ-GTP | 20 | 9-32 IU/L | |||
| Plt. | 26.7 | 15.8-34.8 ×104/μL | ChE | 314 | 201-421 IU/L | |||
| Na | 138 | 138-145 mEq/L | ||||||
| Coagulation | K | 3.2 | 3.6-4.8 mEq/L | |||||
| Values | Normal range | Cl | 104 | 101-108 mEq/L | ||||
| PT | 111 | 70%-130% | P | 2.5 | 2.7-4.6 mg/dL | |||
| PT-INR | 0.94 | 1.0 | Ca | 9.2 | 8.8-10.1 mg/dL | |||
| APTT | 27.4 | 26.9-40.9 sec | CRP | 0.01 | < 0.14 mg/dL | |||
| TG | 60 | 30-117 mg/dL | ||||||
| HDL-C | 63 | 48-103 mg/dL | ||||||
| LDL-C | 88 | 65-163 mg/dL | ||||||
| Hematology | Biochemistry | Marker | ||||||
| Values | Normal range | Values | Normal range | Values | Normal range | |||
| WBC | 12850 | 3300-8600/µL | TP | 6.3 | 6.6-8.1 g/dL | CEA | 97.0 | < 5.8 ng/mL |
| Neutro. | 93.7 | 38.0%-71.0% | Alb | 3.6 | 4.1-5.1 g/dL | CA19-9 | 255 | < 37 IU/mL |
| Lymph. | 4.7 | 21.0%-50.0% | BUN | 10 | 8-20 mg/dL | AFP | 1 | < 9.5 ng/mL |
| Eos. | 0.0 | 7.3% | Cre | 0.76 | 0.46-0.79 mg/dL | AFP-L3 | < 0.5 | < 10 % |
| Bas. | 0.1 | 2.0% | T-Bil | 0.8 | 0.4-1.5 mg/dL | PIVKA-II | 22.0 | < 37.8 ng/mL |
| Mon. | 1.5 | 3.0%-8.0% | AST | 107 | 13-30 IU/L | IL-2R | 1031 | 122-496 U/mL |
| RBC | 395 | 386-492 ×104/μL | ALT | 165 | 7-23 IU/L | |||
| Hb | 11.0 | 11.6-14.8 g/dL | ALP | 1156 | 106-322 IU/L | |||
| Ht. | 35.2 | 35.1%-44.4% | LDH | 1309 | 124-222 IU/L | |||
| Plt. | 36.6 | 15.8-34.8 ×104/μL | γ-GTP | 489 | 9-32 IU/L | |||
| Na | 136 | 138-145 mEq/L | ||||||
| K | 4.0 | 3.6-4.8 mEq/L | ||||||
| Cl | 99 | 101-108 mEq/L | ||||||
| Ca | 9.1 | 8.8-10.1 mg/dL | ||||||
| CRP | 1.96 | < 0.14 mg/dL | ||||||
She had no familial history of cancer but had a history of Henoch–Schönlein purpura (HSP) diagnosed in 2017 via a renal biopsy and was treated with corticosteroids starting at 30 mg/d oral PSL, which was tapered down to 1 mg till February 2019. In March 2019, she presented with neurological symptoms of headache, dizziness, and focal numbness in the right upper and lower extremities, with no evidence of infarction or bleeding in clinical and imaging tests. She was diagnosed with recurrence of the purpura with neurological symptoms and was treated with methylprednisolone pulse therapy, followed by continuation of oral PSL administration.
She had no personal and family history of the malignancies.
Other than the palpable abdominal masses in the epigastric lesion with mild tenderness and a symmetric pitting edema in her lower legs, no abnormal findings in her vital signs and other physical examination were noted.
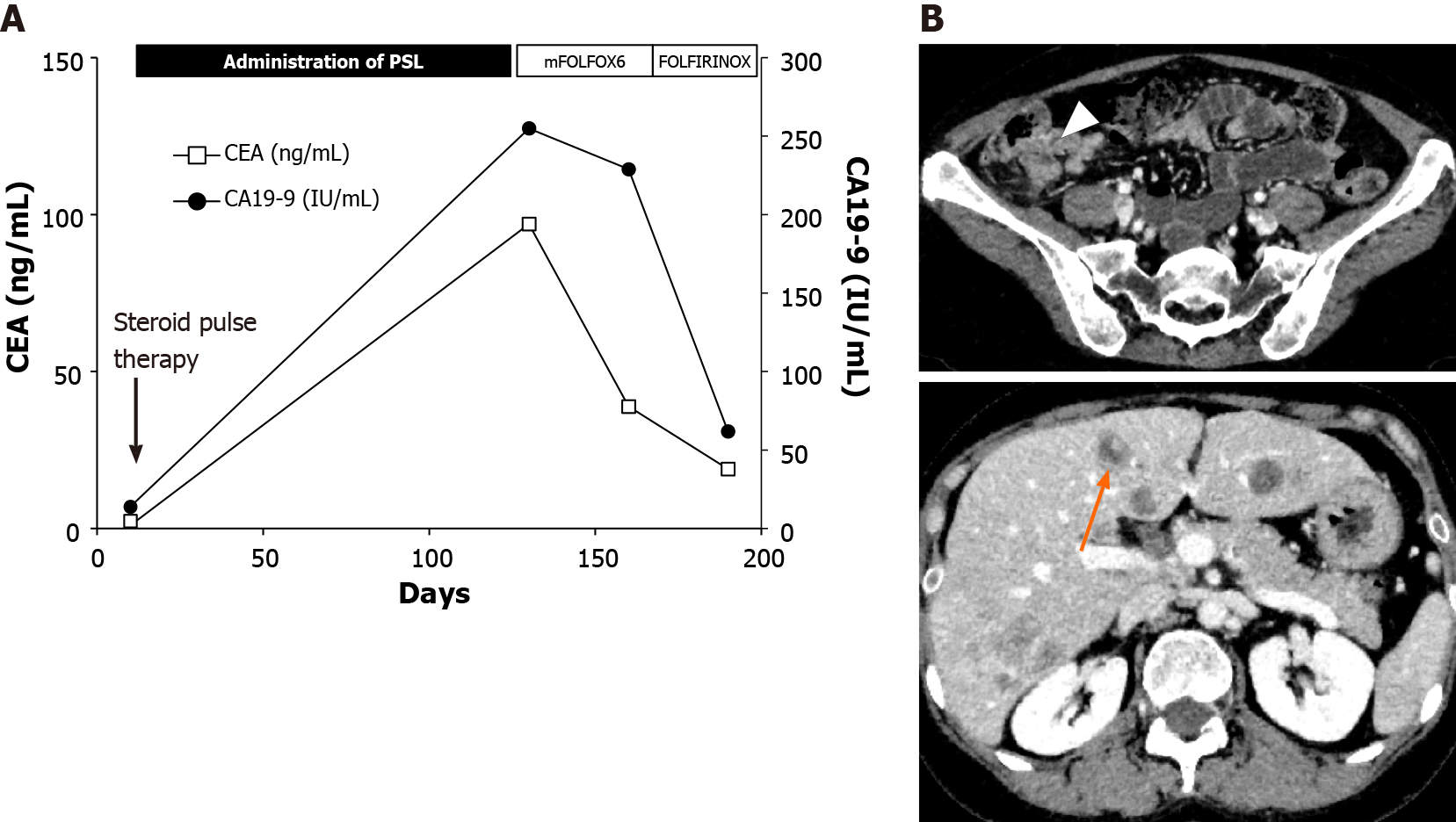
The results of a laboratory test performed on the day of admission revealed elevated white blood cell counts and levels of aspartate aminotransferase, alanine transaminase, alkaline phosphatase, and γ-glutamyl transpeptidase (Table 2). During the four-month period after re-dosing of PSL in March 2019, the levels of the tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 significantly increased from 1.1 ng/mL to 97.0 ng/mL and 14 IU/mL to 255 IU/mL, respectively. To investigate the cause of the elevation in liver enzyme levels, further examinations were conducted.
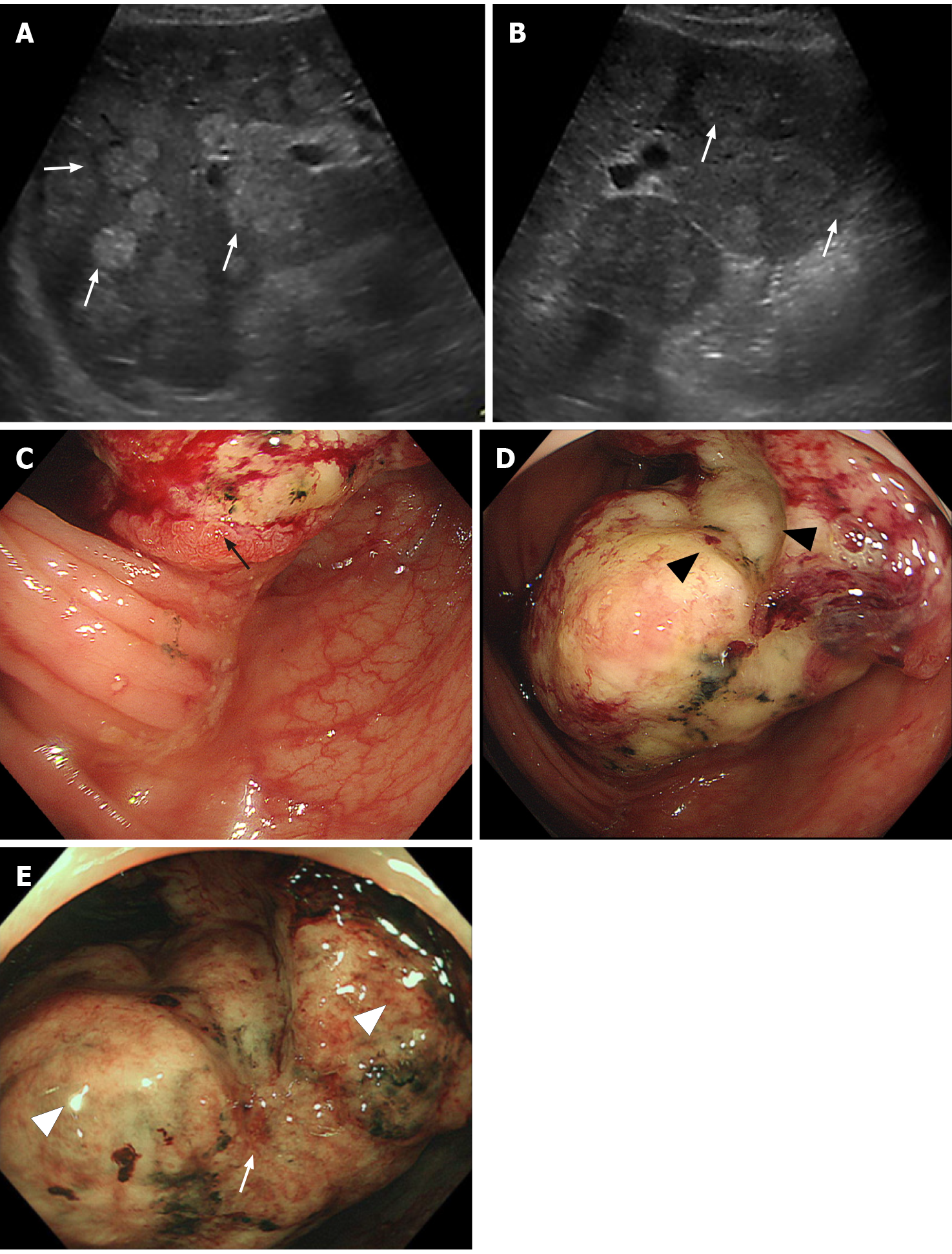
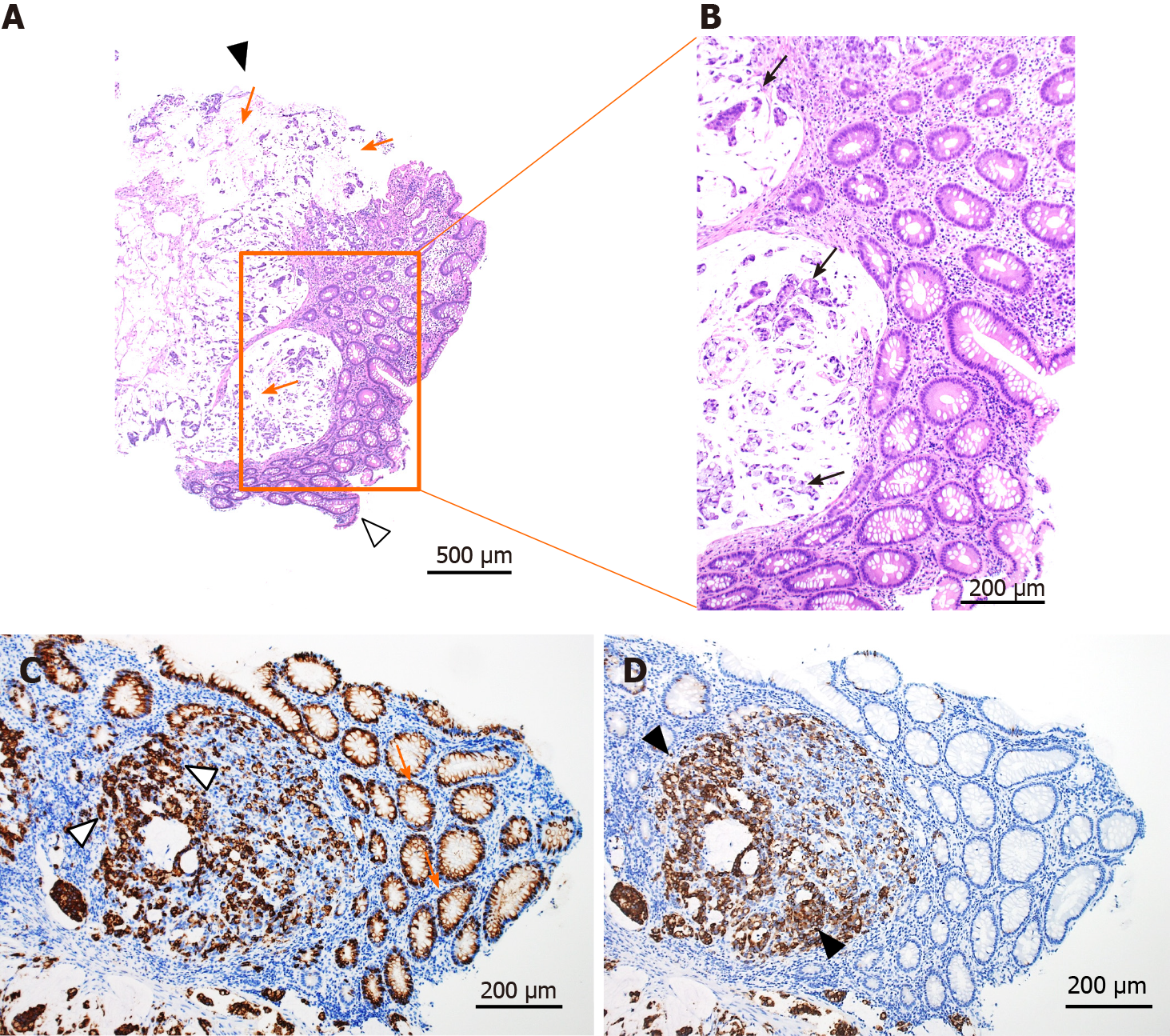
Contrast-enhanced CT revealed a suspicious tumor in the ascending colon with an irregularly thickened intestinal wall and swelling in multiple lymph nodes (six regional lymph nodes) surrounding the ascending colon tumor. In addition, multiple low-density liver tumors showing poor enhancement effect with expanding growth pattern were observed, and the intrahepatic bile duct exhibited mild dilatation due to the tumors (Figure 1C and D). No other suspicious lesions for the primary were seen. Abdominal ultrasonography revealed multiple tumors in the bilateral liver lobes up to 80 mm in size with heterogeneously high echoic patterns (Figure 2A and B). Colonoscopy revealed a large, solid, multinodular epithelial tumor in the ascending colon. The tumor was on the epithelial layer and covered with whitish mucus and debris on its surface with an abnormal vascular structure on its surface and was easily bleeding (Figure 2C). In addition, the tumor showed a semicircular depressive lesion at its center (Figure 2D and E). The histological analyses of the tissue collected from the tumor (black arrow shown in Figure 2C) revealed an abundant amount of extracellular mucin within the tumor on the mucosal epithelia. The tumor cells in the mucin appeared to be adenocarcinoma cells showing a high level of cellular atypia and tended to resemble cells of the glandular tissue (Figure 3 A and B). The tumor cells positively stained for Mucin 2, oligomeric mucus/gel-forming (Figure 3C) and Mucin 5AC (Figure 3D). Based on these CT, endoscopic, and histological analyses, the case was diagnosed with the ascending colon mucinous adenocarcinoma.
Based on this information, the tumor was diagnosed as mucinous adenocarcinoma. Overall, the patient was diagnosed with ascending mucinous colorectal adenocarcinoma with lymph node and liver metastases, and based on the Tumor, Node, Metastasis staging system from the American Joint Committee on Cancer (8th edition), the clinical stage was determined as cT4aN2aM1a.
Chemotherapy was started with the combination of fluorouracil, oxaliplatin, and levofolinate (mFOLFOX6; four courses), followed by the combination of irinotecan and mFOLFOX6 (FOLFIRINOX; two courses), after normalization of the levels of hepatobiliary enzymes, which could be due to the shrinkage of the metastatic liver tumors (Figure 4A).
CT was performed on day 180, after chemotherapy treatment resulted in the shrinkage of the primary colon tumor, lymph node, and liver metastasis (Figure 4B), and chemotherapy was continued. In addition, our case is under the investigation of microsatellite instability and endoscopic follow up will be conducted to screen the tumor progression.
Colorectal mucinous adenocarcinoma is a subtype of colorectal cancer characterized by an abundance of mucus, which accounts for at least 50% of the tumor volume[1]. Statistically, mucinous histological subtypes account for 10%–20% of colorectal cancers[2,3], whereas the rate is lower in Asian countries (4%–5%)[1,4-6]. Colorectal mucinous adenocarcinoma occurs more generally in young women and is more frequently located in the right colon and diagnosed at an advanced stage[1,4-6]. Moreover, colorectal mucinous adenocarcinoma often demonstrates rapid progression and lower curable resection rates compared with colorectal adenocarcinoma[4,5,7-13]. Furthermore, patients with colorectal mucinous adenocarcinoma often show poorer differentiation in the histology of the tumor cells and higher CEA levels than those with adenocarcinoma[4,13-15]. The case presented here was of a young woman with ascending colon mucinous adenocarcinoma diagnosed at stage IV, which developed and progressed rapidly, with an increase in the CEA level within 4 months, which is consistent with the characteristics reported previously[1,4-6,16]. The histological analyses of the tumor cells showed positively stained for Mucin 2 and Mucin 5AC which were reported to be significantly related to the colorectal mucinous adenocarcinoma[17,18]. The overall survival of patients with mucinous adenocarcinoma of the colon tends to be poorer than that of patients with non-mucinous carcinoma of the colon. The prognosis of patients with colorectal mucinous adenocarcinoma was similar to that of patients with non-mucinous carcinoma at stages I and II, whereas the prognosis was significantly poorer at stages III and IV[4]. One of the factors contributing to this poor prognosis is poor response to oxaliplatin, irinotecan, and fluorouracil-based first-line combination chemotherapy. While, fortunately, our patient showed a favorable response to the mFOLFOX6 and FOLFILINOX regimen, as previously reported, colorectal mucinous adenocarcinoma has a higher rate of microsatellite instability than non-mucinous colorectal adenocarcinoma[1,13,19], and the administration of immune checkpoint inhibitors might be useful for these types of cells, our case is under the investigation of microsatellite instability.
In this case, it is noteworthy that rapid progression was observed during the period of significant increase of immunosuppressant medication due to the recurrence of the HSP. Although colonoscopy was not performed before the administration of PSL and the initial missing possibility can’t be excluded, CT revealed no tumor in the colon or in other organs. During these four months, tumor development was seen in the colon along with severe metastatic lesions in the liver and lymph nodes. Long-term use of immunosuppressants has been associated with an increased incidence of various cancers[20-27] and glucocorticoid therapy has been reported to transduce the signal for tumor progression[26]. Among cancers, colorectal cancer is rare, occurring in 0.003%-1.7% of cases treated with immunosuppressant therapy during the study period (Table 3)[20-24,26-31]. Table 3 summarizes the cases of patients who developed colorectal cancer during the period of treatment with immunosuppressants. They received a combination of either an antimetabolite (mycophenolate mofetil or azathioprine) or a calcineurin inhibitor (tacrolimus or cyclosporine) in addition to PSL. The median time from transplantation to diagnosis of colon cancer is 5.3-8.7 years, and tumors were most commonly found in the proximal side colon, including the ascending and transverse colons (Table 3), which was also the primary site in our case. As our case showed rapid progression within four months, it is possible that atypia of the cells with poor differentiation along with immunosuppression affected the growth of tumor cells. The higher risk of the colorectal neoplasia[31] and advanced colonic adenomatous polyps[32] were further reported in the solid organ transplantation recipients under the immunosuppression, it is clear that the earlier surveillance has been recommended for these cases.
| No. | Ref. | Immunosuppressant | No. of cases | CRC (occurrence rate, %) | Yr to diagnosis (median, range) | Lesion | SIR | 95%CI | |||
| Proximal colon (%) | Distal colon (%) | Rectum (%) | UD (%) | ||||||||
| 1 | Safaeian et al[20] | AZA, CsA, MMF, TAC | 224098 | 790 (0.3) | N/A | 408 (51.6) | 195 (24.7) | 146 (18.5) | 41 (5.2) | 1.12 | 1.04-1.20 |
| 2 | Huo et al[21] | N/A | 2105122 | 53 (0.003) | N/A | N/A | N/A | N/A | N/A | 1.82 | 1.59-2.09 |
| 3 | Engels et al[22] | N/A | 175732 | 627 (0.4) | N/A | N/A | N/A | N/A | N/A | 1.24 | 1.15-1.34 |
| 4 | Buell et al[23] | AZA, CsA, MMF, TAC | 13000 | 141 (1.1) | N/A | N/A | N/A | N/A | N/A | 1.94 | 1.64-2.29 |
| 5 | Aberg et al[24] | CsA, TAC, antibody | 540 | 2 (0.4) | N/A | N/A | N/A | N/A | N/A | 1.59 | 0.19-5.74 |
| 6 | Merchea et al[26] | AZA, CsA, MMF, TAC, steroid | 3946 | 20 (0.5) | 8.7 (0.4-19) | 14 (70) | 4 (20) | 2 (10) | 0 (0) | N/A | N/A |
| 7 | Rompianesi et al[27] | N/A | 8178 | 34 (0.4) | 5.6 (3.8-8.8) | 17 (50) | 9 (26.5) | 8 (23.5) | 0 (0) | 0.92 | 0.69-1.20 |
| 8 | Aigner et al[28] | AZA, CsA, MMF, TAC, steroid | 3595 | 9 (0.3) | 5.3 (1.5-10) | 4 | 1 | 4 | 0 (0) | N/A | N/A |
| 9 | Rademacher et al[29] | AZA, CsA, MMF, TAC, steroid | 1616 | 22 (1.3) | 8.2 (0.3-19.9) | N/A | N/A | N/A | N/A | 1.9 | 1.2-2.9 |
| 10 | Haagsma et al[30] | N/A | 174 | 3 (1.7) | 7.9 (5.9-16.7) | N/A | N/A | N/A | N/A | N/A | N/A |
| 11 | Park et al[31] | CsA, TAC, Sirolimus, AZA, MMF | 360 | 4 (1.1) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
In summary, we report a rare case of ascending mucinous colorectal adenocarcinoma with lymphatic and liver metastases that developed within four months of immuno
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Song J, Zhang X S-Editor: Wang LL L-Editor: A P-Editor: Ma YJ
| 1. | Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 2. | Glasgow SC, Yu J, Carvalho LP, Shannon WD, Fleshman JW, McLeod HL. Unfavourable expression of pharmacologic markers in mucinous colorectal cancer. Br J Cancer. 2005;92:259-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Leopoldo S, Lorena B, Cinzia A, Gabriella DC, Angela Luciana B, Renato C, Antonio M, Carlo S, Cristina P, Stefano C, Maurizio T, Luigi R, Cesare B. Two subtypes of mucinous adenocarcinoma of the colorectum: clinicopathological and genetic features. Ann Surg Oncol. 2008;15:1429-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Numata M, Shiozawa M, Watanabe T, Tamagawa H, Yamamoto N, Morinaga S, Watanabe K, Godai T, Oshima T, Fujii S, Kunisaki C, Rino Y, Masuda M, Akaike M. The clinicopathological features of colorectal mucinous adenocarcinoma and a therapeutic strategy for the disease. World J Surg Oncol. 2012;10:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Kanemitsu Y, Kato T, Hirai T, Yasui K, Morimoto T, Shimizu Y, Kodera Y, Yamamura Y. Survival after curative resection for mucinous adenocarcinoma of the colorectum. Dis Colon Rectum. 2003;46:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 6. | Du W, Mah JT, Lee J, Sankila R, Sankaranarayanan R, Chia KS. Incidence and survival of mucinous adenocarcinoma of the colorectum: a population-based study from an Asian country. Dis Colon Rectum. 2004;47:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Bagante F, Spolverato G, Beal E, Merath K, Chen Q, Akgül O, Anders RA, Pawlik TM. Impact of histological subtype on the prognosis of patients undergoing surgery for colon cancer. J Surg Oncol. 2018;117:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Xie L, Villeneuve PJ, Shaw A. Survival of patients diagnosed with either colorectal mucinous or non-mucinous adenocarcinoma: a population-based study in Canada. Int J Oncol. 2009;34:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Nitsche U, Zimmermann A, Späth C, Müller T, Maak M, Schuster T, Slotta-Huspenina J, Käser SA, Michalski CW, Janssen KP, Friess H, Rosenberg R, Bader FG. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg. 2013;258:775-782; discussion 782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 10. | Catalano V, Loupakis F, Graziano F, Bisonni R, Torresi U, Vincenzi B, Mari D, Giordani P, Alessandroni P, Salvatore L, Fornaro L, Santini D, Baldelli AM, Rossi D, Giustini L, Silva RR, Falcone A, D'Emidio S, Rocchi M, Luzi Fedeli S. Prognosis of mucinous histology for patients with radically resected stage II and III colon cancer. Ann Oncol. 2012;23:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Okuno M, Ikehara T, Nagayama M, Kato Y, Yui S, Umeyama K. Mucinous colorectal carcinoma: clinical pathology and prognosis. Am Surg. 1988;54:681-685. [PubMed] |
| 12. | Nozoe T, Anai H, Nasu S, Sugimachi K. Clinicopathological characteristics of mucinous carcinoma of the colon and rectum. J Surg Oncol. 2000;75:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Park JS, Huh JW, Park YA, Cho YB, Yun SH, Kim HC, Lee WY, Chun HK. Prognostic comparison between mucinous and nonmucinous adenocarcinoma in colorectal cancer. Medicine (Baltimore). 2015;94:e658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Li ZP, Liu XY, Kao XM, Chen YT, Han SQ, Huang MX, Liu C, Tang XY, Chen YY, Xiang D, Huang YD, Lei ZJ, Chu XY. Clinicopathological characteristics and prognosis of colorectal mucinous adenocarcinoma and nonmucinous adenocarcinoma: a surveillance, epidemiology, and end results (SEER) population-based study. Ann Transl Med. 2020;8:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Chew MH, Yeo SA, Ng ZP, Lim KH, Koh PK, Ng KH, Eu KW. Critical analysis of mucin and signet ring cell as prognostic factors in an Asian population of 2,764 sporadic colorectal cancers. Int J Colorectal Dis. 2010;25:1221-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Karasawa Y, Karasawa G, Kamiya K, Miyokawa N, Ootsuka S, Hoshi K. A case of early-stage (pM) mucinous carcinoma of the large intestine. Gastroenterological Endoscopy. 2009;51:362-367. |
| 17. | Pigny P, Guyonnet-Duperat V, Hill AS, Pratt WS, Galiegue-Zouitina S, d'Hooge MC, Laine A, Van-Seuningen I, Degand P, Gum JR, Kim YS, Swallow DM, Aubert JP, Porchet N. Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics. 1996;38:340-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 170] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Imai Y, Yamagishi H, Fukuda K, Ono Y, Inoue T, Ueda Y. Differential mucin phenotypes and their significance in a variation of colorectal carcinoma. World J Gastroenterol. 2013;19:3957-3968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Papaconstantinou HT, Sklow B, Hanaway MJ, Gross TG, Beebe TM, Trofe J, Alloway RR, Woodle ES, Buell JF. Characteristics and survival patterns of solid organ transplant patients developing de novo colon and rectal cancer. Dis Colon Rectum. 2004;47:1898-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Safaeian M, Robbins HA, Berndt SI, Lynch CF, Fraumeni JF Jr, Engels EA. Risk of Colorectal Cancer After Solid Organ Transplantation in the United States. Am J Transplant. 2016;16:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Huo Z, Li C, Xu X, Ge F, Wang R, Wen Y, Peng H, Wu X, Liang H, Peng G, Li R, Huang D, Chen Y, Zhong R, Cheng B, Xiong S, Lin W, He J, Liang W. Cancer Risks in Solid Organ Transplant Recipients: Results from a Comprehensive Analysis of 72 Cohort Studies. Oncoimmunology. 2020;9:1848068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1093] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 23. | Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80:S254-S264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 412] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 24. | Aberg F, Pukkala E, Höckerstedt K, Sankila R, Isoniemi H. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008;14:1428-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Volden PA, Conzen SD. The influence of glucocorticoid signaling on tumor progression. Brain Behav Immun. 2013;30 Suppl:S26-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Merchea A, Abdelsattar ZM, Taner T, Dean PG, Colibaseanu DT, Larson DW, Dozois EJ. Outcomes of colorectal cancer arising in solid organ transplant recipients. J Gastrointest Surg. 2014;18:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Rompianesi G, Ravikumar R, Jose S, Allison M, Athale A, Creamer F, Gunson B, Manas D, Monaco A, Mirza D, Owen N, Roberts K, Sen G, Srinivasan P, Wigmore S, Fusai G, Fernando B, Burroughs A, Tsochatzis E. Incidence and outcome of colorectal cancer in liver transplant recipients: A national, multicentre analysis on 8115 patients. Liver Int. 2019;39:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Aigner F, Boeckle E, Albright J, Kilo J, Boesmueller C, Conrad F, Wiesmayr S, Antretter H, Margreiter R, Mark W, Bonatti H. Malignancies of the colorectum and anus in solid organ recipients. Transpl Int. 2007;20:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Rademacher S, Seehofer D, Eurich D, Schoening W, Neuhaus R, Oellinger R, Denecke T, Pascher A, Schott E, Sinn M, Neuhaus P, Pratschke J. The 28-year incidence of de novo malignancies after liver transplantation: A single-center analysis of risk factors and mortality in 1616 patients. Liver Transpl. 2017;23:1404-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Haagsma EB, Hagens VE, Schaapveld M, van den Berg AP, de Vries EG, Klompmaker IJ, Slooff MJ, Jansen PL. Increased cancer risk after liver transplantation: a population-based study. J Hepatol. 2001;34:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 246] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Park HY, Chang BJ, Lim SW, Kim J, Kim JY, Chang DK, Son HJ, Rhee PL, Kim JJ, Rhee JC, Kim YH. Risk of colorectal neoplasia in patients with solid organ transplantation. Clin Transplant. 2012;26:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Ashkar MH, Chen J, Shy C, Crippin JS, Chen CH, Sayuk GS, Davidson NO. Increased Risk of Advanced Colonic Adenomas and Timing of Surveillance Colonoscopy Following Solid Organ Transplantation. Dig Dis Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |