Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9108
Peer-review started: January 27, 2021
First decision: August 18, 2021
Revised: August 18, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 26, 2021
Processing time: 266 Days and 21.3 Hours
As immune checkpoint inhibitors (ICIs) have become widely used in lung cancer treatment, immune-related adverse events (irAEs) warrant sufficient attention. Checkpoint inhibitor-related pneumonitis (CIP) is one of the most concerning adverse events as it is uncommon but life threatening.
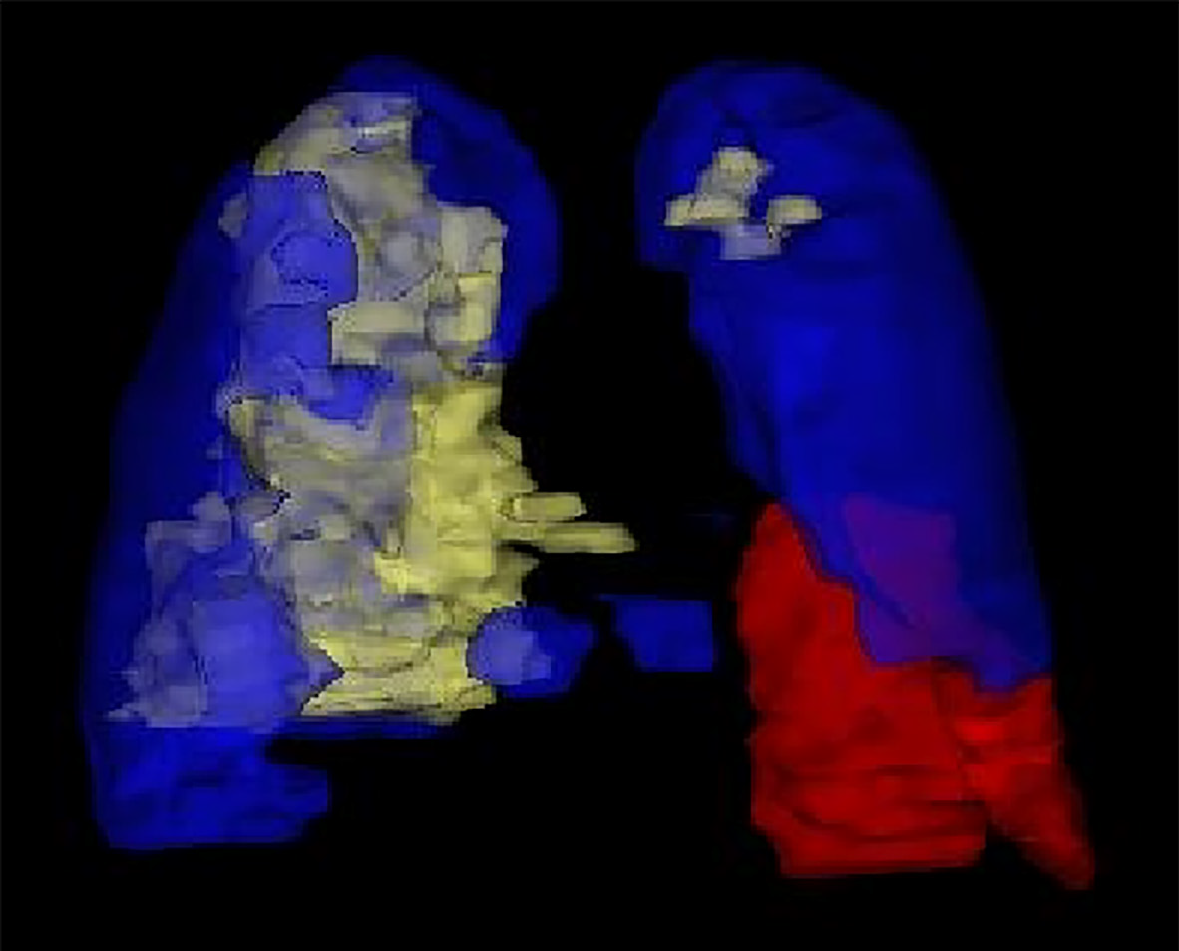
The patient whose case is reported here experienced three episodes of CIP in a span of 4 mon. Interestingly, the three episodes of CIP involved different regions of the lung separately. Taking these pneumonitis areas together makes nearly a whole lung area.
This case showed that recurrent CIPs may occur repeatedly until the whole lung is involved, suggesting that the follow-up period of CIP should be long enough, and the rechallenge of ICI should be done with due caution.
Core Tip: Checkpoint inhibitor-related pneumonitis (CIP) is one of the most concerning adverse events as it is uncommon but life threatening. This is the first case report on the unique dynamic changes in the radiologic features of CIP. This case showed that recurrent CIPs may occur repeatedly until the whole lung is involved, indicating that the follow-up period of CIP should be long enough, and the rechallenge of immune checkpoint inhibitor should be done with due caution.
- Citation: Tan PX, Huang W, Liu PP, Pan Y, Cui YH. Dynamic changes in the radiologic manifestation of a recurrent checkpoint inhibitor related pneumonitis in a non-small cell lung cancer patient: A case report. World J Clin Cases 2021; 9(30): 9108-9113
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9108.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9108
In recent years, immune checkpoint inhibitors (ICIs) have become a rising star in cancer therapy. It has revolutionized the treatment of lung cancer, including non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), from the early stages to the advanced stages of the disease[1,2,3]. Currently, clinical trials are still ongoing to investigate the potential clinical benefit of ICI in lung cancer therapy.
As ICIs become widely used in clinical practice, immune-related adverse events (irAEs) should be given sufficient attention. Checkpoint inhibitor-related pneumonitis (CIP) is one of the most concerning adverse events as it is uncommon but life threatening. The incidence rate of CIP in NSCLC is 4.1% in any grade and 0.8% in grade 3 or higher[4], which is slightly higher than in other cancers. Prior thoracic radiotherapy, pulmonary comorbidities, smoking status, and treatment with PD-1 inhibitors may be risk factors of CIP[5]. The timing of onset of CIP can vary from 9 d to 24 mon after the first dose of immunotherapy[6]. The typical symptoms are non-productive cough and unresolving dyspnea, while fever and chest pain are rare[6]. The diagnosis of CIP mainly depends on the combination of clinical symptoms and radiological manifestations. The radiographic patterns present in CIP are diverse, including cryptogenic organizing pneumonia (COP), non-specific interstitial pneumonia, hypersensitivity pneumonitis (HP), and acute interstitial pneumonia[7]. High-dose corticosteroids are recommended for the treatment of CIP greater than grade 2 (CTCAE5.0). Recurrent CIP may occur after steroid treatment with or without continuing ICI. The clinical features and underlying mechanism of CIP are scantily reported. Here, we present a recurrent CIP in a post-operative NSCLC patient with interesting dynamic changes in the radiologic findings in the lungs.
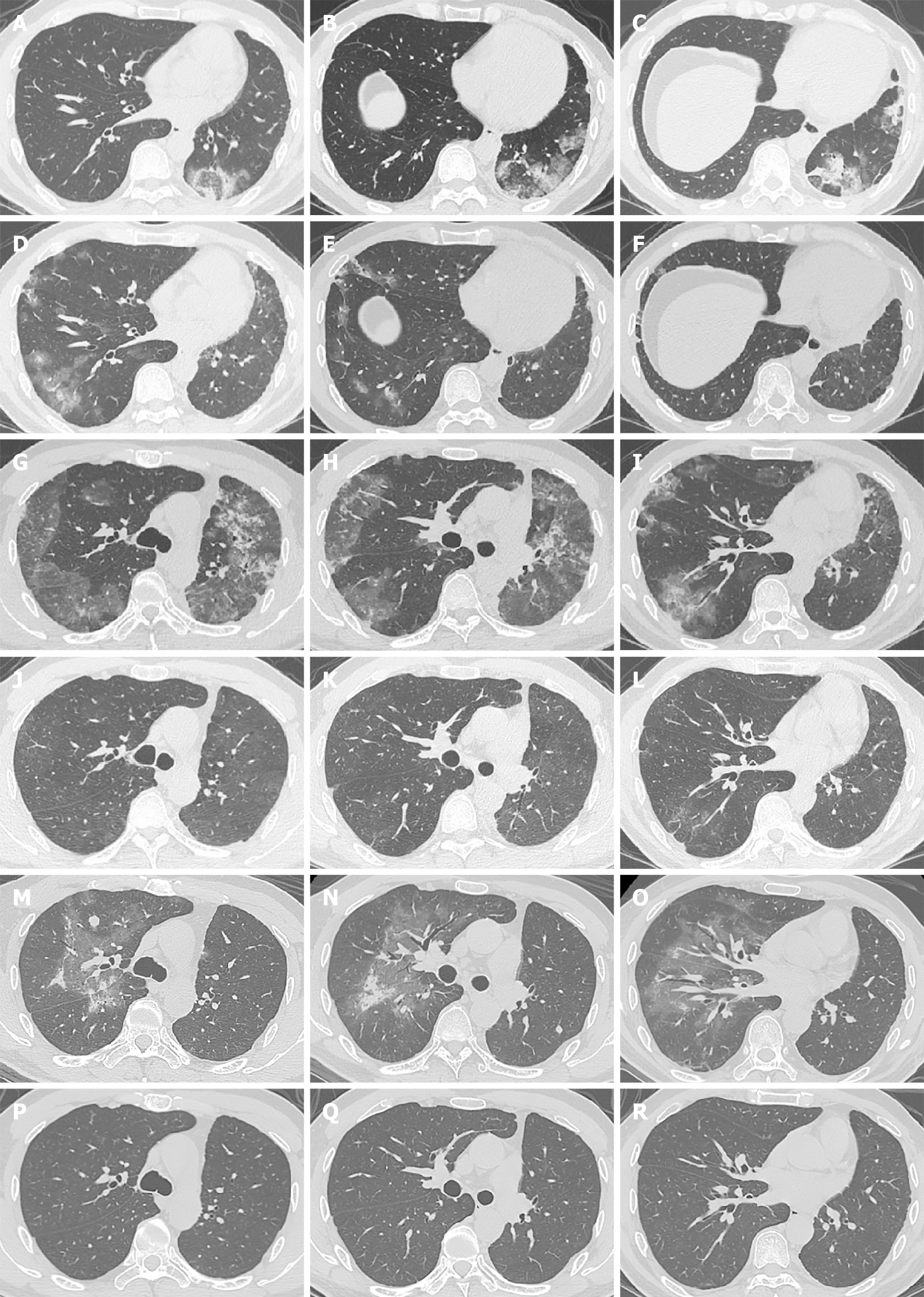
A chest computed tomography (CT) scan showed pneumonitis after ten cycles of immune checkpoint inhibitor treatment.
A 40-year-old man, who has no history of smoking, was diagnosed with locally advanced (pT3N2M0 stage IIIA) left-lower lung adenocarcinoma. The status of driver genes and PD-L1 expression were unknown. He underwent radical left-lower lobe resection plus mediastinal lymph node dissection followed by four cycles of adjuvant chemotherapy with pemetrexed plus cisplatin. After the operation and chemotherapy, he was administered with durvalumab (anti-PD-L1, 1500 mg q4w) as consolidation immunotherapy. After ten cycles of durvalumab injection, pneumonitis with a COP pattern in the left upper lung was detected by CT for regular follow-up (Figures 1A-C). Physical examination showed no positive signs. Considering that the patient did not have a history of interstitial pneumonia and did not complain of any relevant symptoms, he was diagnosed with CIP grade 1. Treatment with durvalumab was continued for two cycles until the patient complained of moderate dry cough. An interesting finding in the chest CT scan was that the pneumonitis in the left upper lobe completely disappeared (Figures 1D-F) without any anti-CIP treatment. In addition, new consolidations and ground-glass opacities representing again a COP pattern in combination with an HP pattern were newly observed in multifocal distributions in the rest of the lung (Figures 1G-I). The values of β-D glucan and procalcitonin were normal, which indicated that it was a non-infectious pneumonia. He was therefore diagnosed with recurrent CIP grade 2 and was treated with intravenous injection of methylprednisolone (1 mg/kg/d). After a week of methylprednisolone treatment, the dry cough was significantly improved and CT showed resolution of consolidations and ground-glass opacities (Figures 1J-L). Methylprednisolone was tapered slowly over 8 wk. After oral corticosteroid treatment, a chest CT was performed for routine follow-up. Again, interestingly, in the absence of durvalumab retreatment, new diffuse ground-glass and consolidative opacities appeared in the lung exactly where the first and second episode pneumonitis were not involved (Figures 1M-O). With only radiographic changes, the patient was again diagnosed with recurrent CIP grade 1. He refused to restart the steroid treatment. Durvalumab was discontinued permanently because of recurrent CIP.
The patient had on history of past illness.
The patient had no notable personal or family history.
Physical examination showed no positive signs.
The values of β-D glucan and procalcitonin were normal.
CT scan images of the patient during CIP presentation and follow-up in Figure 1. The first episode of CIP in Figures 1A-C; chest CT showed that the changes of the former in Figures 1D-I; CIP in the left upper lobe disappeared in Figures 1D-F; the second episode of CIP in Figures 1G-I; a significant improvement in the CIP in Figures 1J-L; the third episode of CIP in Figures 1M-O; resolution of CIP in Figures 1P-R.
CIP.
At the second episode of CIP, Methylprednisolone (1 mg/kg/d) was administrated and was tapered slowly over 8 wk.
A follow-up CT scan performed 5 mon later showed that the CIP was completely cured without any treatment (Figures 1P-R). The patient harboring EGFR exon 19 mutations is administered osimertinib because of disease progression with bilateral lungs and multiple brain metastases. No symptoms or side effects were observed.
The most interesting aspect of this case is the dynamic changes in radiographic findings. The first episode of CIP was limited to the left lung, while the second episode of CIP was distributed to the rest of the left lung and peripheral area of the right lung. As for the third episode of CIP, the radiologic changes were mainly localized in the central area of the right lung. Obviously, the areas of the three CIPs involved in the lung were different from each other. Interestingly, taking these pneumonitis areas together makes nearly a whole lung area (Figure 2). The dynamic radiographic changes in this case are different from those of the other cases reported in recurrent CIP, as most of the CIP cases were recurrent in the former pneumonitis area or in different areas overlapping the former[8].
It was reported that half of the patients had recurrent CIP in the absence of ICI retreatment[9]. Recurrent CIP, which is considered to be related to the persistent response after ICI discontinuation, is a unique phenomenon for ICI treatment and may be explained by the durable nature of the effect of ICI[8]. In this case, the patient suffered from a second recurrent CIP just at the end of steroid tapering, which indicated the durable effect of durvalumab continued for at least 8 wk. What is more interesting is that the third episode of CIP started after the whole oral corticosteroid treatment and that it was relieved completely without any anti-ICI treatment. This observation indicates that some types of CIP may be resistant to steroids, and that wait-and-see may be a choice for them.
The mechanism of CIP remains to be investigated. By targeting the PD-L1 expressed on cancer cells, anti-PD-L1 inhibitors are considered to cause fewer irAEs, especially immune-related pneumonitis, than anti-PD-1 inhibitors[10]. Based on the dynamic changes of radiologic findings in our case, a possible mechanism of CIP is that some specific immune factors targeting antigens expressed on the surface of normal lung cells are generated. This may provoke the immune system to attack normal lung cells until the whole lung is involved. It may be some kind of reversible self-healing allergic reaction.
This is the first case report on the unique dynamic changes in the radiologic features of CIP. This case showed that recurrent CIPs may occur repeatedly until the whole lung is involved, indicating that the follow-up period of CIP should be long enough, and the rechallenge of ICI should be done with due caution.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mazzei MA S-Editor: Wang JJ L-Editor: A P-Editor: Guo X
| 1. | Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC; KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3449] [Cited by in RCA: 4805] [Article Influence: 686.4] [Reference Citation Analysis (0)] |
| 2. | Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Özgüroğlu M; PACIFIC Investigators. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379:2342-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1573] [Cited by in RCA: 2061] [Article Influence: 294.4] [Reference Citation Analysis (0)] |
| 3. | Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Kazarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Shire N, Jiang H, Goldman JW; CASPIAN investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 1429] [Article Influence: 238.2] [Reference Citation Analysis (0)] |
| 4. | Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;2:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 560] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 5. | Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, Pennell NA, Velcheti V. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest. 2017;152:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 363] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 6. | Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss MH, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon RA, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2017;35:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 825] [Article Influence: 91.7] [Reference Citation Analysis (1)] |
| 7. | Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, Hatabu H, Ott PA, Armand PF, Hodi FS. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin Cancer Res. 2016;22:6051-6060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (1)] |
| 8. | de Jong C, Peters BJM, Schramel FMNH. Recurrent Episodes of Nivolumab-Induced Pneumonitis after Nivolumab Discontinuation and the Time Course of Carcinoembryonic Antigen Levels: A Case of a 58-Year-Old Woman with Non-Small Cell Lung Cancer. Chemotherapy. 2018;63:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Nishino M, Chambers ES, Chong CR, Ramaiya NH, Gray SW, Marcoux JP, Hatabu H, Jänne PA, Hodi FS, Awad MM. Anti-PD-1 Inhibitor-Related Pneumonitis in Non-Small Cell Lung Cancer. Cancer Immunol Res. 2016;4:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6286] [Article Influence: 483.5] [Reference Citation Analysis (0)] |