Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9077
Peer-review started: April 14, 2021
First decision: June 3, 2021
Revised: June 27, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 26, 2021
Processing time: 189 Days and 19.3 Hours
The standard treatment of locally advanced rectal cancers (LARC) consists on neoadjuvant chemoradiotherapy followed by total mesorectal excision. Different data in literature showed a benefit on tumor downstaging and pathological complete response (pCR) rate using radiotherapy dose escalation, however there is shortage of studies regarding dose escalation using the innovative techniques for LARC (T3-4 or N1-2).
To analyze the role of neoadjuvant radiotherapy dose escalation for LARC using innovative radiotherapy techniques.
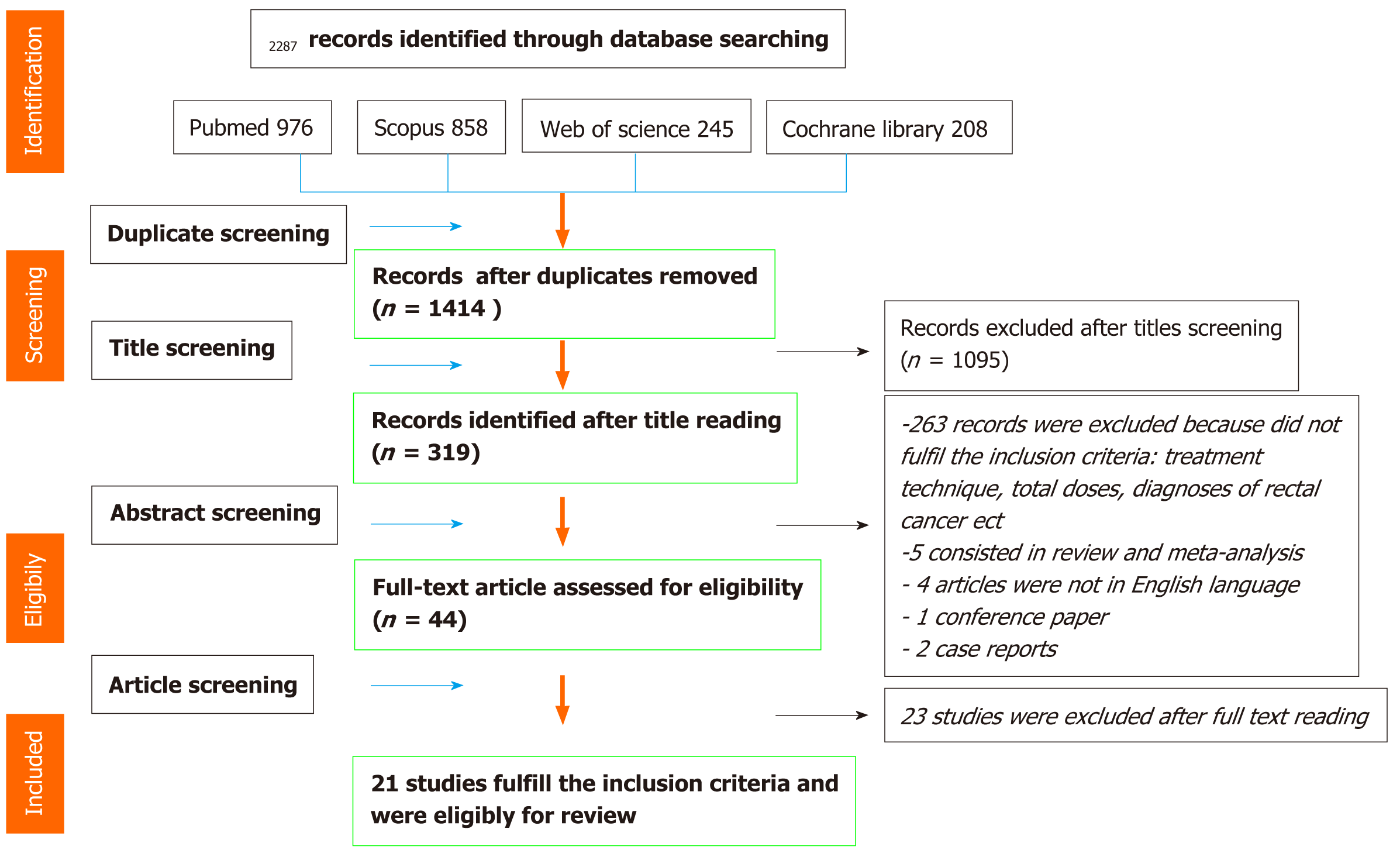
In December 2020, we conducted a comprehensive literature search of the following electronic databases: PubMed, Web of Science, Scopus and Cochrane library. The limit period of research included articles published from January 2009 to December 2020. Screening by title and abstract was carried out to identify only studies using radiation doses equivalent dose 2 Gy fraction (EQD2) ≥ 54 Gy and Volumetric Modulated Arc Therapy (VMAT), intensity-modulated radiotherapy or image-guided radiotherapy (IGRT) techniques. The authors’ searches generated a total of 2287 results and, according to PRISMA Group (2009) screening process, 21 publications fulfil selection criteria and were included for the review.
The main radiotherapy technique used consisted in VMAT and IGRT modality. The mainly dose prescription was 55 Gy to high risk volume and 45 Gy as prophylactic volume in 25 fractions given with simultaneous integrated boosts technique (42.85%). The mean pCR was 28.2% with no correlation between dose prescribed and response rates (P value ≥ 0.5). The R0 margins and sphincter preservation rates were 98.88% and 76.03%, respectively. After a mean follow-up of 35 months local control was 92.29%. G3 or higher toxicity was 11.06% with no correlation between dose prescription and toxicities. Patients receiving EQD2 dose > 58.9 Gy and BED > 70.7 Gy had higher surgical complications rates compared to other group (P value = 0.047).
Dose escalation neoadjuvant radiotherapy using innovative techniques is safe for LARC achieving higher rates of pCR. EQD2 doses > 58.9 Gy is associated with higher rate of surgical complications.
Core Tip: We analyzed the role of neoadjuvant radiotherapy dose escalation for locally advanced rectal cancers (LARC) using innovative radiotherapy techniques. A comprehensive literature search was performed on electronic database with a period limit from January 2009 to December 2020. According to PRISMA Group (2009) screening process only studies using equivalent dose 2 Gy fraction (EQD2) ≥ 54 Gy and Volumetric Modulated Arc Therapy, image-guided radiotherapy or image-guided radiotherapy techniques were included for the review. Neoadjuvant radiotherapy dose escalation using innovative techniques is safe for LARC with acceptable acute toxicity, achieving higher pathological complete response compared to standard treatment. EQD2 doses > 58.9 Gy with a BED > 70.7 Gy was associated with higher rate of surgical complications.
- Citation: Delishaj D, Fumagalli IC, Ursino S, Cristaudo A, Colangelo F, Stefanelli A, Alghisi A, De Nobili G, D’Amico R, Cocchi A, Ardizzoia A, Soatti CP. Neoadjuvant radiotherapy dose escalation for locally advanced rectal cancers in the new era of radiotherapy: A review of literature. World J Clin Cases 2021; 9(30): 9077-9089
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9077.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9077
The incidence of colorectal cancer (CRC) from 2020 declined rapidly among screening-aged individuals, but increased in adults aged younger than 55 years old. CRC represent the third most common cancer and often is diagnosed in an advanced stage. Despite an improve of survival rate in patients with CRC in last years, CRC remain the second case of death in the United States[1].
The standard treatment of locally advanced rectal cancers (LARC) consists on neoadjuvant chemoradiationtherapy (CRT) followed by total mesorectal excision[2-3].
Neoadjuvant radiotherapy improve local control of LARC (T3-4 or N1-2 disease) and subsequently can influence survival rate improving overall survival (OS). The advantages of neoadjuvant radiotherapy were recognized from 1997 by the Swedish Rectal Cancer Study Group, which found a significant reduction in local recurrence rates in 1168 patients analyzed[4].
These findings were confirmed afterwards by other randomized studies, consolidating the important role of neoadjuvant radiotherapy treatment in locally advanced rectal cancer[5-18].
The standard neoadjuvant radiotherapy treatment in rectal cancer consist in a total dose of 45-50.4 Gy delivered in 25-28 daily fractions[2,3].
Typically, up to the 2010s the main radiotherapy technique used for the treatment of rectal cancer was three-dimensional CRT (3DCRT)[5-9].
The clinical outcome after 3DCRT is largely dependent on tumor response and it is estimate that overall 15% of patients experience a pathological complete response (pCR) at the standard radiation dose[4-10].
It is also known that a higher dose to tumor consists in a better tumor rate response, but this could often lead to a higher dose in surrounding tissue and a risk of increased side effects and surgical complications[11-18].
In the last few decades there was a technological improvements in radiotherapy treatment with the introduction of new innovative techniques such as intensity-modulated radiotherapy (IMRT), Volumetric Modulated Arc Therapy (VMAT) and image-guided radiotherapy (IGRT)[19-21].
Using this innovative radiotherapy inverse planning techniques is possible to deliver a higher dose to the target avoiding surrounding tissue improving tumor response rate and disease control with the reduction of acute and late toxicity[22-26].
For these reasons in last years 3DCRT has been abandoned and replaced by new innovative techniques for the treatment of rectal cancer[22-57].
Different data in literature showed a benefit in terms of tumor downstaging and complete response rate of radiotherapy dose escalation, however there are not a lot of studies regarding the dose escalation using the innovative techniques such as IMRT, VMAT and IGRT for the treatment of LARC.
The aim of our review was to analyze the role of neoadjuvant radiotherapy dose escalation for the treatment of locally advanced rectal cancer, using innovative radiotherapy techniques.
In December 2020 we conducted a comprehensive literature search of the following electronic databases: PubMed, Web of Science, Scopus and Cochrane library. The databases research was made with a combination of following keywords: “neoadjuvant” AND “radiotherapy” AND “rectal” AND “cancer” in title and abstract fields of each databases research. The limit period of research included the articles published from January 2009 to December 2020.
We included in this review randomized trials, non-randomized trials, prospective studies, retrospective studies and case series in patients affected by rectal cancer underwent neodjuvant radiotherapy treatment (with or without chemotherapy), using a radiotherapy dose escalation and innovative technique. Single case reports and small case series with less than 10 cases were excluded. Moreover, we excluded studies reporting on patients with diagnoses different from rectal cancer, palliative treatment, if radiotherapy (± chemotherapy) was given with adjuvant intent or exclusive intent.
In case of duplicated datasets (e.g., multiple articles from the same study group or institution, related to the same treatment on the same cohort of patient), only the work with the longest follow-up and the greatest number of patients were included.
Screening by title and abstract was carried out to identify only studies using a total radiation equivalent dose 2 Gy fraction (EQD2) ≥ 54 Gy in patients affected by LARC underwent neoadjuvant radiotherapy.
For each study following exclusion criteria were applied: (1) Studies using 3DCRT radiotherapy delivering technique, brachytherapy or proton beam radiotherapy were excluded; (2) Studies of previously irradiated patients, or recurrent disease patients; (3) Studies that did not routinely schedule definitive surgery (i.e., palliative-intent or watch-and-wait strategies); (4) Studies using short-course regimen neoadjuvant radiotherapy; (5) Total dose of pelvic irradiation lower than standard EBRT dose (< 45 Gy); (6) Studies using brachytherapy boost; (7) Case report; (8) Case series with a number of patients < 10; (9) Review or letter to editor; and (10) Age of study population < 18 years old.
The inclusion criteria for each study were: (1) Clinical investigations using innovative radiotherapy technique such us IMRT, VMAT or IGRT; (2) Clinical investigations using long-course radiotherapy and a total dose of pelvic irradiation ≥ 45 Gy; (3) Clinical investigations with age of study population ≥ 18 years old; (4) Clinical investigations with radiotherapy dose escalation of EQD2 ≥ 54 (using SIB, concomitant or sequential RTE boost); and (5) Studies which radiotherapy treatment was given with neoadjuvant intent and routinely scheduled definitive surgery.
Data extraction was performed by one reviewer and checked by a second reviewer. Subsequently all papers obtained after database research were selected by two reviewers. All Screening process was performed according to PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses. In detail, the first screening was performed by title reading of each reviewer independently. The second screening was performed after abstract reading of each article by each reviewer. Finally, after the first and second selection by title and abstract reading, full text of all retrieved papers was reviewed suitable articles were selected for this review according to selection criteria established research process. After carefully selection of articles suitable for the review, we obtained the following information from each report: author identification, year of publication, medical center, study design characteristics, study population, number of patients, age, sex, histological diagnoses, radiotherapy treatment, total dose, dose for fraction, delivered dose, chemotherapy treatment, sphincter preservation rate, R0 resection rate, local control, post-surgical complications, anastomotic leakage, toxicity, grading scale of toxicity used for each study, and follow-up time. In Figure 1 is showed the flow chart of systematic literature search process according to PRISMA group guidelines. Late Radiation Morbidity Scoring Schema of the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer (RTOG/EORTC)[30] and NCI Common Terminology Criteria for Adverse Events (CTCAE) scale version 4 and 5 were used for description of late and acute toxicities[31,32].
All the data extracted from the selected review were processed in excel software (Microsoft office 2010 professional of © Microsoft company). At first, an exploratory phase of data was carried out; the categorical data were described by frequency and percentage, whereas continuous data by mean, median and range. If necessary, after data exploration, analysis and calculation of frequencies, median and range was performed due to description of end-points of the review.
All analyses were performed using excel software and the Statistical Package for Social Science (SPSS) version 22 technology.
The authors’ searches generated a total of 2287 results. Through a process of screening 21 publications fulfil selection criteria and were selected for the review. Figure 1 shows in detail the flowchart of the review literature search process.
Regarding the device used for radiotherapy treatment, in majority of studies (85.7%) the radiotherapy treatment was performed by a standard LINAC device and only in 3 studies (14.3%) radiotherapy treatment was performed with Tomotherapy machine.
The main radiotherapy technique used for the treatment of LARC consisted in VMAT technique (47.6%), followed by IMRT technique (38.1%) and in three studies were used both VMAT and IMRT techniques (14.3%).
Due to avoid target missing, organ motion and set-up errors, an IGRT modality for radiotherapy dose delivery was used in 74.12% of studies by a CBCT/MVCT (87.3%) or EPID (13.3%) image guided modalities. In reaming 25.88% studies set-up verify was performed weekly or twice a week according to protocol centre.
We found a heterogeneity regarding total dose and dose for fraction dose used between the studies analyzed for this review for treatment of LARG for both prophylactic to the pelvis and as a boost dose escalation.
The main dose for fraction used for the treatment of LARC was 55 Gy to high risk volume and 45 Gy as prophylactic volume in 25 fractions (42.85% of studies) delivered with SIB technique (Table 1).
| Ref. | Nr of pts | Mean age (range) | Prescription dose / number of fraction | CR rate (%) | Local control (%) | R0 rate (%) | Sphincter preservation rate (%) | Toxicity (%) | Surgery complications (%, any grade) | ||
| Boost dose | Prophylactic dose | G2 | G3 OR > | ||||||||
| Alongi et al[37], 2016 | 40 | 69 (47-83) | 60/30 | 54/30 | 17.5 | 100 | 100.0 | 92.5 | GI 15, GU 12.5 | 0 | 10.0 |
| Couwenberg et al[38], 2020 | 51 | 64.5 (55-69) | 50/25, + 15/5 | 50/25 | 35.9 | - | - | 56.3 | GI 20, GU 7.8, Sk 9.4 | GI 9.3, GU 1.6 | 26.4 |
| Bertocchi et al[39], 2020 | 31 | 68.7 (47-81) | 60/30 | 50.4/30 | 22.6 | - | 100.0 | 93.5 | GI 16.1, GU 19.3 | 0 | 41.9 |
| Engels et al[40], 2014 | 57 | 69 (32-85) | 55.2/23 | 46/23 | - | 97 | - | - | GI 12.2, GU 12.2, Sk 36.8 | 14 | -- |
| Hernando-Requejo et al[41], 2014 | 74 | 61.7 (33-80) | 57.6/23 | 46/23 | 30.6 | 100 | 97.2 | 77.7 | GI 28.4, GU 9.5, Sk 21.6 | GI 9.5, GU 5.4, Sk 2.7 | 25.7 |
| Zhu et al[42], 2014 | 78 | 54 (30-76) | 55/25 | 50/25 | 23.7 | 85.4 | 100.0 | 36.8 | GI 14.1, Sk 20.5 | GI 10.3, Sk 17.9 | 17.1 |
| Wang et al[43], 2019 | 60 | 56 (22-75) | 55/25 | 50/25 | 28.1 | 90.6 | 100.0 | 38.6 | / | 25 | 24.6 |
| Lima et al[44], 2019 | 11 | 45.9 (28-59) | 54/30 | 28.5 | - | - | - | 40 | 20 | - | |
| Jankarashvili et al[45], 2019 | 22 | 59 (36-84) | 57.5/23 | 46/23 | 59.1 | - | 100.0 | - | GI 40.9, GU 22.7, Sk 45.5 | GI 0GU 13.6, Sk 9.1 | - |
| Parikh et al[46], 2019 | 44 | 67 (47-84) | 55.8/31 | 40.9 | 93.2 | - | 100.0 | 6.8 | 0 | 43.0 | |
| Passoni et al[47], 2013 | 25 | 59 (37-77) | 27.6/12 + 18/6 | 41.4/18 | 30.0 | 100 | 96.0 | 87.0 | - | GI 12 | 26.0 |
| Picardi et al[48], 2016 | 18 | 62 (39-79) | 57.5/25 | 45/25 | 25.0 | 100 (1y), 68.6 (3y), 68.6 (5y) | 100.0 | 62.5 | - | 44.4 | - |
| Spatola et al[49], 2019 | 62 | 61.5 (36-84) | 45/25 + 9/6 | 45/25 | 19.0 | 96.5 | 100.0 | 85.0 | - | GI 10, GU 0, Sk 3 | 5.0 |
| Liu et al[50], 2020 | 85 | 80 (75-85) | 55/25 | 45-50/25 | 21.4 | 83.9 | 78.6 | - | GI 5.2, GU 1.8 | 12.5 | |
| Yamashita et al[51], 2017 | 60 | 66 (44-88) | 55/25 | 45/25 | 17.0 | 90 | 100.0 | 88.0 | GU 49 | 0 | 3.0 |
| Yang et al[52], 2019 | 26 | 55 (18-75) | 58.75/25 | 50/25 | 32.0 | 100.0 | 60.0 | GI 30.8, Sk 7.7 | Sk 7.7 | 8.0 | |
| Alsuhaibani et al[53], 2018 | 79 | 59.7 (28–102) | 55/25 | 45 | 20.0 | - | 100.0 | 72 ? | - | 0 | - |
| Chiloiro et al[54], 2019 | 22 | 64 (41–86) | 55/25 | 45 | 27.3 | - | 100.0 | 89.5 | GU 0, GI 40 | GI 22.7 | - |
| Lupattelli et al[55], 2016 | 60 | 64 (29–84) | 57-55-54/25 | 45 | 27.8 | - | 96.0 | 85.7 | - | 10.5 | 18.1 |
| Tey et al[56], 2017 | 20 | - | 55/25 | 45 | 35.0 | 100 | 95.0 | 85.0 | 0 | 5 | 0.0 |
| Zhao et al[57], 2017 | 141 | 59 (50–67) | 55/25 | 45-30 | 22.7 | 95.5 | 97.9 | 80.0 | - | GI 7.8 | 10.6 |
In detail, the radiotherapy EQD2 dose delivered as prophylactic doses to the pelvis varied in a range from 45-55.8 Gy (mean 48 and median 46 Gy) with a fractionation range from 1.8 to 3 Gy for fraction. High risk volume (T or N +) was treated with a total EQD2 dose (alfa/beta 10) from 54 Gy to 66.3 Gy (mean 56.4; median 55.9 Gy) and a calculated BED (alfa/beta 10) with a range wide from 63.7 Gy to 76.2 Gy (mean 70 Gy; median 67.1 Gy).
Table one shows treatment characteristics with respective outcomes for each study included in the review.
The complete response rate was reported in twenty studies selected (95.24%) and the mean pCR was 28.2%, with a range wide from 17% to 59%. In a regression analysis there was not any correlation between dose prescribed and pCR (P value = 0.234).
Regarding T down staging rate it was reported in seventeen of twenty-one studies analyzed (80.95%) with a mean of 66.96% (range 55%-97.7%). Lymph node down staging rate was described by ten authors and the mean of N down staging resulted 66.67%, with a rage wide from 10.7% to 94%.
In all 16 studies which reported R0 margins rate was observed an excellent negative margins rate with a mean of 98.88% (range 95%-100%).
Finally, sixteen of twenty-one analyzed studies described sphincter preservation an the mean rate was 76.03% with a range wide from 36.8% to 100%. There was not any correlation between down staging, R0, sphincter preservation and total EQD2 dose prescribed to both prophylactic pelvis and as a boost dose escalation to high risk volumes in regression analyses (P value > 0.05).
Unfortunately survival rates often were not endpoints of studies and for this reasons were analyzed only by few authors. Local control was reported by thirteen authors (61.9%) and resulted 92.29% (range 68.6%-100%) after a mean follow-up of 35 mo. As expected we found lower rates of PFS (mean 74.16%; range 57-100) and was described by 13 authors.
Unfortunately, OS was reported by only 7 authors (33%) and three years median OS resulted 83.82% with a range wide from 68% to 100%; CSS was described only from three authors and three years median CSS resulted 96.66%.
There was not any correlation between calculated EQD2 dose prescription and survival outcomes (LC, PFS OS, and CSS) in regression analyses (P value > 0,05 ).
All studies reported the rates of acute toxicity which consisted in GU, GI or skin toxicity. According to RTOG/CTCAE scale the mean ≥ G3 toxicity was 11.06% (range 0-44%). The mean G2 toxicity resulted 27.08% with a range wide from 6.8% to 49%. There was not any correlation between dose prescription and toxicities.
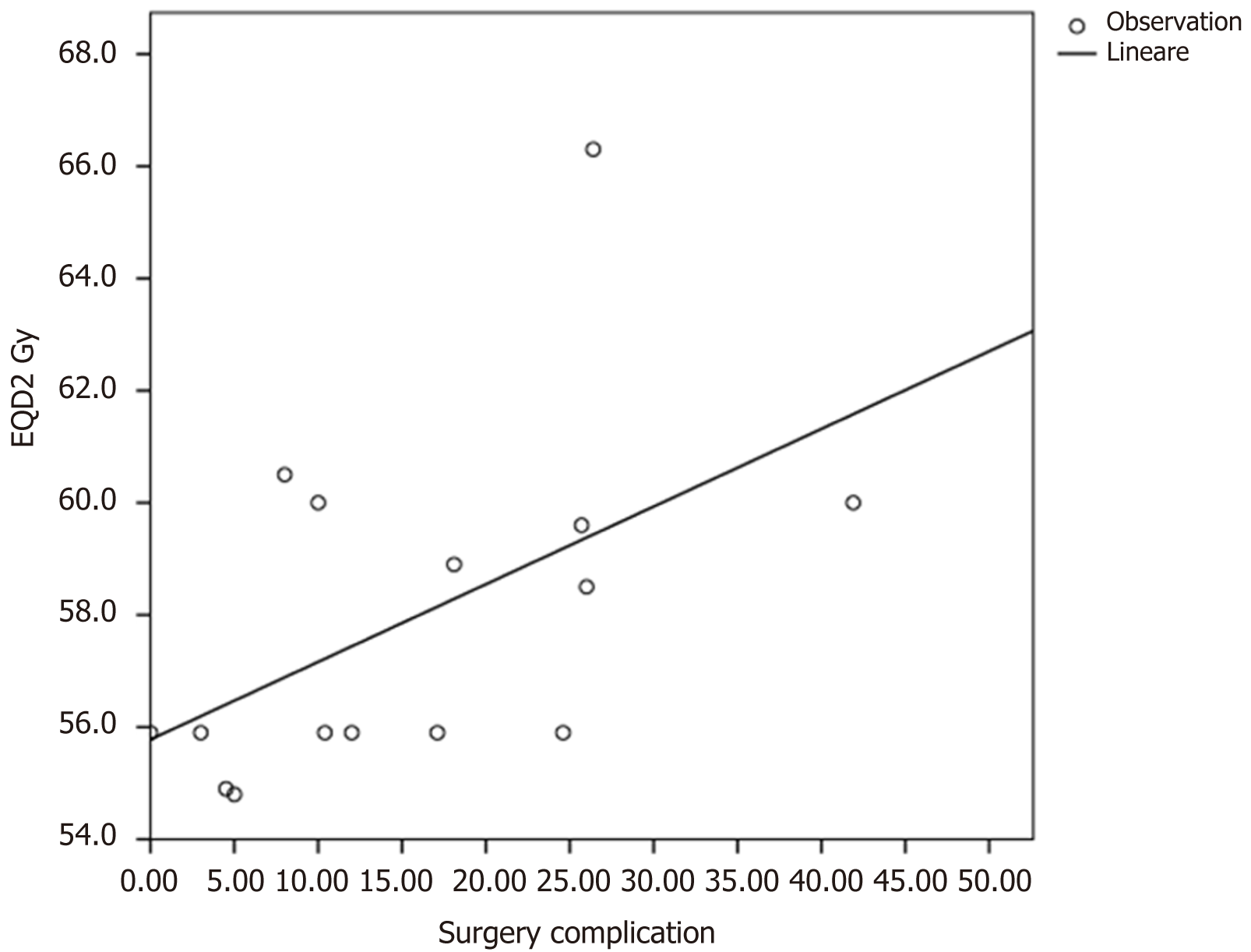
Overall the surgical complications and anastomotic leakage were described by fifteen authors. The mean surgical complications rate was 15.51% with a range wide from 0% to 41.9%. In patients receiving a EQD2 doses < 58.9 Gy with a BED < 70.7 Gy the surgical complications rate were lower (mean 12.01%) compared to patients receiving a EQD2 dose > 58.9 Gy with a BED > 70.7 (mean 22.4%). This differences resulted statistically significant in a linear regression analyses (P value = 0.047) (Figure 2).
Finally, the mean of anastomotic leakage or fistula was 4.26 % with a range wide from 0% to 29%. There was not any correlation between dose prescription and anastomotic leakage at the regression analysis (P value = 0.354).
To our knowledge this is the first review that systematically examine outcomes of LARC underwent neoadjuvant radiotherapy dose escalation with inverse-planning modality and innovative radiotherapy technique in new era of radiotherapy.
The benefit of achievement a pathological complete response in both disease-free and OS has been demonstrated[21-23].
The results of our review showed a high rates of pCR (28.2%), tumor down staging (66.96%) and R0 margins rate using a dose escalation EQD2 (alfa/beta 10 Gy) ≥ 54 Gy with innovative radiotherapy techniques and inverse planning modality.
It is known that in previously controlled trial the pCR rate of standard neoadjuvant RT-CT for the treatment of LARC is approximating 15%[2-11].
This outcomes are higher even compared to previously reviews and meta-analysis in patients with LARC underwent neoadjuvant and dose escalation radiotherapy with 3DCRT techniques.
In fact, in a previous systematic review and meta-analysis in 2014 Burbach et al[24] reported a pCR of 20.4% in studies using 3DCRT technique and escalating dose to ≥ 60 Gy. Unfortunately, authors did not report the R0 resection rate and sphincter preservation. Resectability rate and pooled acute grade 3 toxicity were 89.5% and 10.3%, respectively.
An interesting systematic review and meta-analysis was performed recently by Hearn et al[25] which analyzed dose escalation for the treatment of neoadjuvant LARC, screening studies for radiotherapy prescription dose > 54 Gy. In this meta-analysis authors reported a pool estimated of pCR of 24.1% in all studies and 25.7% in inverse planning studies without a statistically differences between techniques. Moreover, as reported in results of our review, authors did not found any factor significantly related with pCR rates in regression analysis.
Nevertheless, pCR rates using dose escalation in our review and results of above reviews are significantly higher compared to standard neoadjuvant RT-CT doses for the treatment of LARC[2-11].
Different authors analyzed factors improving pCR in patients with LARC underwent neoadjuvant RT-CT.
Appelt et al[36] described, in a predictive model study, a highly significant dose-response relationship for pCR after neoadjuant external-beam radiation therapy and brachytherapy in locally advanced rectal cancer for tumor dose levels in the range of 50.4-70 Gy. A correlation between increasing rates of pCR with escalating radiotherapy doses was confirmed in a recent meta-analysis by Teo et al[26] in a phase 2 neoadjuvant treatment intensification trials.
Furthermore, some authors reported a correlation between pCR and extension of surgical interval. The evidence for longer surgical intervals evolved in last years and often is recommended a minimal surgical interval of 8 wk after neoadjuvant RT-CT treatment[27,28,29]. However, in GRECCAR-6 randomized multicenter trial 265 patients were treated with standard nCRT and underwent surgical treatment. There was not a benefit regarding pCR rate and surgical interval between to arms (7 wk vs 11 wk)[29].
The data in literature regarding a correlation between chemotherapy escalation and pCR are controversial due to some authors described a benefit improved pCR rates with the addition of concurrent oxaliplatin and/or bevacizumab compared with fluoropyrimidine treatments alone[33,34] and other studies did not show any benefit in terms of pCR, even reporting higher incidences of acute toxicity[33].
According to RTOG/CTCAE scale in our review the mean ≥ G3 toxicity was 11.06% and, differently of Hearn et al[25] review, we did not find any correlation between EQD2 (alpha/beta 10 Gy) dose prescription and ≥ G3 toxicities.
These data in literature support that the use of innovative techniques and inverse-planning techniques lead to delivery an higher dose to tumour avoiding the dose to surrounding tissue with the reduction of acute and late toxicity. For these reasons in last years a moderately dose-escalated treatment with inverse-planning techniques is often used and reported by different authors with higher pCR and acceptable acute and late toxicities[48-56].
Additionally, a real benefit of radiotherapy dose escalation with innovative technique achieving pCR can be considered in patients with low, very-low rectal cancer candidate to organ preservation with watch and wait strategy due to avoid definitive stoma. This benefit can be extended to cases of low rectal distal T2N0 disease, where minimally invasive surgical techniques may be viable to reduce procedural complications and improve sphincter preservation as reported by INTERACT protocol[35].
In our review R0 rates were higher (98.88%) in confront of Hearn et al[25] results (90.7%). This finding can be explain because we included only studies which used inverse-planning technique, in fact, in Hearn et al[25] meta-analyses was reported a significantly positively correlation between the use of inverse planning techniques and R0 rate in univariate regression analysis.
Moreover, we found a surgical complications rate comparable with other tails in literature which used standard doses of RT treatment (mean 15.51%) with a range wide from 0% to 41.9%. Some authors described an increase surgical complications with radiotherapy boost[39,43,46]. However, the data were heterogeneous due to radiotherapy techniques used, total dose prescription, dose for fractions and kind of concomitant chemotherapy used. In a regression analyses we found an higher rate surgical complications in patients receiving a EQD2 doses > 58.9 Gy with a BED > 70.7 (median 22.4%) compared to patients receiving a EQD2 doses < 58.9 Gy with a BED < 70.7 Gy (mean 12.01%).
Nevertheless, more data with randomized trials are needed in order to clarify and identify the optimal EQD2 dose escalation and surgical complication in LARC. In fact, the data reported in literature are often confounding due to retrospective analysis, complications are described as anastomotic leak, often are second end points of the studies and are not described by authors at all.
Finally, the mean of anastomotic leakage or fistula was 4.26%, as reported in previously in different data in literature. There was not any correlation between dose prescription and anastomotic leakage at the regression analysis (P value = 0.354).
Unfortunately, with the limitation of a mean follow-up of 35 mo, survival rates often were not described by authors and we did not found any correlation between survival rates, dose prescription and pCR (P value > 0.05 ).
Additionally, a lot of other authors have questioned the importance of achieving pCR in the context of survival and other treatment outcomes[17,23].
Gunther et al[14] analyzed 76 patients receiving modestly escalated treatment (52.5 Gy vs 45 Gy), and reported higher 10-year PFS rates (71.9% vs 57.6%, P value < 0.01) and OS rates (71.6% vs 62.4%, P value < 0.01) despite similar pCR rates between groups.
Otherwise, Hearn et al[25] in a systematic review and meta-analyses did not identified a survival benefit with radiotherapy dose escalation.
We encourage authors to describe and analyze and survival rates in patient affected by LARC treated with neoadjuvant dose escalation radiotherapy.
The limitation of our review consist on low number of patients analyzed for each study, the majority of eligible trials included in our review consisted in retrospective studies, shorter follow-up, heterogeneity between studies regarding modality and imaging used for definition of gross tumor volumes, different schedules of concomitant chemotherapy used and surgical intervals used in different studies.
Based on results of our review, with above mentioned limitations, we believe that neoadjuvant radiotherapy dose escalation using innovative techniques with inverse planning modality will be the standard for the treatment pf LARC in a prospective future, especially in low-very low rectal cancer patients candidate of sphincter preservation and/or “watch and wait strategy”.
Based on our systematic review results, neoadjuvant radiotherapy dose escalation using innovative techniques with inverse planning modality is safe with acceptable acute toxicity achieving higher pCR compared to standard treatment of locally advanced rectal cancer. EQD2 doses > 58.9 Gy with a BED > 70.7 given with SIB technique seems to be associated with higher rate of surgical complications.
Finally, a real benefit in achieving higher pCR rates can be essential in patients with LARC candidate to organ preservation with “watch and wait” strategy, patients with low rectal cancer reaching R0 margins and patients with low-very low rectal cancer candidate to definitive stoma or sphincter preservation.
Preoperative radiochemotherapy had an important role in locally advanced rectal cancers (LARC) improving local and disease control. A benefit on tumor downstaging and pathological complete response (pCR) rate was reported by authors using radiotherapy dose escalation.
Considering the progress of radiation therapy in last decades we decided to analyzed the role of neoadjuvant radiotherapy dose escalation for LARC using innovative radiotherapy techniques such as VMAT, intensity-modulated radiotherapy (IMRT) or image-guided radiotherapy (IGRT).
To evaluate clinical outcomes and toxicity for neoadjuvant radiotherapy dose escalation using innovative radiotherapy techniques.
In December 2020 we conducted a comprehensive literature search of the following electronic databases: PubMed, Web of Science, Scopus and Cochrane library. According to PRISMA Group (2009) screening process only studies using radiation doses EQD2 ≥ 54 Gy and VMAT, IMRT or IGRT techniques were analyzed included for the review.
At the analyses we found high pCR rates (28.2%), local control (92.29%), R0 margins (98.88%) and sphincter preservation rates (76.03%).
Patients receiving EQD2 dose > 58.9 Gy and BED > 70.7 Gy had higher surgical complications rates compared to other group (P value = 0.047). G3 or higher toxicity was 11.06 % with no correlation between dose prescription and toxicities.
We believe that dose escalation neoadjuvant radiotherapy using innovative techniques is safe for LARC and can be considered the standard radiotherapy treatment in a future perspective.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin Q S-Editor: Wang LL L-Editor: A P-Editor: Zhang YL
| 1. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3269] [Article Influence: 653.8] [Reference Citation Analysis (2)] |
| 2. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1195] [Article Influence: 149.4] [Reference Citation Analysis (0)] |
| 3. | Clinical Practice Guidelines in Oncology (NCCN Guidelines), Rectal Cancer. Version 1 2021. [cited 20 February 2021]. Available from: https://www.nccn.org/professionals/physician_gls/PDF/rectal.pdf. |
| 4. | Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1818] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 5. | Chen ET, Mohiuddin M, Brodovsky H, Fishbein G, Marks G. Downstaging of advanced rectal cancer following combined preoperative chemotherapy and high dose radiation. Int J Radiat Oncol Biol Phys. 1994;30:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 131] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Meade PG, Blatchford GJ, Thorson AG, Christensen MA, Ternent CA. Preoperative chemoradiation downstages locally advanced ultrasound-staged rectal cancer. Am J Surg. 1995;170:609-612; discussion 612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Mohiuddin M, Regine WF, John WJ, Hagihara PF, McGrath PC, Kenady DE, Marks G. Preoperative chemoradiation in fixed distal rectal cancer: dose time factors for pathological complete response. Int J Radiat Oncol Biol Phys. 2000;46:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 143] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Rouanet P, Saint-Aubert B, Lemanski C, Senesse P, Gourgou S, Quenet F, Ycholu M, Kramar A, Dubois J. Restorative and nonrestorative surgery for low rectal cancer after high-dose radiation: long-term oncologic and functional results. Dis Colon Rectum. 2002;45:305-313; discussion 313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Chao M, Gibbs P, Tjandra J, Cullinan M, McLaughlin S, Faragher I, Skinner I, Jones I. Preoperative chemotherapy and radiotherapy for locally advanced rectal cancer. ANZ J Surg. 2005;75:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, Miccichè F, Ricci R, Morganti AG, Gambacorta MA, Maurizi F, Coco C. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:752-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 330] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 11. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4462] [Article Influence: 212.5] [Reference Citation Analysis (1)] |
| 12. | Movsas B, Diratzouian H, Hanlon A, Cooper H, Freedman G, Konski A, Sigurdson E, Hoffman J, Meropol NJ, Weiner LM, Coia L, Lanciano R, Stein J, Kister D, Eisenberg B. Phase II trial of preoperative chemoradiation with a hyperfractionated radiation boost in locally advanced rectal cancer. Am J Clin Oncol. 2006;29:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Jakobsen A, Mortensen JP, Bisgaard C, Lindebjerg J, Hansen JW, Rafaelsen SR. Preoperative chemoradiation of locally advanced T3 rectal cancer combined with an endorectal boost. Int J Radiat Oncol Biol Phys. 2006;64:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Gunther JR, Chadha AS, Shin US, Park IJ, Kattepogu KV, Grant JD, Weksberg DC, Eng C, Kopetz SE, Das P, Delclos ME, Kaur H, Maru DM, Skibber JM, Rodriguez-Bigas MA, You YN, Krishnan S, Chang GJ. Preoperative radiation dose escalation for rectal cancer using a concomitant boost strategy improves tumor downstaging without increasing toxicity: A matched-pair analysis. Adv Radiat Oncol. 2017;2:455-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 474] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 16. | Arias F, Eito C, Asín G, Mora I, Cambra K, Mañeru F, Ibáñez B, Arbea L, Viudez A, Hernández I, Arrarás JI, Errasti M, Barrado M, Campo M, Visus I, Flamarique S, Ciga MA. Fecal incontinence and radiation dose on anal sphincter in patients with locally advanced rectal cancer (LARC) treated with preoperative chemoradiotherapy: a retrospective, single-institutional study. Clin Transl Oncol. 2017;19:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Gentile M, Bucci L, Cerbone D, D'Antonio D, Guarino V. Evaluation of DOWNSTAGING as leading concept in sphincter-saving surgery for rectal cancer after preoperative radio-chemotherapy (Preop RCT). Ann Ital Chir. 2003;74:555-558. [PubMed] |
| 18. | Appelt AL, Vogelius IR, Pløen J, Rafaelsen SR, Lindebjerg J, Havelund BM, Bentzen SM, Jakobsen A. Long-term results of a randomized trial in locally advanced rectal cancer: no benefit from adding a brachytherapy boost. Int J Radiat Oncol Biol Phys. 2014;90:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Zhao J, Hu W, Cai G, Wang J, Xie J, Peng J, Zhang Z. Dosimetric comparisons of VMAT, IMRT and 3DCRT for locally advanced rectal cancer with simultaneous integrated boost. Oncotarget. 2016;7:6345-6351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Pollard JM, Wen Z, Sadagopan R, Wang J, Ibbott GS. The future of image-guided radiotherapy will be MR guided. Br J Radiol. 2017;90:20160667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 21. | Freedman GM, Meropol NJ, Sigurdson ER, Hoffman J, Callahan E, Price R, Cheng J, Cohen S, Lewis N, Watkins-Bruner D, Rogatko A, Konski A. Phase I trial of preoperative hypofractionated intensity-modulated radiotherapy with incorporated boost and oral capecitabine in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2007;67:1389-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Kim MJ, Jeong SY, Park JW, Ryoo SB, Cho SS, Lee KY, Park KJ. Oncologic Outcomes in Patients Who Undergo Neoadjuvant Chemoradiotherapy and Total Mesorectal Excision for Locally Advanced Rectal Cancer: A 14-Year Experience in a Single Institution. Ann Coloproctol. 2019;35:83-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Zorcolo L, Rosman AS, Restivo A, Pisano M, Nigri GR, Fancellu A, Melis M. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol. 2012;19:2822-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Burbach JP, den Harder AM, Intven M, van Vulpen M, Verkooijen HM, Reerink O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;113:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Hearn N, Atwell D, Cahill K, Elks J, Vignarajah D, Lagopoulos J, Min M. Neoadjuvant Radiotherapy Dose Escalation in Locally Advanced Rectal Cancer: a Systematic Review and Meta-analysis of Modern Treatment Approaches and Outcomes. Clin Oncol (R Coll Radiol). 2021;33:e1-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Teo MTW, McParland L, Appelt AL, Sebag-Montefiore D. Phase 2 Neoadjuvant Treatment Intensification Trials in Rectal Cancer: A Systematic Review. Int J Radiat Oncol Biol Phys. 2018;100:146-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann Surg. 2016;263:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 28. | Ryan ÉJ, O'Sullivan DP, Kelly ME, Syed AZ, Neary PC, O'Connell PR, Kavanagh DO, Winter DC, O'Riordan JM. Meta-analysis of the effect of extending the interval after long-course chemoradiotherapy before surgery in locally advanced rectal cancer. Br J Surg. 2019;106:1298-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. |
Lefevre JH, Mineur L, Cachanado M, Rullier E, Rouanet P, De Chaisemartin C, et al Does a longer waiting period after neoadjuvant radiochemotherapy improve the oncological prognosis of rectal cancer?: three-year follow-up results of the GRECCAR-6 randomized multicenter trial.
|
| 30. | Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3186] [Cited by in RCA: 3508] [Article Influence: 116.9] [Reference Citation Analysis (0)] |
| 31. | CTCAE v.4.0 NIH Publication No. 09-5410. [cited 20 February 2021]. Available from: https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5x7.pdf. |
| 32. | CTCAE v.5.0 NIH Publication – November 27, 2017. [cited 20 February 2021]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. |
| 33. | Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, Hofheinz RD, Ghadimi M, Wolff HA, Lang-Welzenbach M, Raab HR, Wittekind C, Ströbel P, Staib L, Wilhelm M, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R, Liersch T; German Rectal Cancer Study Group. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 536] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 34. | Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Peng J, Ren D, Wang J. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol. 2016;34:3300-3307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 35. | Valentini V, Gambacorta MA, Cellini F, Aristei C, Coco C, Barbaro B, Alfieri S, D'Ugo D, Persiani R, Deodato F, Crucitti A, Lupattelli M, Mantello G, Navarria F, Belluco C, Buonadonna A, Boso C, Lonardi S, Caravatta L, Barba MC, Vecchio FM, Maranzano E, Genovesi D, Doglietto GB, Morganti AG, La Torre G, Pucciarelli S, De Paoli A. The INTERACT Trial: Long-term results of a randomised trial on preoperative capecitabine-based radiochemotherapy intensified by concomitant boost or oxaliplatin, for cT2 (distal)-cT3 rectal cancer. Radiother Oncol. 2019;134:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 36. | Appelt AL, Pløen J, Harling H, Jensen FS, Jensen LH, Jørgensen JC, Lindebjerg J, Rafaelsen SR, Jakobsen A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16:919-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 402] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 37. | Alongi F, Fersino S, Mazzola R, Fiorentino A, Giaj-Levra N, Ricchetti F, Ruggieri R, Di Paola G, Cirillo M, Gori S, Salgarello M, Zamboni G, Ruffo G. Radiation dose intensification in pre-operative chemo-radiotherapy for locally advanced rectal cancer. Clin Transl Oncol. 2017;19:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Couwenberg AM, Burbach JPM, Berbee M, Lacle MM, Arensman R, Raicu MG, Wessels FJ, Verdult J, Roodhart J, Reerink O, Hoendervangers S, Buijsen J, Grabsch HI, Pronk A, Consten ECJ, Smits AB, Heikens JT, Appelt AL, van Grevenstein WMU, Verkooijen HM, Intven MPW. Efficacy of Dose-Escalated Chemoradiation on Complete Tumor Response in Patients with Locally Advanced Rectal Cancer (RECTAL-BOOST): A Phase 2 Randomized Controlled Trial. Int J Radiat Oncol Biol Phys. 2020;108:1008-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 39. | Bertocchi E, Barugola G, Nicosia L, Mazzola R, Ricchetti F, Dell'Abate P, Alongi F, Ruffo G. A comparative analysis between radiation dose intensification and conventional fractionation in neoadjuvant locally advanced rectal cancer: a monocentric prospective observational study. Radiol Med. 2020;125:990-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 40. | Engels B, Platteaux N, Van den Begin R, Gevaert T, Sermeus A, Storme G, Verellen D, De Ridder M. Preoperative intensity-modulated and image-guided radiotherapy with a simultaneous integrated boost in locally advanced rectal cancer: report on late toxicity and outcome. Radiother Oncol. 2014;110:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Hernando-Requejo O, López M, Cubillo A, Rodriguez A, Ciervide R, Valero J, Sánchez E, Garcia-Aranda M, Rodriguez J, Potdevin G, Rubio C. Complete pathological responses in locally advanced rectal cancer after preoperative IMRT and integrated-boost chemoradiation. Strahlenther Onkol. 2014;190:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Zhu J, Liu F, Gu W, Lian P, Sheng W, Xu J, Cai G, Shi D, Cai S, Zhang Z. Concomitant boost IMRT-based neoadjuvant chemoradiotherapy for clinical stage II/III rectal adenocarcinoma: results of a phase II study. Radiat Oncol. 2014;9:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Wang J, Guan Y, Gu W, Yan S, Zhou J, Huang D, Tong T, Li C, Cai S, Zhang Z, Zhu J. Long-course neoadjuvant chemoradiotherapy with versus without a concomitant boost in locally advanced rectal cancer: a randomized, multicenter, phase II trial (FDRT-002). Radiat Oncol. 2019;14:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Lima MA, Moraes ED, Saito EY, Barros DG, Malta PSA, Alves IM, Oliva A. Assessment of the non-surgical treatment of patients with rectal cancer who underwent neoadjuvant treatment with chemotherapy and radiotherapy at the oncology department. J coloproctol (rio j). 2019;39:127-131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Jankarashvili N, Kakhadze S, Topeshashvili M, Turkiasvili L, Tchiabrishvili M. Neoadjuvant volumetric modulated arc radiochemotherapy with a simultaneous integrated boost technique compared to standard chemoradiation for locally advanced rectal cancer. Turk J Med Sci. 2019;49:1484-1489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Parikh K, DeNittis AS, Marks G, Zeger E, Cho D, Marks J. Neoadjuvant chemotherapy and high-dose radiation using intensity-modulated radiotherapy followed by rectal sparing TEM for distal rectal cancer. J Radiat Oncol. 2019;8:217-224. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 47. | Passoni P, Fiorino C, Slim N, Ronzoni M, Ricci V, Di Palo S, De Nardi P, Orsenigo E, Tamburini A, De Cobelli F, Losio C, Iacovelli NA, Broggi S, Staudacher C, Calandrino R, Di Muzio N. Feasibility of an adaptive strategy in preoperative radiochemotherapy for rectal cancer with image-guided tomotherapy: boosting the dose to the shrinking tumor. Int J Radiat Oncol Biol Phys. 2013;87:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Picardi V, Deodato F, Guido A, Giaccherini L, Macchia G, Gambacorta MA, Arcelli A, Farioli A, Cellini F, Cuicchi D, DI Fabio F, Poggioli G, Ardizzoni A, Frezza G, Cilla S, Caravatta L, Valentini V, Fuccio L, Morganti AG. Concurrent Chemoradiation with Concomitant Boost in Locally Advanced Rectal Cancer: A Phase II Study. Anticancer Res. 2016;36:4081-4087. [PubMed] |
| 49. | Spatola C, Raffaele L, Tocco A, Acquaviva G, Milazzotto R, Bevilacqua R, Acquaviva G, Salamone V. Intensified neoadjuvant radio-chemotherapy for locally advanced rectal cancer: mono-istitutional experience and long-term results. Int J Radiat Res. 2019;17:265-273. [DOI] [Full Text] |
| 50. | Liu X, Wang J, Hu K, Zhang F, Hou X, Xiao Y, Lian X, Sun S, Liu Z, Yan J, Miao Z. Neoadjuvant chemoradiotherapy or radiotherapy in patients aged 75 years or older with locally advanced rectal cancer. J Cancer. 2020;11:3536-3542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Yamashita H, Ishihara S, Nozawa H, Kawai K, Kiyomatsu T, Okuma K, Abe O, Watanabe T, Nakagawa K. Comparison of volumetric-modulated arc therapy using simultaneous integrated boosts (SIB-VMAT) of 45 Gy/55 Gy in 25 fractions with conventional radiotherapy in preoperative chemoradiation for rectal cancers: a propensity score case-matched analysis. Radiat Oncol. 2017;12:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Yang Y, Liu Q, Jia B, Du X, Dai G, Liu H, Chen J, Zeng M, Wen K, Zhu Y, Wang Y, Feng L. Preoperative Volumetric Modulated Arc Therapy With Simultaneous Integrated Boost for Locally Advanced Distal Rectal Cancer. Technol Cancer Res Treat. 2019;18:1533033818824367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Alsuhaibani A, Elashwah A, Mahmood R, Abduljabbar A, Alhomoud S, Ashari L, Bazarbashi S, Aljubran A, Alzahrani A, Mohiuddin M, Almanea H, Alhussaini H, AlSanea N. Dose Escalation with Simultaneous Integrated Boost (SIB) Using Volumetric Modulated Arc Therapy (VMAT) in Rectal Cancer. J Gastrointest Cancer. 2018;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 54. | Chiloiro G, Boldrini L, Meldolesi E, Re A, Cellini F, Cusumano D, Corvari B, Mantini G, Balducci M, Valentini V, Gambacorta MA. MR-guided radiotherapy in rectal cancer: First clinical experience of an innovative technology. Clin Transl Radiat Oncol. 2019;18:80-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 55. | Lupattelli M, Matrone F, Gambacorta MA, Osti M, Macchia G, Palazzari E, Nicosia L, Navarria F, Chiloiro G, Valentini V, Aristei C, De Paoli A. Preoperative intensity-modulated radiotherapy with a simultaneous integrated boost combined with Capecitabine in locally advanced rectal cancer: short-term results of a multicentric study. Radiat Oncol. 2017;12:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Tey J, Leong CN, Cheong WK, Sze TG, Yong WP, Keong Tham IW. A phase II trial of preoperative concurrent chemotherapy and dose escalated intensity modulated radiotherapy (IMRT) for locally advanced rectal cancer. J Cancer. 2017;8:3114-3121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Zhao J, Liu X, Wang W, Hu K, Zhang F, Hou X. Concomitant dose escalation with image-guided tomotherapy in locally advanced midelow rectal cancer: a single-center study. Cancer Manag Res. 2019;11:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |