Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9090
Peer-review started: April 1, 2021
First decision: June 23, 2021
Revised: June 25, 2021
Accepted: September 2, 2021
Article in press: September 2, 2021
Published online: October 26, 2021
Processing time: 205 Days and 5.4 Hours
The clinical significance of breast cancer susceptibility gene 1 (BRCA1) in non-small cell lung cancer (NSCLC) patients undergoing surgery remains unclear up to now.
To explore the relation of BRCA1 expression with clinicopathological characteristics and survival in patients with resected NSCLC.
EMBASE, PubMed, Web of Science, and The Cochrane Library databases were searched to identify the relevant articles. To assess the correlation between the expression of BRCA1 and clinicopathological characteristics and prognosis of patients with resected NSCLC patients, the combined relative risks or hazard ratios (HRs) with their corresponding 95% confidence intervals [CIs] were estimated.
Totally, 11 articles involving 1041 patients were included in the meta-analysis. The results indicated that the expression of BRCA1 was significantly correlated with prognosis of resected NSCLC. Positive BRCA1 expression signified a shorter overall survival (HR = 1.60, 95%CI: 1.25-2.05; P < 0.001) and disease-free survival (HR = 1.78, 95%CI: 1.42-2.23; P < 0.001). However, no significant association of BRCA1 expression with any clinicopathological parameters was observed.
BRCA1 expression indicates a poor prognosis in resected NSCLC patients. BRCA1 might serve as an independent biomarker to predict clinical outcomes and help to customize optimal adjuvant chemotherapy for NSCLC patients who had received surgical therapy.
Core Tip: Based on 11 included articles involving 1041 patients, we demonstrated that the expression of the breast cancer susceptibility gene 1 (BRCA1) was significantly correlated with prognosis of resected non-small cell lung cancer (NSCLC). Furthermore, positive BRCA1 expression signified a shorter overall survival and disease-free survival. However, no significant association of BRCA1 expression with any clinicopathological parameters was observed. Overall, BRCA1 expression indicates a poor prognosis in resected NSCLC patients. BRCA1 might serve as an independent biomarker to predict clinical outcomes and help to customize optimal adjuvant chemotherapy for NSCLC patients who had received surgical therapy.
- Citation: Gao Y, Luo XD, Yang XL, Tu D. Clinical significance of breast cancer susceptibility gene 1 expression in resected non-small cell lung cancer: A meta-analysis . World J Clin Cases 2021; 9(30): 9090-9100
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9090.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9090
Lung cancer has become the leading cause of cancer death among men and women globally[1-3]. According to the pathology, lung cancer is classified into two subgroups: Small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC). And approximately 85% of all lung cancer cases belong to NSCLC. For most NSCLC patients, surgical resection is still the most important therapy. Although the diagnosis and treatment techniques have been improved greatly in recent years, the overall 5-year survival rate for NSCLC in China was only 19.8%[4].
The tumor-node-metastasis (TNM) staging remains the most popular tool to assess the progression and predict the prognosis of NSCLC patients. However, a number of indicators have been reported to be significantly associated with clinicopathological characteristics and survival of NSCLC patients in recent years, including many genetic markers[5,6]. Increasing evidence indicates that DNA repair genes can be used as predictive and prognostic markers in NSCLC[7]. Breast cancer susceptibility gene 1 (BRCA1) is a 220-kD multifunctional nuclear phosphoprotein that has a vital role in DNA repair[8]. As a member of the DNA repair genes, BRCA1 plays an important role in the pathway to repair fractured double-stranded DNA. It interacts with the MRE1/RAD50/NBS1 complex which is related to homologous recombination repair and non-homologous recombination repair[9].
BRCA1 is expressed in about 40% of inherited breast cancers and more than 80% of inherited ovarian cancers[10]. As for NSCLC, the proportion of BRCA1 positive cases ranged from 13.1% to 66.7%[11,12]. Meanwhile, several studies have explored the clinicopathological and prognostic significance of BRCA1 expression in NSCLC patients who received surgical therapy. Nevertheless, their results were inconclusive and inconvincible because of limited data.
Therefore, we aimed to conduct the current meta-analysis to further investigate the correlation of BRCA1 expression with clinicopathological parameters and long-term outcomes in resected NSCLC.
A systematic research was conducted in EMBASE, Web of Science, Cochrane Library, and PubMed for relevant studies published from the inception of the databases to July 4, 2020 using the following terms: BRCA1, breast cancer susceptibility gene 1, breast cancer 1, lung, pulmonary, cancer, neoplasm, tumor, and carcinoma. The specific search strategy was: (breast cancer susceptibility gene 1 OR BRCA1) AND (lung OR pulmonary) AND (tumor OR cancer OR carcinoma OR neoplasm). The references listed in the included articles were also reviewed for eligibility. The literature retrieval was conducted by two independent authors (Gao Y and Luo XD).
The inclusion criteria were as follows: (1) Patients who were diagnosed with NSCLC pathologically and received surgical therapies; (2) Articles that assessed the correlation of BRCA1 expression with prognosis; (3) The interest outcomes included overall survival (OS) or progression-free survival (PFS) with hazard ratios (HRs) and corresponding 95% confidence intervals (CIs); (4) Enough data like Kaplan-Meier curves were provided to calculate the HRs with 95%CIs for OS or PFS when they were not reported directly; (5) Articles were published in English with full-texts; and (6) Newcastle–Ottawa scale score ≥ 6[13].
The exclusion criteria were as follows: (1) Expert opinions, reviews, meeting abstracts, letters, case reports, and animal trials; (2) The HRs with 95%CIs could not be calculated because of the lack of relevant information; and (3) Duplicated or overlapped studies.
The literature selection was performed by two investigators independently (Gao Y and Luo XD).
The following information was extracted from each included study: The name of the first author, publication year, country, sample size, number of patients with positive BRCA1 expression, TNM stage, end-point events, source of HR, HRs with corresponding 95%CIs, and other necessary data to calculate the association of BRCA1 expression with several clinicopathological parameters including gender, age, tumor size, TNM stage, differentiation status, and histological type.
The quality of included studies was assessed using the Newcastle-Ottawa scale score and studies that earned a score of 6 or higher were regarded as high-quality studies[13].
All statistical analyses were conducted using STATA 12.0 software (StataCorp, College Station, TX, United States). The relation of BRCA1 expression with the clinicopathological characteristics and prognosis was assessed by the pooled relative ratios or HRs with 95%CIs. HRs with 95%CIs from multivariable models were used whenever available. If the articles did not report them directly, they would be estimated from the Kaplan-Meier curves using the method reported by Tierney et al[14]. The χ2-based Q-test and the I2 statistic were applied to calculate the heterogeneity among the included studies[15]. If there was significant heterogeneity (P < 0.10 and/or I2 > 50%), the random-effects model was used; otherwise, the fixed-effects model was used[16]. The stability of pooled results was assessed by sensitivity analysis. Potential publication bias was detected using Begg’s funnel plot and Egger’s test[17]. A log-rank P value < 0.05 was considered statistically significant.
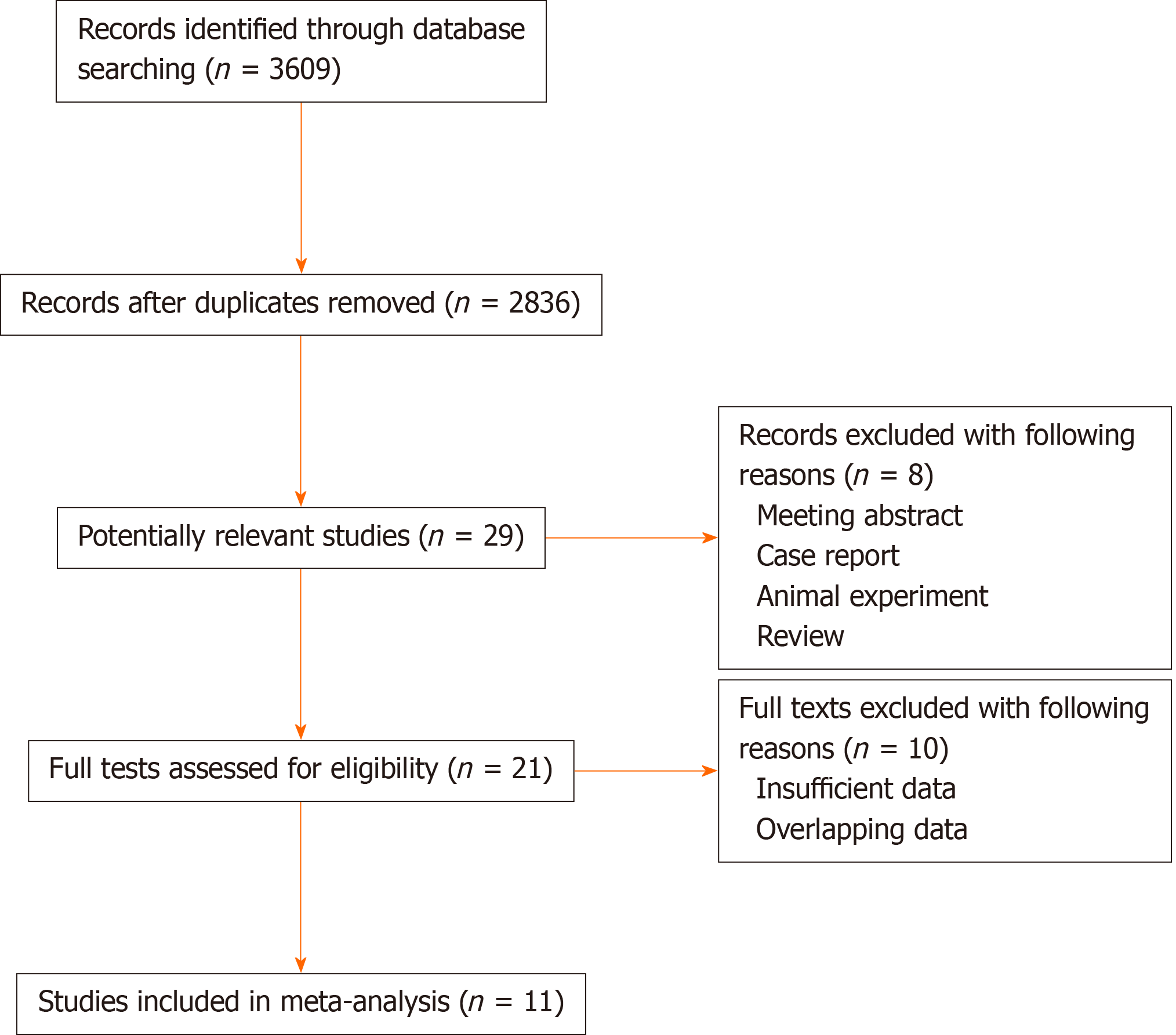
As shown in Figure 1, initially 3609 records were identified through database searching. After removing the duplicates, 2836 records were left. Then 29 publications were reviewed with full-texts for eligibility after excluding 2807 irrelevant records. Finally, 11 articles involving 1041 patients were included in the meta-analysis [11,12,18-26]. The sample sizes ranged from 32 to 221 and the proportion of patients with BRCA1 expression ranged from 13.1% to 66.7%. Furthermore, Gachechiladze et al[21] performed subgroup analysis based on the TNM stage and provided sufficient data of the two groups, so we considered them as two studies during the process of statistical analyses. Other specific information is summarized in Table 1.
| Ref. | Year | Country | Sample size | Positive, n (%) | TNM stage | Outcome | Source of HR | NOS score |
| Rosell et al[18] | 2007 | Spain | 123 | 40 (32.5) | IB-IIIA | OS | R | 6 |
| Bartolucci et al[11] | 2009 | Italy | 54 | 36 (66.7) | IB-IIB | OS/DFS | R | 7 |
| Ma et al[19] | 2010 | China | 89 | 53 (59.6) | IIIA | OS/DFS | E | 8 |
| Leng et al[20] | 2012 | China | 85 | 14 (16.5) | I- IV | OS/DFS | R | 7 |
| Gachechiladze et al[21] | 2013 | Israel | 32 | 9 (28.1) | I- II | OS/DFS | E | 7 |
| Gachechiladze et al[21] | 2013 | Israel | 58 | 15 (25.9) | III- IV | OS/DFS | E | 7 |
| Yu et al[22] | 2013 | China | 80 | 36 (45) | I- IV | OS/DFS | E | 7 |
| Grossi et al[23] | 2015 | Italy | 81 | 45 (55.6) | I A-IIIB | OS | R | 6 |
| Lafuente-Sanchis et al[24] | 2015 | Spain | 64 | 30 (46.9) | I | DFS | R | 7 |
| Huang et al[12] | 2016 | China | 84 | 11 (13.1) | II-III | DFS | R | 8 |
| Wang et al[25] | 2017 | China | 70 | NR | II-III | OS | R | 6 |
| Levallet et al[26] | 2017 | France | 221 | 129 (58.4) | I- IV | OS/DFS | R | 8 |
Based on the information provided by included studies, we explored the relationship of BRCA1 expression with gender, age (≥ 60 vs < 60 years), tumor size (T3/4 vs T1/2), TNM stage (IV/III vs I/II), histology (adenocarcinoma vs squamous cell carcinoma), differentiation (poor vs moderate/well). However, no significant association between BRCA1 expression and these parameters was observed (Table 2).
| Ref. | Gender (M vs F) | Age (≥ 60 vs < 60) | Tumor size (T3/4 vs T1/2) | TNM stage (III /IV vs I/II) | Histology (AC vs SCC) | Differentiation (poor vs moderate/well) |
| Rosell et al[18] | - | - | - | - | - | |
| Bartolucci et al[11] | - | - | - | - | - | |
| Ma et al[19] | 1.050 (0.740-1.489) | - | 1.046 (0.734-1.489) | - | 0.916 (0.636-1.319) | 1.204 (0.860-1.687) |
| Leng et al[20] | - | - | - | - | - | - |
| Gachechiladze et al[21] | - | - | - | 0.920 (0.454-1.861) | - | - |
| Gachechiladze et al[21] | 0.857 (0.608-1.210) | 1.273 (0.917-1.768) | - | 1.020 (0.727-1.433) | 0.990 (0.704-1.392) | 1.238 (0.890-1.723) |
| Yu et al[22] | - | - | - | - | - | - |
| Grossi et al[23] | - | - | - | - | - | - |
| Lafuente-Sanchis et al[24] | 0.829 (0.265-2.592) | 0.367 (0.050-2.678) | - | 3.375 (0.776-14.678) | 1.660 (0.383-7.204) | - |
| Huang et al[12] | - | - | - | - | - | - |
| Wang et al[25] | 0.948 (0.735-1.224) | 0.889 (0.711-1.112) | - | - | - | - |
| Overall | 0.94 (0.79-1.12), P = 0.522; I2 = 0.0, P = 0.871 | 1.01 (0.72-1.42), P = 0.948; I2 = 51.2, P = 0.129 | - | 1.05 (0.78-1.42), P = 0.739; I2 = 22.7, P = 0.274 | 0.97 (0.76-1.24), P = 0.806; I2 = 0.0, P = 0.732 | 1.22 (0.97-1.55), P = 0.097; I2 = 0.0, P = 0.908 |
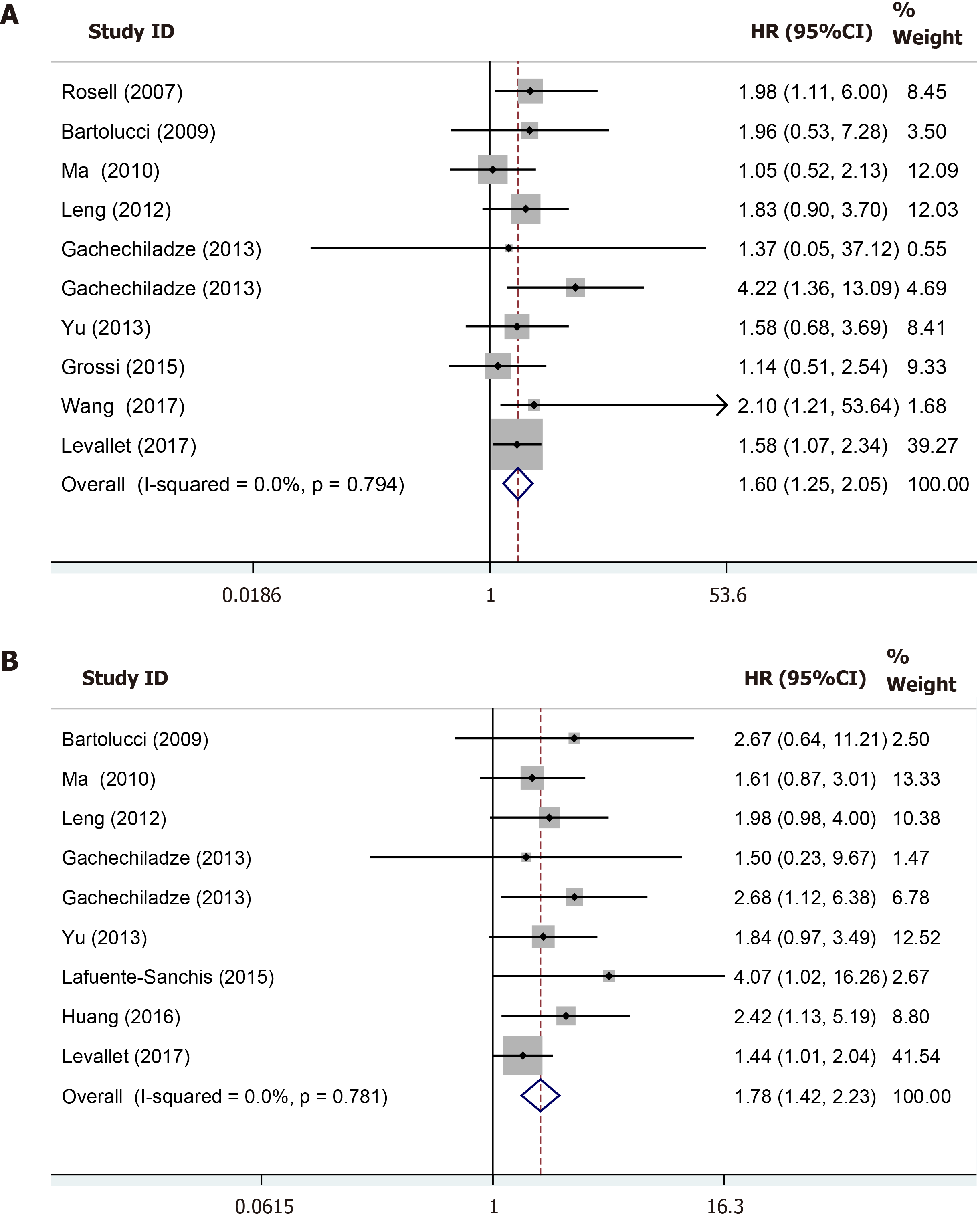
Totally ten studies of 893 patients compared OS between the BRCA1-positive group and BRCA1-negative group. The fixed-effects model was adopted because of the low heterogeneity (I2 = 0.0%, P = 0.794) among those studies included. The pooled HR was 1.60 (95%CI: 1.25-2.05, P < 0.001), which indicated that BRCA1 positive expression was an independent risk factor for OS of resected NSCLC patients (Figure 2A). The results of subgroup analysis stratified by the country was similar with the overall pooled results (Table 3).
| Analysis | No. of studies | HR (95%CI) | Log-rank P value | I2 (%) | P value |
| Overall survival | 10 | 1.60 (1.25-2.05) | < 0.001 | 0 | 0.794 |
| Country | |||||
| Non-Asian countries | 4 | 1.57 (1.15-2.15) | 0.005 | 0.0 | 0.798 |
| Asian countries | 6 | 1.66 (1.12-2.45) | 0.011 | 7.5 | 0.495 |
| Disease-free survival | 9 | 1.78 (1.42-2.23) | < 0.001 | 0.0 | 0.781 |
| Country | |||||
| Non-Asian countries | 3 | 1.58 (1.13-2.20) | 0.007 | 22.3 | 0.276 |
| Asian countries | 6 | 1.97 (1.44-2.69) | < 0.001 | 0.0 | 0.936 |
In total, nine studies of 767 patients explored the impact of BRCA1 expression on the disease-free survival (DFS). The patients with BRCA1 negative expression had a significantly prolonged DFS compared to patients with BRCA1 positive expression (HR = 1.78, 95%CI: 1.42-2.23, P < 0.001) with no heterogeneity (I2 = 0.0%, P = 0.781) (Figure 2B). Subgroup analysis based on the country further verified the above result (Table 3).
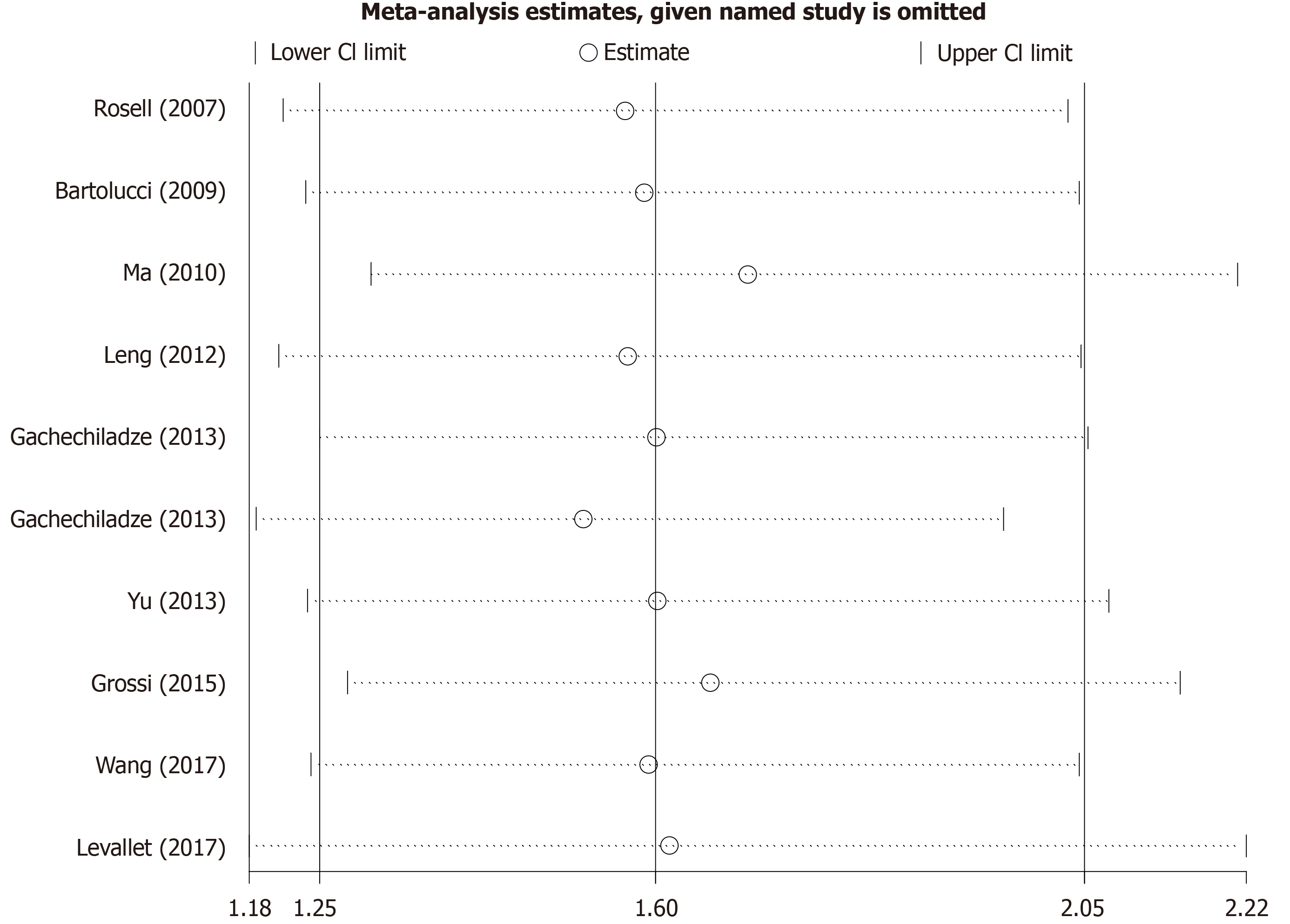
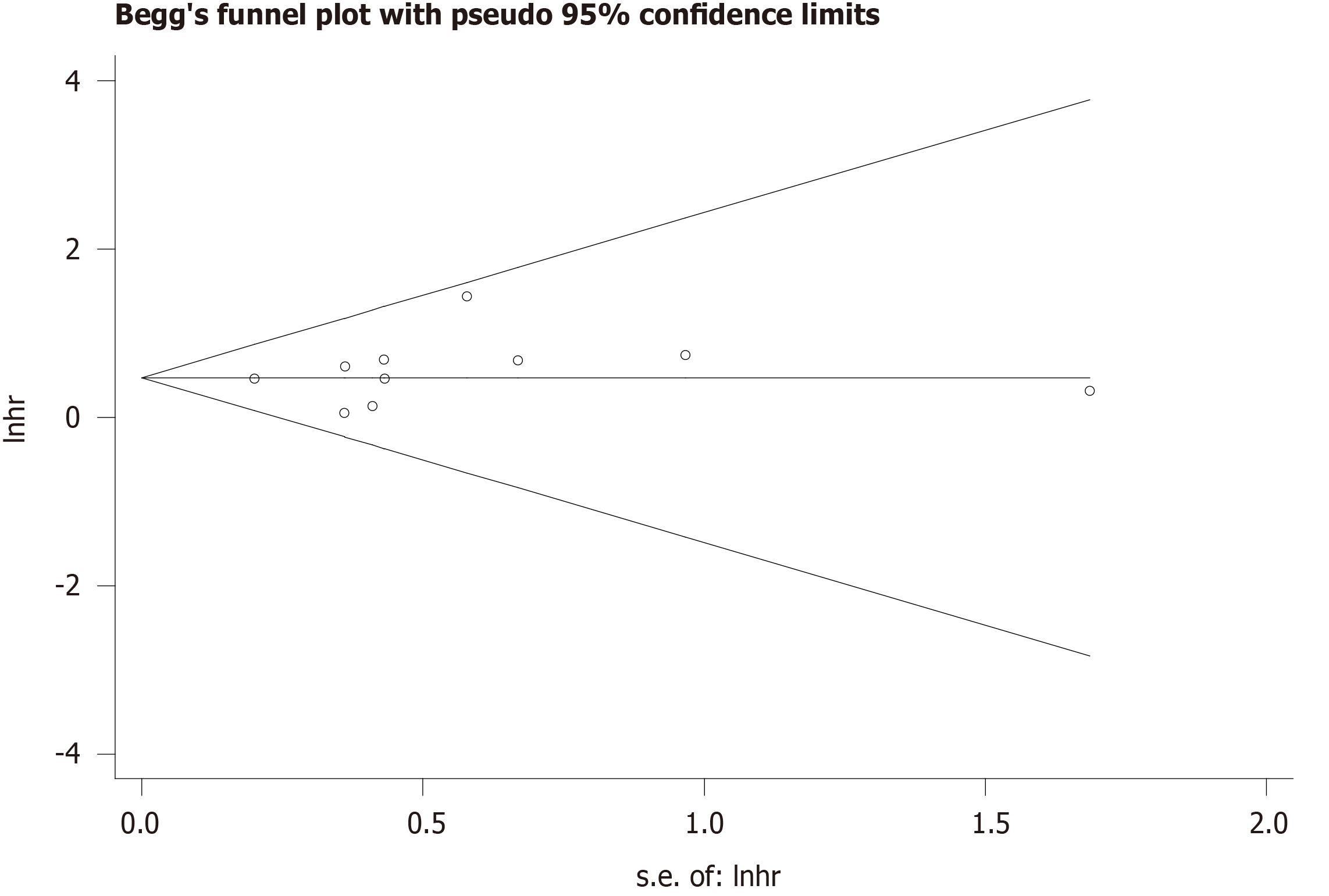
The sensitivity analysis performed by excluding each study individually signified that the results of our meta-analysis were stable (Figure 3). We conducted Begg’s funnel plot and Egger’s test to identify potential publication bias. As a result, Begg’s funnel plot was symmetrical (Figure 4) and the P value for Egger’s test was 0.451, which both indicated that no significant publication bias existed.
The results of the current meta-analysis which summarized 11 studies involving 1041 patients showed that positive BRCA1 expression was associated with a shorter OS and DFS without regional disparities. The conclusion was consistent with the results of the previous studies[18,21,25,26]. BRCA1 expression status could serve as a novel indicator for the assessment of prognosis and formulation of therapy strategy for resected NSCLC patients. No significant correlation between BRCA1 expression and clinicopathological characteristics was observed in our study. However, more than half of included studies did not report the relation of BRCA1 expression with clinicopathological parameters including gender, age, tumor size, TNM stage, histology type, and differentiation. Therefore, we believe that more investigations are still needed to further determine the association between BRCA1 expression status and these parameters.
Cellular DNA is continuously attacked by intracellular active substances and environmental factors. Toxicity and mutagenesis are minimized by different repair pathways. Approximately130 known human genes are involved during DNA repair process[27]. As a part of them, the BRCA1 gene was identified to have normal cellular functions, such as replication, transcription regulation, cell cycle regulation, and higher chromatin hierarchical control[8]. Furthermore, BRCA1 plays a crucial role in DNA damage response (DDR) through direct interaction with DDR proteins[28].
BRCA1 mutation is known as a risk factor for breast and ovarian cancers. And breast cancer patients with BRCA1 mutation account for 51% to 75% of all cases[29]. It has also been reported that prostate carcinomas with BRCA1 positive expression showed a high tumor proliferation index due to the cell-cycle regulation, and signified a worse prognosis compared to BRCA1 negative tumors[30]. Although BRCA1 mutations have not been found in NSCLC, the downregulation of BRCA1 mRNA and protein expression was found in 30% of NSCLC cases (mostly adenocarcinoma)[31].
One of the main targets of BRCA1 was the DNA damage-responsive gene GADD45. Expression of BRCA1 could trigger apoptosis through the c-Jun N-terminal kinase/ stress-activated protein kinase pathway (JNK/SAPK)[32], which is activated by GADD45. Nevertheless, the JNK/SAPK pathway could mediate a physiological response to cisplatin-induced DNA damage[33]. It was plausible that the absence of BRCA1 increased a high sensitivity to cisplatin and there was a survival advantage for patients with a low level of BRCA1 mRNA treated with platinum-based drugs[34,35], which was consistent with our conclusion.
About the clinical significance of BRCA1 in NSCLC, there are still some fields which deserve further investigations. First of all, the inner mechanisms of the platinum-resistance in NSCLC patients with positive BRCA1 expression are still not clear. It is necessary and valuable to seek more potent agents to reverse platinum resistance in platinum-resistant NSCLC patients. It has been published that curcumin may serve as a chemosensitizer to enhance cisplatin-induced proliferation inhibition and apoptosis of A549/cisplatin cells by inhibiting DNA repair in the FA/BRCA pathway[36], which is a DNA cross-link damage repair pathway that regulates cellular resistance to DNA cross-link agents[37]. More rigorous and well-designed studies are warranted to investigate the possibility and feasibility of agents to reverse platinum resistance. Second, as mentioned above, only five of included studies did not report the association of BRCA1 expression with clinicopathological characteristics, which led to an indistinct relationship between BRCA1 expression and tumor development and progression in NSCLC. Third, BRCA1 is usually expressed in breast and ovarian cancers[10], so is there any difference in the clinical significance of BRCA1 expression between men and women?
There were some limitations in our meta-analysis. First of all, only 11 studies with small sample sizes were included. Second, the methods (reverse transcription-polymerase chain reaction and immunohistochemistry) to calculate BRCA1 expression were inconsistent in the studies involved. Lastly, we were unable to conduct subgroup analysis based on age, sex, and TNM stage due to the lack of original data.
In conclusion, the level of BRCA1 expression is associated with a poor prognosis in resected NSCLC. BRCA1 may be a promising and independent prognostic factor in NSCLC. This information could be useful to customize the adjuvant chemotherapy for NSCLC patients who received surgical therapy.
The clinical role of breast cancer susceptibility gene 1 (BRCA1) expression in non-small cell lung cancer (NSCLC) patients undergoing surgery remains unclear.
This study explored the clinical value of BRCA1 expression in resected NSCLC patients.
This study aimed to explore the relation of BRCA1 expression with clinicopathological characteristics and survival in patients with resected NSCLC.
Several electronic databases were searched to identify the relevant articles. The combined relative risks or hazard ratios with their corresponding 95%CIs were estimated.
The expression of the BRCA1 was significantly correlated with the prognosis of resected NSCLC. Positive BRCA1 expression signified a shorter overall survival and disease-free survival. However, no significant association of BRCA1 expression with any clinicopathological parameters was observed.
BRCA1 expression indicated a poor prognosis in resected NSCLC patients. BRCA1 might serve as an independent biomarker to predict clinical outcomes and help to customize optimal adjuvant chemotherapy for NSCLC patients who received surgical therapy.
BRCA1 could serve as a novel biomarker in resected NSCLC for the evaluation of prognosis and formulation of treatment strategy.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gebbia V, Kim HS S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Li JH
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64667] [Article Influence: 16166.8] [Reference Citation Analysis (176)] |
| 2. | Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol. 2016;893:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1259] [Article Influence: 125.9] [Reference Citation Analysis (0)] |
| 3. | Thill PG, Goswami P, Berchem G, Domon B. Lung cancer statistics in Luxembourg from 1981 to 2008. Bull Soc Sci Med Grand Duche Luxemb. 2011;43-55. [PubMed] |
| 4. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3426] [Article Influence: 489.4] [Reference Citation Analysis (1)] |
| 5. | Parmigiani G, Garrett-Mayer ES, Anbazhagan R, Gabrielson E. A cross-study comparison of gene expression studies for the molecular classification of lung cancer. Clin Cancer Res. 2004;10:2922-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Potti A, Mukherjee S, Petersen R, Dressman HK, Bild A, Koontz J, Kratzke R, Watson MA, Kelley M, Ginsburg GS, West M, Harpole DH Jr, Nevins JR. A genomic strategy to refine prognosis in early-stage non-small-cell lung cancer. N Engl J Med. 2006;355:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 418] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 7. | Cobo M, Isla D, Massuti B, Montes A, Sanchez JM, Provencio M, Viñolas N, Paz-Ares L, Lopez-Vivanco G, Muñoz MA, Felip E, Alberola V, Camps C, Domine M, Sanchez JJ, Sanchez-Ronco M, Danenberg K, Taron M, Gandara D, Rosell R. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. J Clin Oncol. 2007;25:2747-2754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 341] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 8. | Rosen EM, Fan S, Isaacs C. BRCA1 in hormonal carcinogenesis: basic and clinical research. Endocr Relat Cancer. 2005;12:533-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst. 2004;96:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 329] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 10. | Karami F, Mehdipour P. A comprehensive focus on global spectrum of BRCA1 and BRCA2 mutations in breast cancer. Biomed Res Int. 2013;2013:928562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Bartolucci R, Wei J, Sanchez JJ, Perez-Roca L, Chaib I, Puma F, Farabi R, Mendez P, Roila F, Okamoto T, Taron M, Rosell R. XPG mRNA expression levels modulate prognosis in resected non-small-cell lung cancer in conjunction with BRCA1 and ERCC1 expression. Clin Lung Cancer. 2009;10:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Huang ZL, Cao X, Luo RZ, Chen YF, Zhu LC, Wen Z. Analysis of ERCC1, BRCA1, RRM1 and TUBB3 as predictors of prognosis in patients with non-small cell lung cancer who received cisplatin-based adjuvant chemotherapy: A prospective study. Oncol Lett. 2016;11:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12664] [Article Influence: 844.3] [Reference Citation Analysis (0)] |
| 14. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4954] [Article Influence: 275.2] [Reference Citation Analysis (0)] |
| 15. | Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1858] [Cited by in RCA: 1996] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 16. | Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21:3672-3673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 363] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 17. | Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1560] [Article Influence: 82.1] [Reference Citation Analysis (1)] |
| 18. | Rosell R, Skrzypski M, Jassem E, Taron M, Bartolucci R, Sanchez JJ, Mendez P, Chaib I, Perez-Roca L, Szymanowska A, Rzyman W, Puma F, Kobierska-Gulida G, Farabi R, Jassem J. BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS One. 2007;2:e1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Ma J. Expression of ERCC1, BRCA1, β-tubulin III, RRM1 and Clinical Response to Adjuvant Chemotherapy in Completely Resected Staged III A-N2 Non-Small-Cell Lung Cancer. M.Sc. Thesis, Zhongshan University. 2010. Available from: https://d.wanfangdata.com.cn/thesis/Y1691870. |
| 20. | Leng XF, Chen MW, Xian L, Dai L, Ma GY, Li MH. Combined analysis of mRNA expression of ERCC1, BAG-1, BRCA1, RRM1 and TUBB3 to predict prognosis in patients with non-small cell lung cancer who received adjuvant chemotherapy. J Exp Clin Cancer Res. 2012;31:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Gachechiladze M, Uberall I, Kolek V, Klein J, Krejci V, Stastna J, Radova L, Fridman E, Skarda J. Correlation between BRCA1 expression and clinicopathological factors including brain metastases in patients with non-small-cell lung cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Yu JJ, Zhu LL, Zhang W, Bai W. [Role of ERCC1 and BRCA1 in prognosis of NSCLC patients receiving postoperative adjuvant chemotherapy]. Chongqi Yike Daxue Xuebao. 2013;38:517-522. |
| 23. | Grossi F, Dal Bello MG, Salvi S, Puzone R, Pfeffer U, Fontana V, Alama A, Rijavec E, Barletta G, Genova C, Sini C, Ratto GB, Taviani M, Truini M, Merlo DF. Expression of Ribonucleotide Reductase Subunit-2 and Thymidylate Synthase Correlates with Poor Prognosis in Patients with Resected Stages I-III Non-Small Cell Lung Cancer. Dis Markers. 2015;2015:302649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Lafuente-Sanchis A, Zúñiga Á, Galbis JM, Cremades A, Estors M, Martínez-Hernández NJ, Carretero J. Prognostic value of ERCC1, RRM1, BRCA1 and SETDB1 in early stage of non-small cell lung cancer. Clin Transl Oncol. 2016;18:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Wang M, Li W, Xing X, Zhang D, Lei J, Li G. BRCA1 and STMN1 as prognostic markers in NSCLCs who received cisplatin-based adjuvant chemotherapy. Oncotarget. 2017;8:80869-80877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Levallet G, Dubois F, Fouret P, Antoine M, Brosseau S, Bergot E, Beau-Faller M, Gounant V, Brambilla E, Debieuvre D, Molinier O, Galateau-Sallé F, Mazieres J, Quoix E, Pujol JL, Moro-Sibilot D, Langlais A, Morin F, Westeel V, Zalcman G. MSH2/BRCA1 expression as a DNA-repair signature predicting survival in early-stage lung cancer patients from the IFCT-0002 Phase 3 Trial. Oncotarget. 2017;8:4313-4329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Wang C, Nie H, Li Y, Liu G, Wang X, Xing S, Zhang L, Chen X, Chen Y. The study of the relation of DNA repair pathway genes SNPs and the sensitivity to radiotherapy and chemotherapy of NSCLC. Sci Rep. 2016;6:26526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Yarden RI, Papa MZ. BRCA1 at the crossroad of multiple cellular pathways: approaches for therapeutic interventions. Mol Cancer Ther. 2006;5:1396-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Abdulrashid K, AlHussaini N, Ahmed W, Thalib L. Prevalence of BRCA mutations among hereditary breast and/or ovarian cancer patients in Arab countries: systematic review and meta-analysis. BMC Cancer. 2019;19:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Fiorentino M, Judson G, Penney K, Flavin R, Stark J, Fiore C, Fall K, Martin N, Ma J, Sinnott J, Giovannucci E, Stampfer M, Sesso HD, Kantoff PW, Finn S, Loda M, Mucci L. Immunohistochemical expression of BRCA1 and lethal prostate cancer. Cancer Res. 2010;70:3136-3139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Marsit CJ, Liu M, Nelson HH, Posner M, Suzuki M, Kelsey KT. Inactivation of the Fanconi anemia/BRCA pathway in lung and oral cancers: implications for treatment and survival. Oncogene. 2004;23:1000-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Harkin DP, Bean JM, Miklos D, Song YH, Truong VB, Englert C, Christians FC, Ellisen LW, Maheswaran S, Oliner JD, Haber DA. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97:575-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 428] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 33. | Potapova O, Haghighi A, Bost F, Liu C, Birrer MJ, Gjerset R, Mercola D. The Jun kinase/stress-activated protein kinase pathway functions to regulate DNA repair and inhibition of the pathway sensitizes tumor cells to cisplatin. J Biol Chem. 1997;272:14041-14044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 179] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Taron M, Rosell R, Felip E, Mendez P, Souglakos J, Ronco MS, Queralt C, Majo J, Sanchez JM, Sanchez JJ, Maestre J. BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet. 2004;13:2443-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 35. | Quinn JE, Kennedy RD, Mullan PB, Gilmore PM, Carty M, Johnston PG, Harkin DP. BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res. 2003;63:6221-6228. [PubMed] |
| 36. | Chen P, Li J, Jiang HG, Lan T, Chen YC. Curcumin reverses cisplatin resistance in cisplatin-resistant lung caner cells by inhibiting FA/BRCA pathway. Tumour Biol. 2015;36:3591-3599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Wang L, Wang H, Wang T, Liu J, Chen W, Wang Y, Chen C, Zhu H, Dai P. Analysis of polymorphisms in genes associated with the FA/BRCA pathway in three patients with multiple primary malignant neoplasms. Artif Cells Nanomed Biotechnol. 2019;47:1101-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |