Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9114
Peer-review started: March 14, 2021
First decision: July 14, 2021
Revised: July 26, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: October 26, 2021
Processing time: 220 Days and 14.9 Hours
Mucinous cystic neoplasm of the liver (MCN-L) and intraductal papillary neoplasm of the bile duct (IPN-B) are two different types of mucin-producing bile duct tumour that may complicate the course of pregnancy. To the best of our knowledge, we describe herein the first case of MCN-L with spontaneous rupture during pregnancy necessitating complex surgical treatment.
A 24-year-old woman was initially admitted to another hospital in October 2018 with signs of jaundice (serum bilirubin level 12 mg/dL) and upper abdominal pain radiating to the left shoulder. Initial magnetic resonance imaging (MRI) of the abdominal cavity revealed a multilocular cystic tumour of the liver hilum (37 mm × 40 mm in diameter) located between segments 3 and 4 of the left liver lobe. Six weeks later (December 2018), the patient was found to be 12 wk pregnant and was referred to our institution for further diagnostics and treatment. At admission, a soft, palpable, and tender mass in the left upper abdomen was found. It was determined via MRI (with no intravenous contrast in view of the first-trimester pregnancy) to be a large collection of fluid (19 cm × 17 cm × 10 cm) located close to the liver hilum and below the left liver lobe. The patient did not undergo any diagnostic or therapeutic procedures nor did they have any abdominal trauma in the preceding weeks. The fluid collection proved to be of biliary origin following percutaneous drainage. Therefore, we concluded this was a spontaneous rupture of an MCN-L with the formation of a biloma. The MRI study also revealed the previously found cystic tumour of the liver hilum communicating with the left hepatic duct, which, together with left hepatic duct dilatation, suggested the diagnosis of IPN-B. The follow-up MRI with intravenous gadolinium contrast performed in the second trimester of pregnancy (week 14) showed, in turn, some features of MCN-L, including enhancement of the internal septations within the cystic liver mass. A precise preoperative differential diagnosis between IPN-B and MCN-L was therefore not possible. The patient was submitted to surgery in the second trimester of pregnancy (week 18). Surgery included a cholecystectomy, left hepatectomy, and concomitant resection of the extrahepatic bile ducts followed by anastomosis of the right hepatic duct with the Roux limb of the jejunum. The post-operative period was uneventful and the patient was discharged 8 days after surgery. The histopathological examination of the resected specimen revealed a final diagnosis of MCN-L with low-grade dysplasia and epithelium surrounded by ovarian-type stromal tissue. The patient delivered a healthy baby girl and both remain well at present, after 2 years of follow-up since surgery.
The differential diagnosis and management of MCN-L and IPN-B may be very challenging, particularly in the setting of pregnancy. When indications for surgery are obvious, the final diagnosis is based on histopathological examination, with ovarian-type stroma being pathognomonic for MCN-L. We believe that the growth of this subepithelial stroma secondary to the high levels of sex hormones produced during pregnancy might have been the main causative factor leading to the tumour rupture with the formation of a biloma in our patient.
Core Tip: The differential diagnosis and management of biliary cystic neoplasms of the liver may be very challenging, especially in the setting of pregnancy. To the best of our knowledge, we describe herein the first case of a mucinous cystic neoplasm of the liver (MCN-L) with spontaneous rupture leading to the formation of a huge biloma in a pregnant woman. This was treated with percutaneous drainage of the biloma, followed by MCN-L removal via left hepatectomy with extrahepatic bile duct resection and anastomosis of the right hepatic duct with the Roux limb of the jejunum in the second trimester of pregnancy. We believe that the growth of this subepithelial stroma due to the high levels of sex hormones during pregnancy might have been the main causative factor that led to the tumour rupture and the formation of a biloma in our patient.
- Citation: Kośnik A, Stadnik A, Szczepankiewicz B, Patkowski W, Wójcicki M. Spontaneous rupture of a mucinous cystic neoplasm of the liver resulting in a huge biloma in a pregnant woman: A case report. World J Clin Cases 2021; 9(30): 9114-9121
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9114.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9114
Recent advances in pathology have enabled the classification of mucin-producing bile duct tumours of the liver into two different groups: Mucinous cystic neoplasm of the liver (MCN-L) and intraductal papillary neoplasm of the bile duct (IPN-B). They are distinguished based on the presence of subepithelial ovarian-type stroma (MCN-L) or bile ductal communication (IPN-B). MCN-L is an extremely rare, cyst-forming epithelial tumour that is typically observed in women (> 90%) and is usually characterised by having no communication with the biliary tree. Pathologically, the cyst is composed of mucin-producing cuboidal or columnar epithelium. On the other hand, IPN-B shows a slight male predominance, usually grows inside the bile duct, and secretes mucin in only about one-third of all cases. It shows communication with the bile duct and does not exhibit ovarian-type subepithelial stroma, which, as already mentioned, is pathognomonic for MCN-L. The possible influence of sex hormones on the transformation of the ovarian-like stroma of MCNs may be responsible for their rapid growth during pregnancy[1].
A 24-year-old woman who was 12 wk pregnant was referred to our institution in December 2018 with an initial diagnosis of a cystic mass of the liver hilum.
The patient was initially admitted to a regional hospital in October 2018 (i.e., 6 wk before referral to our centre) with signs of jaundice (serum bilirubin level 12 mg/dL) and upper abdominal pain radiating to the left shoulder. Within a week, the abdominal pain had gradually decreased, with serum bilirubin coming down to 3 mg/dL. When found to be pregnant, the patient was referred to our centre for further diagnostics and treatment.
The patient had no relevant previous medical history.
The patient had no relevant family medical history.
On admission to our unit, the patient was slightly jaundiced with a soft and tender mass palpable in the left upper abdomen.
Laboratory tests revealed bilirubin of 2.3 mg/dL (normal range 0.2–1.2 mg/dL); alkaline phosphatase of 194 U/L (normal 38–126 U/L); gamma-glutamyl transpeptidase of 117 (normal 7–50); and GOT, GPT, amylase, and lipase within the normal ranges. The serum markers demonstrated normal values of carbohydrate antigen Ca 19-9 and carcinoembryonic antigen CEA, and slightly elevated alfa-fetoprotein (AFP) of 16 ng/mL (normal < 7 ng/mL). Hydatid cystic disease was excluded following negative serologic tests.
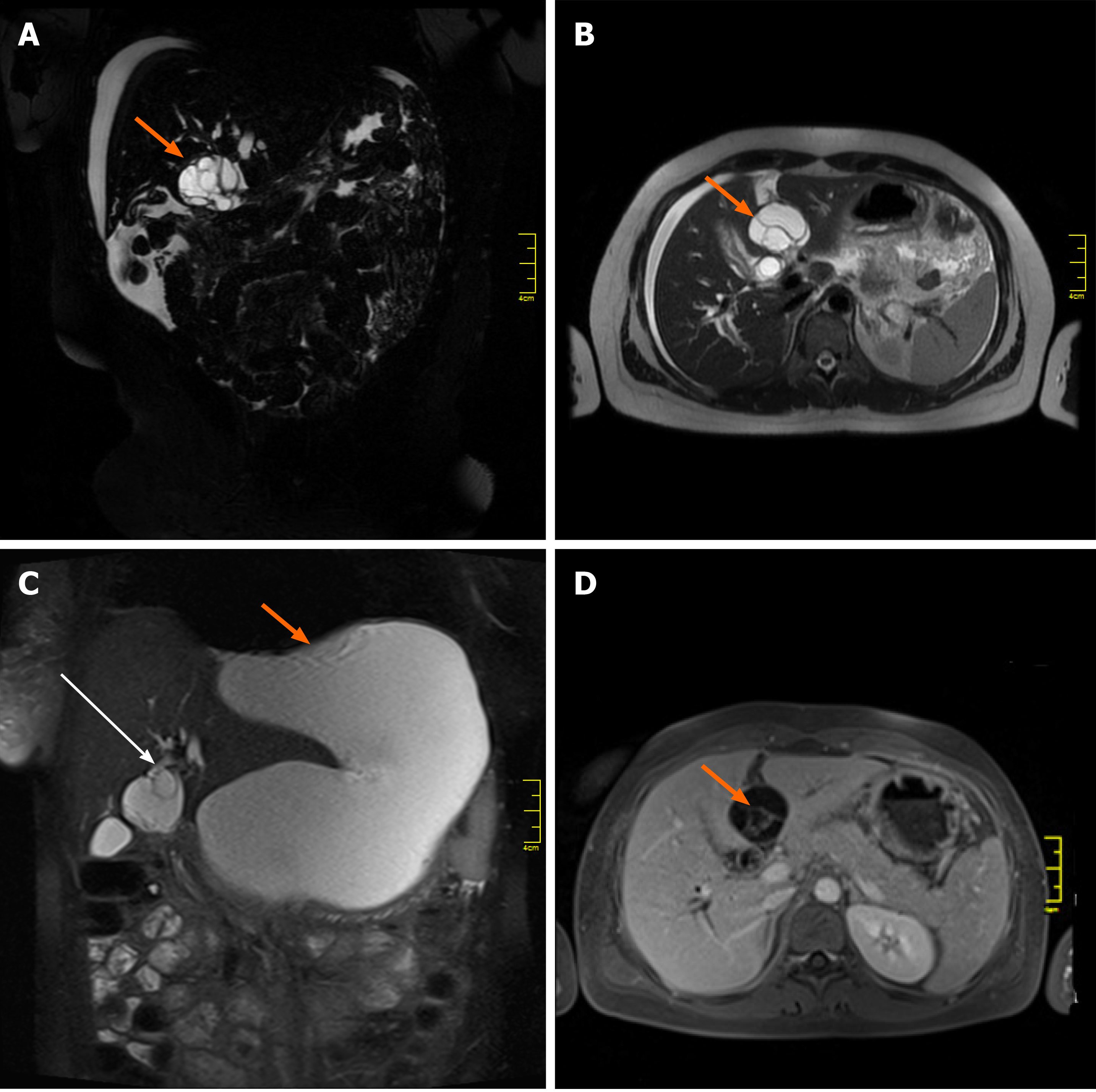
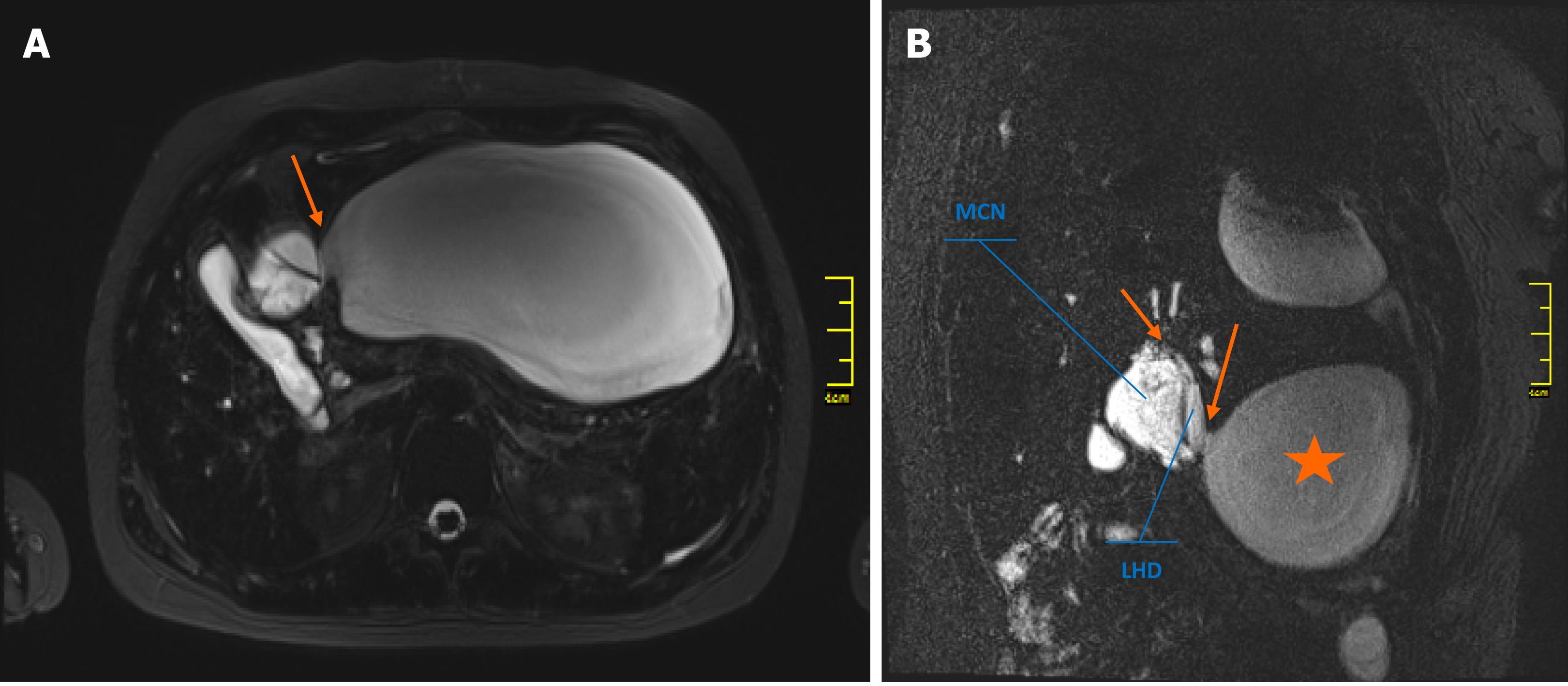
The initial magnetic resonance imaging (MRI) of the abdominal cavity, performed in another hospital in October 2018, revealed a multilocular cystic mass of the liver hilum located between segments 3 and 4 of the left liver lobe (Figure 1A, 1B) and measuring approximately 37 mm × 40 mm in diameter. Dilatation of the left hepatic duct and common hepatic duct (up to 12 and 15 mm in diameter, respectively) was also visible. Meanwhile, the right hepatic duct (9 mm in diameter) had no signs of intraluminal pathology. The common bile duct was also normal, measuring 5 mm in diameter. After admission to our unit, abdominal MRI showed the presence of a large fluid collection of 19 cm × 17 cm × 10 cm in size, located anteriorly and below the left liver lobe. The cystic mass appeared to have a direct communication with the left hepatic duct and the intrahepatic biliary ducts of segments 2 and 3 (Figure 1C). Because of the early stage of pregnancy (first trimester), the use of a hepatospecific contrast agent was contraindicated, so confirmation of the possible communication between the collection and the biliary tree could not be visualized based on MRI. However, the fluid collection was directly adjacent to the MCN-L, as shown in the axial T2-weighted images (Figure 2A). Moreover, the communication between the cystic mass and the fluid collection was visible on a coronal T2-weighted MRI study (Figure 2B). Percutaneous fluid drainage revealed mainly biliary content (fluid bilirubin of 18 mg/dL vs serum bilirubin of 2 mg/dL), which led to the diagnosis of a ruptured biliary cyst of the liver. The concentration of Ca 19-9 in the drained biliary fluid was 52652 IU/mL while it was normal (< 34) in the serum. External biliary leakage of 1000–1500 mL daily continued for nine consecutive days before it finally stopped. A follow-up MRI study with intravenous gadolinium contrast was performed in the second trimester of pregnancy (week 14) and demonstrated enhancement of the internal septations within the cystic liver mass (Figure 1D). While the above features appeared typical for MCN-L, the multilocular cystic appearance with marked bile duct dilatation and communication with the biliary tree were more suggestive of IPN-B. Therefore, a precise preoperative differential diagnosis between those two entities was not possible. Each of these two diagnoses was considered an indication for immediate surgical treatment despite the patient’s pregnancy, mainly due to the symptomatic nature of the disease and the risk of malignancy.
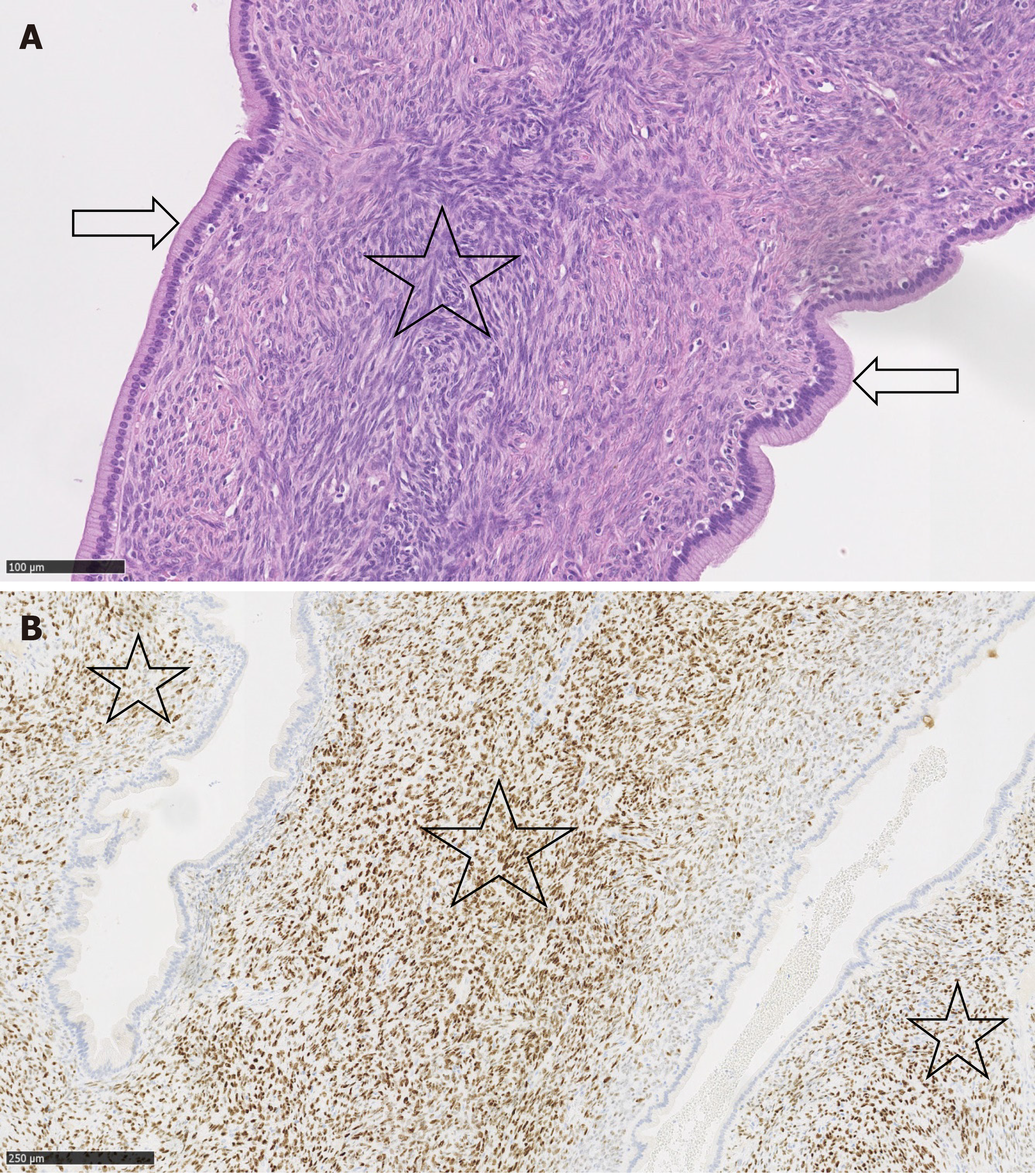
The final diagnosis was made following surgery, based on a histopathological examination of the resected tumour. This examination revealed an MCN-L with low-grade dysplasia with epithelium surrounded by ovarian-type stromal tissue (Figure 3A). Immunohistochemical studies showed positive staining for progesterone receptors in the stroma cell nuclei (Figure 3B).
Surgery was performed on 4 February 2019, when the patient was in her 18th week of pregnancy. Intraoperatively, no signs of residual biliary fluid collection were present following the percutaneous drainage 5 wk prior. After opening the confluence of the hepatic ducts, the cystic tumour was found originating from the left hepatic duct and entering into the lumen of the common hepatic duct. The tumour was resected with a cholecystectomy, left hepatectomy, and concomitant resection of the extrahepatic bile ducts, followed by anastomosis of the right hepatic duct with the Roux-en-Y loop of the jejunum.
The postoperative course was uneventful, and the patient was discharged 8 d after surgery. The patient delivered a healthy baby girl following 43 wk of pregnancy. Over 2 years of follow-up, there has been no MCN-L recurrence. The patient remains well at present, with normal liver function test results and a healthy 18-month-old daughter.
Differential diagnosis between MCN-L and IPN-B is challenging, especially in the setting of pregnancy with the associated limited diagnostic possibilities. MCN-L is diagnosed almost entirely (93%–100%) in women and is most frequently found within the left liver lobe. IPN-B, in contrast, occurs slightly more often in males within the intra- and extrahepatic biliary tree at similar frequency, with the left lobe being involved more often in intrahepatic cases[1-3]. IPN-B tends to grow in communication with the bile duct, which is not typical for MCN-L. However, MCN-L has also been found to grow expansively into the bile duct, causing obstructive jaundice in some cases. This is particularly true for tumours originating within the left liver lobe (segment 4), in which prolapse into the left hepatic duct and expansive growth may be a characteristic behaviour of such tumours[4]. This was exactly the case in our patient, in whom tumour spread into both the left hepatic duct and the common hepatic duct was confirmed during surgery. Takano et al[4], in their interesting review, described 17 cases of MCN-L, 12 of which were located in the left liver lobe. The left hepatic duct within segment 4 of the liver was the primary tumour site in 10 of those individuals. Most of them initially presented with signs of obstructive jaundice, which, together with upper abdominal pain, was also the primary presentation of the disease in our patient.
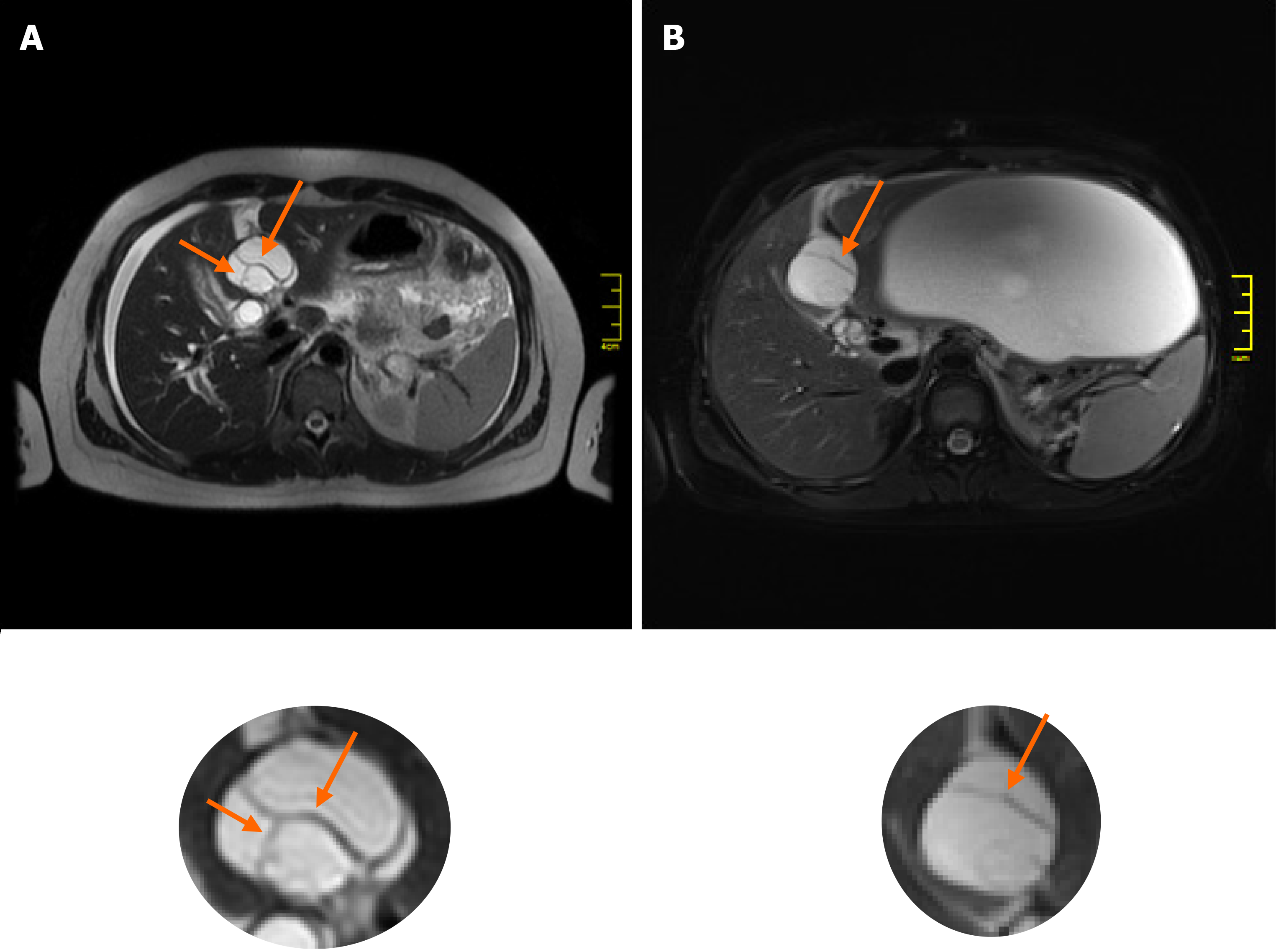
The imaging features of MCN-L include a multiloculated cystic mass with enhancing septations (Figure 1D); solid components rarely observed. In typical cases, there is no communication with the biliary tree and no downstream ductal dilatation. Conversely, the presence of bile duct dilatation and communication with an enhanced mural nodule is more typical of IPN-B[1-3]. Magnetic resonance cholangiopancreatography depicts the relationship of the lesion to the bile duct quite well and thereby aids in diagnosis. In our patient, a multilocular cystic appearance with communication with the biliary tree, as well as marked bile duct dilatation (Figure 1A–C), was highly suggestive of IPN-B. However, the enhanced internal septations were more indicative of MCN-L (Figure 1D). Comparing the baseline with post-rupture MRI examinations, the shape and architecture of the internal septations were clearly changed, as shown in Figure 4. The final diagnosis was made based on the presence of ovarian-type stroma upon histopathological evaluation of the resected specimen (Figure 3A). Besides typical mucin-secreting biliary-type epithelium, the presence of dense subepithelial ovarian-type stroma with spindle cells expressing female sex hormone receptors is a characteristic feature of MCN-L[3,4]. The presence of such stroma is also a defining feature of MCN of the pancreas[5], which has been observed to grow more rapidly during pregnancy, sometimes achieving extraordinary size[5,6]. This female hormonal influence might also explain the growth of the MCN-L and its eventual rupture at the beginning of pregnancy (Weeks 8–10) in our patient.
There are conflicting reports regarding the application of cyst fluid carbohydrate Ca 19-9 Levels for the differentiation of MCN-L from simple cysts[7,8]. The concentration of the Ca 19-9 marker in the drained cyst fluid from our patient was very high (> 52000 IU/mL). Serum and cyst fluid Ca 19-9 Levels have been reported to be elevated in MCN-L[7,8]. This can probably be explained by the biliary origin of these tumours.
Surgery remains the first-line treatment for both MCN-L and IPN-B[1]. Although rare, MCN-L has the potential to become malignant, and its malignant counterpart, i.e., MCN-L with associated invasive carcinoma, cannot be fully excluded by imaging alone[3]. Therefore, early resection of such lesions without preoperative biopsy, even in asymptomatic or minimally symptomatic patients, should be considered. Preoperative biopsy is also known to carry a significant risk of false negative results in such cases. In the setting of pregnancy, the second trimester is believed to be a relatively safe time for surgery and anaesthesia[5]. A complete resection of MCN-L offers universal cure (100% 5-year survival rate) as compared to 82%–84% 5-year survival following radical resection of IPN-B[1,9]. Our case is an example of a fully successful surgical treatment with no detrimental effects for either the patient or her baby. Abdominal MRI with intravenous contrast was performed both at week 6 (when the patient was not yet aware of being pregnant) and at week 14 (second trimester) of pregnancy. This had no detrimental effect on the development of the foetus. Both the patient and her daughter remain well and in good condition after 24 mo of follow-up since surgery.
To the best of our knowledge, we describe herein the first case of a symptomatic MCN-L diagnosed in a pregnant woman. Its spontaneous rupture early in the pregnancy resulted in the need for emergency percutaneous drainage of an extremely large biloma as the first step in treatment and major hepatobiliary surgery later on to remove the tumour. We believe that the perforation of the MCN-L in our patient might have been due to the growth of the subepithelial ovarian-type stroma with its final rupture due to the high levels of sex hormones produced during pregnancy. Preoperative radiologic differential diagnosis between MCN and IPN-B may be difficult. Such diagnosis can often only be made based on histopathological evaluation of a resected specimen.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vernuccio F, Wu SZ S-Editor: Ma YJ L-Editor: A P-Editor: Xing YX
| 1. | Kubota K, Nakanuma Y, Kondo F, Hachiya H, Miyazaki M, Nagino M, Yamamoto M, Isayama H, Tabata M, Kinoshita H, Kamisawa T, Inui K. Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: a multi-institutional study by the Japan Biliary Association. J Hepatobiliary Pancreat Sci. 2014;21:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Zen Y, Pedica F, Patcha VR, Capelli P, Zamboni G, Casaril A, Quaglia A, Nakanuma Y, Heaton N, Portmann B. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol. 2011;24:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Zen Y, Jang KT, Ahn S, Kim DH, Choi DW, Choi SH, Heo JS, Yeh MM. Intraductal papillary neoplasms and mucinous cystic neoplasms of the hepatobiliary system: demographic differences between Asian and Western populations, and comparison with pancreatic counterparts. Histopathology. 2014;65:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Takano Y, Nagahama M, Yamamura E, Maruoka N, Mizukami H, Tanaka J, Ohike N, Takahashi H. Prolapse into the bile duct and expansive growth is characteristic behavior of mucinous cystic neoplasm of the liver: report of two cases and review of the literature. Clin J Gastroenterol. 2015;8:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Herring AA, Graubard MB, Gan SI, Schwaitzberg SD. Mucinous cystadenocarcinoma of the pancreas during pregnancy. Pancreas. 2007;34:470-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Revoredo F, de Vinatea J, Reaño G, Villanueva L, Kometter F, Arenas J, Polanco PM. Mucinous cystic neoplasms of the pancreas associated with pregnancy: Two case reports. Medicine (Baltimore). 2020;99:e21471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Choi HK, Lee JK, Lee KH, Lee KT, Rhee JC, Kim KH, Jang KT, Kim SH, Park Y. Differential diagnosis for intrahepatic biliary cystadenoma and hepatic simple cyst: significance of cystic fluid analysis and radiologic findings. J Clin Gastroenterol. 2010;44:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Koffron A, Rao S, Ferrario M, Abecassis M. Intrahepatic biliary cystadenoma: role of cyst fluid analysis and surgical management in the laparoscopic era. Surgery. 2004;136:926-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 9. | Li T, Ji Y, Zhi XT, Wang L, Yang XR, Shi GM, Zhang W, Tang ZY. A comparison of hepatic mucinous cystic neoplasms with biliary intraductal papillary neoplasms. Clin Gastroenterol Hepatol. 2009;7:586-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |