Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8852
Peer-review started: May 18, 2021
First decision: June 15, 2021
Revised: June 22, 2021
Accepted: August 9, 2021
Article in press: August 9, 2021
Published online: October 16, 2021
Processing time: 150 Days and 7.4 Hours
Patients with Becker muscular dystrophy (BMD) have a high risk of developing hyperkalemia, rhabdomyolysis, and malignant hyperthermia when exposed to volatile anesthetics and depolarizing muscle relaxants. Patients with BMD are also prone to respiratory depression after general anesthesia. Thus, it is extremely challenging for anesthesiologists to manage anesthesia in BMD patients, particularly in pediatric BMD patients. Here, we present successful anesthesia management using transversus abdominis plane block (TAPB) combined with total intravenous anesthesia (TIVA) in a pediatric BMD patient undergoing laparoscopic inguinal hernia repair.
A 2-year-old boy, weighing 15 kg, with BMD, was scheduled for laparoscopic inguinal hernia repair. TIVA was used for induction, and continuous infusions of short-acting intravenous anesthetics combined with TAPB were performed for anesthesia maintenance. Moreover, TAPB provided good postoperative analgesia. The patient underwent uneventful surgery and anesthesia, and over the 17 mo follow-up period showed no anesthesia-induced complications.
TAPB combined with TIVA, using short-acting intravenous anesthetic agents, can provide safe and effective anesthesia management in pediatric BMD patients undergoing short-term abdominal surgery.
Core Tip: Becker muscular dystrophy (BMD) is a rare disease induced by genetic mutations. Anesthesia management is extremely challenging in patients with BMD, as they are at high risk of developing hyperkalemia, rhabdomyolysis, or malignant hyperthermia. The mortality rate is very high in patients with these complications. In this report, we describe the use of total intravenous anesthesia combined with transversus abdominis plane block for laparoscopic inguinal hernia repair in a pediatric patient with BMD. This anesthesia technique is considered a safe and effective strategy for pediatric BMD patients. Our pediatric BMD patient achieved a good outcome.
- Citation: Peng L, Wei W. Anesthesia management in a pediatric patient with Becker muscular dystrophy undergoing laparoscopic surgery: A case report. World J Clin Cases 2021; 9(29): 8852-8857
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8852.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8852
Becker muscular dystrophy (BMD) is an X-linked autosomal recessive disease resulting in partial absence or abnormality of dystrophin[1]. It is a rare disease with an incidence of approximately 1 in 18000 live male births[2]. Although the progression of BMD is slow, the disease eventually results in dilated cardiomyopathy or respiratory failure that may not be consistent with muscular weakness[3]. Patients with BMD have a high risk of hyperkalemia, rhabdomyolysis, and malignant hyperthermia when they are exposed to volatile anesthetics and depolarizing muscle relaxants[4]. Malignant hyperthermia is a severe anesthesia-related complication with a poor prognosis and high mortality rate. Therefore, it is highly challenging for anesthesiologists to manage anesthesia in BMD patients, particularly in pediatric patients. Herein, we present successful anesthesia management using transversus abdominis plane block (TAPB) combined with total intravenous anesthesia (TIVA) in a pediatric BMD patient undergoing laparoscopic inguinal hernia repair to provide experience regarding anesthesia management in BMD patients.
A 2-year-old boy, weighing 15 kg and 93 cm in height, was hospitalized due to a left inguinal hernia (Figure 1A).
The patient developed a reducible mass in the left groin area after crying at the age of 1 year.
The child was diagnosed with BMD at the age of 18 mo based on genetic testing, which showed a deletion of exon 45-47 in the Duchenne muscular dystrophy (DMD) gene.
No data were available.
Physical examination revealed that the patient was in good general condition, except for congenital malformations of the fingers (Figure 1B) and toes (Figure 1C).
Laboratory tests showed elevated levels of creatine kinase (CK, 4891 IU/L), lactate dehydrogenase (LDH, 745 IU/L), and hydroxybutyrate dehydrogenase (HBDH, 668 IU/L).
Preoperative electrocardiogram, transthoracic echocardiography, and chest X-ray were normal. Electromyography suggested possible myogenic damage in bilateral musculus biceps brachii.
The patient was diagnosed with inguinal hernia combined with BMD.
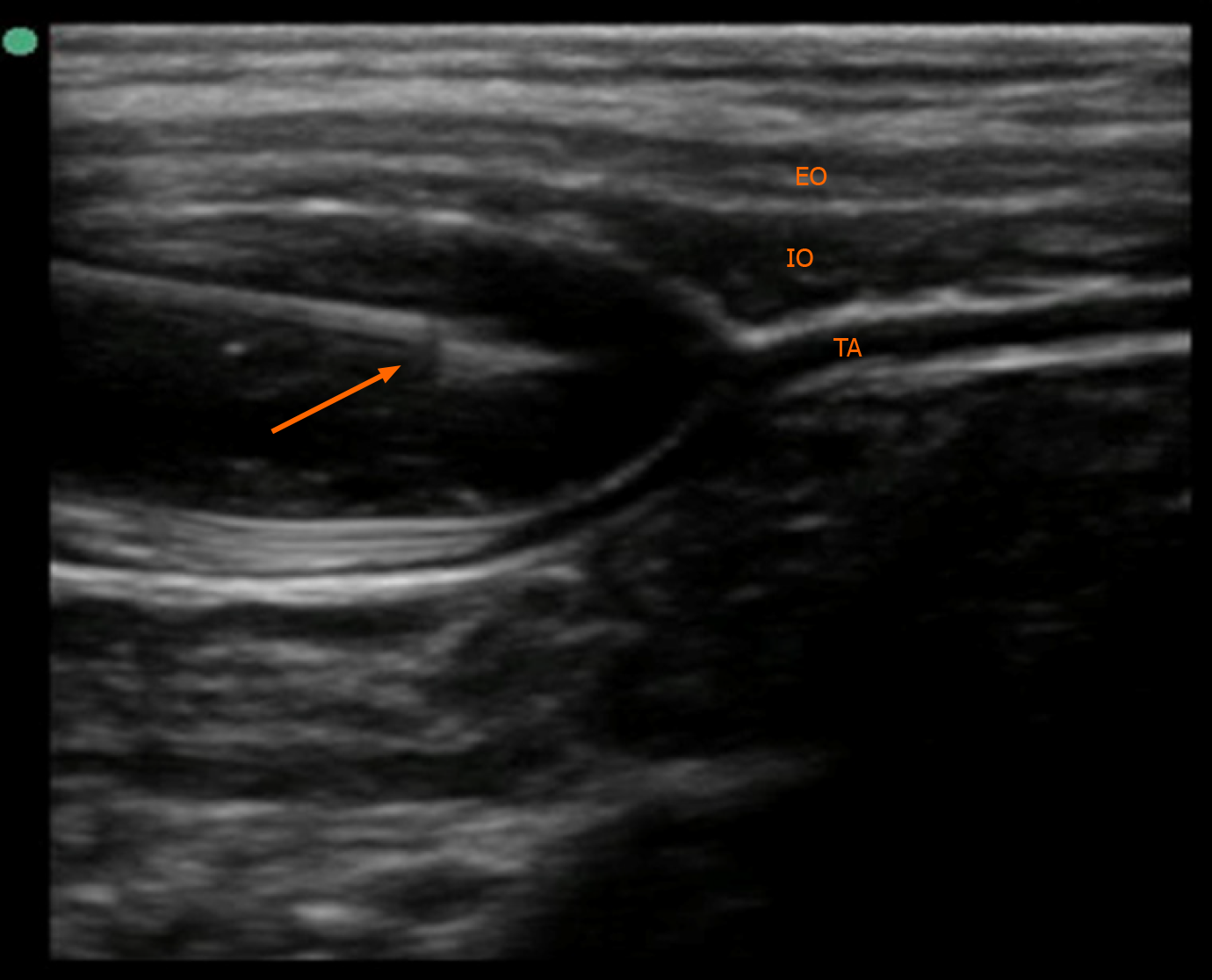
The patient was scheduled for laparoscopic inguinal hernia repair. Before surgery, we prepared an anesthesia machine without recent use of volatile anesthetics. The machine was also flushed with fresh oxygen for approximately 30 min. Dantrolene was also prepared for emergency administration. Following admission to the operating room, the patient was monitored using non-invasive blood pressure measurement, electrocardiogram, and pulse oxygen saturation. Peripheral venous access was previously established in the ward. General anesthesia was induced with 0.15 mg atropine, 0.5 mg midazolam, 45 mg propofol, 30 μg fentanyl, and 1 mg cisatracurium besylate. A cuffed endotracheal tube 4.5 mm in size was inserted using a video laryngoscope for mechanical ventilation. At 13 cm H2O inspiratory pressure, 8-10 mL/kg of tidal volume could be achieved. End-tidal carbon dioxide (EtCO2) and nasopharynx temperature were continuously monitored. Ventilator parameters were adjusted to maintain EtCO2 in the normal range. Bilateral TAPB with 0.25% ropivacaine 6 mL under ultrasound guidance was then performed (Figure 2). The TAPB can provide analgesia for iliac and inguinal regions for approximately 6 h. Anesthesia was maintained with 2-4 mg/kg/h propofol and 0.05-0.1 μg/kg/min remifentanil with an oxygen-air mixture. During the operation, the patient’s heart rate ranged between 100 and 130 bpm, while the mean blood pressure was between 60 and 80 mmHg. The EtCO2 fluctuated between 35 and 45 mmHg, and the nasopharyngeal temperature was 36.4-36.6 °C. The operation lasted approximately 20 min. The patient recovered spontaneous breathing 10 min after cessation of anesthetics infusion, and then 0.2 mg neostigmine combined with 0.1 mg atropine was administered to reverse cisatracurium besylate-induced neuromuscular block. After 5 min of neostigmine administration, the patient’s tidal volume increased to approximately 6-8 mL/kg, and he recovered consciousness. The endotracheal tube was immediately removed.
The patient recovered from anesthesia uneventfully and was transferred to the postoperative care unit (PACU). There were no episodes of decreased oxygen saturation or nausea in the PACU, and he did not require additional analgesics during the postoperative period. He was discharged on postoperative day 1. The 17 mo follow-up period revealed no complications related to anesthesia.
BMD is a congenital muscle disorder caused by genetic mutations[1]. The diagnosis usually depends on a muscle biopsy or genetic testing[4]. BMD differs from DMD in terms of slower progression and less severe muscle weakness. BMD patients rarely have clinical manifestations in early childhood but often have other deformities, such as scoliosis, tracheal stenosis, macroglossia, or inguinal hernia[3,5,6]. These deformities usually require surgical correction in childhood. Anesthesia management is extremely challenging for anesthesiologists, as patients with BMD have a high risk of developing hyperkalemia, rhabdomyolysis, or malignant hyperthermia, and the incidence of respiratory depression after anesthesia is also increased[4]. Cardiac arrest and malignant hyperthermia episodes have been reported in young BMD patients who inhaled isoflurane during anesthesia induction or maintenance[7-9]. Therefore, anesthesia management is more difficult in children with BMD, as the available anesthesia methods for children are limited.
Intraspinal anesthesia, such as spinal anesthesia, epidural anesthesia, caudal block, and saddle block, using local anesthetics is considered a good option for patients with BMD[6]. When intraspinal anesthesia was performed as the only anesthesia technique, BMD patients were less likely to have rhabdomyolysis or malignant hyperthermia. However, intraspinal anesthesia is limited in severe BMD patients with impaired respiratory or cardiac function, as it may further decrease the patient’s breathing capacity or cardiac output.
Regional anesthesia is considered a safe approach in patients with muscular dystrophy[10]. Regional anesthesia has a reduced impact on hemodynamics and respiratory function. Therefore, it might have more advantages in high-risk patients with cardiac or respiratory dysfunction induced by muscular dystrophy. Moreover, regional anesthesia could provide effective postoperative analgesia without respiratory depression, postoperative nausea, or vomiting. However, both intraspinal anesthesia and regional anesthesia are difficult to implement as the only anesthetic technique in a conscious pediatric patient because of non-cooperation.
Previous reports have shown that TIVA with or without a non-depolarizing muscle relaxant is a safe approach for anesthesia management in BMD patients[1,2,11]. It has been proven that propofol, etomidate, and opioids did not trigger hyperkalemia, rhabdomyolysis, or malignant hyperthermia[12,13]. Nevertheless, when TIVA was used as the only anesthetic technique, the occurrence of postoperative respiratory depression may increase in BMD patients due to high sensitivity to opioids and muscle relaxants. Moreover, propofol may lead to profound hypotension, reduced organ perfusion, and violent coughing[14]. Due to high sensitivity and prolonged duration of action of muscle relaxants in muscular dystrophy patients, it is even suggested that muscle relaxants should be avoided[2]. Another report revealed that muscle relaxant antagonists could effectively reverse muscle relaxation in progressive BMD patients, and the risk of respiratory depression induced by residual neuromuscular blockade after endotracheal extubation could be decreased[11]. Train-of-four (TOF) stimuli may be helpful in monitoring neuromuscular blockade when muscle relaxants are used. Thus, it is possible that non-depolarizing muscle relaxants can be used in patients with BMD. Cisatracurium besylate is a non-depolarizing muscle relaxant without the requirement of dose-dependent histamine release. In our patient, we used a small dose of cisatracurium besylate to provide muscle relaxation for endotracheal intubation and surgery. In addition, different to using TIVA as the only anesthetic technique, we performed TAPB after anesthesia induction to allow a reduction in the dose of the intravenously administered anesthetics and muscle relaxant during anesthesia maintenance. The respiratory depression induced by opioids and residual muscle relaxants might also decrease.
After surgery, particularly in the PACU, close attention should be paid to respiratory depression, analgesia, and mechanical muscle injury in BMD pediatric patients. Episodes of decreased oxygen saturation may occur due to postoperative respiratory depression. As opioids are considered to result in a high risk of respiratory depression, postoperative nausea, and vomiting, opioid-based patient-controlled intravenous analgesia should be avoided in BMD patients. Although non-steroidal anti-inflammatory drugs are used for postoperative pain management in adult BMD patients, their safety and effectiveness during analgesia are still uncertain[2,6,15]. Regional techniques, such as TAPB in our case, could provide an effective and safe postoperative analgesia for BMD patients[10]. The excellent analgesia provided by regional anesthesia could also decrease the restlessness of pediatric patients after surgery. To prevent mechanical muscle injury, the noninvasive blood pressure monitoring interval may need to be extended.
Although dantrolene is effective in treating anesthesia-induced rhabdomyolysis or malignant hyperthermia, prevention is still the most important factor in the anesthesia management of patients with BMD[3]. Volatile anesthetics and depolarizing muscle relaxants should be contraindicated in patients with BMD or with a positive family history. It is also necessary to flush the anesthetic machine with fresh oxygen before anesthesia. Comprehensive perioperative monitoring, including electrocardiogram, blood pressure, pulse oxygen saturation, EtCO2, and body temperature, are very important in BMD patients. Blood gas analysis should also be performed if necessary.
There were some limitations during the anesthesia management of our patient. First, TOF stimuli were not applied during anesthesia or in the PACU in our patient. However, we reduced the dose of intravenous anesthetic agents and muscle relaxant. In addition, a muscle relaxant antagonist was administered to reverse the neuromuscular block. Second, considering the duration of surgery was short, we did not perform invasive blood pressure monitoring and blood gas analysis. We extended noninvasive blood pressure monitoring to every 5 min to prevent mechanical muscle injury.
TAPB combined with TIVA, using short-acting intravenous anesthetic agents, could provide safe and effective anesthesia management and postoperative analgesia for short-term abdominal operations in pediatric BMD patients. Comprehensive perioperative monitoring and care are crucial for muscular dystrophy patients undergoing general anesthesia.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abubakar MS S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wu RR
| 1. | Parish M, Farzin H. Adult patient with Becker dystrophy undergoing orthopedic surgery: an anesthesia challenge. Int Med Case Rep J. 2018;11:33-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Iwata M, Kuzumoto N, Akasaki Y, Morioka M, Nakayama K, Matsuzawa N, Kimoto K, Shimomura T. The ultrasound-guided nerve blocks of abdominal wall contributed to anesthetic management of cholecystectomy in a patient with Becker muscular dystrophy without using muscle relaxants. JA Clin Rep. 2017;3:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Gurnaney H, Brown A, Litman RS. Malignant hyperthermia and muscular dystrophies. Anesth Analg. 2009;109:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 4. | Segura LG, Lorenz JD, Weingarten TN, Scavonetto F, Bojanić K, Selcen D, Sprung J. Anesthesia and Duchenne or Becker muscular dystrophy: review of 117 anesthetic exposures. Paediatr Anaesth. 2013;23:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Lee DK, Lim BG, Lee IO, Oh HR, Lim SH, Lee MK. Unexpected tracheal narrowing during general anesthesia in the prone position of Duchenne muscular dystrophy patient -A report of two cases-. Korean J Anesthesiol. 2013;64:456-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Tatulli F, Caraglia A, Delcuratolo A, Cassano S, Chetta GS. Repair of an inguinoscrotal hernia in a patient with Becker muscular dystrophy. G Chir. 2017;37:216-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Poole TC, Lim TY, Buck J, Kong AS. Perioperative cardiac arrest in a patient with previously undiagnosed Becker's muscular dystrophy after isoflurane anaesthesia for elective surgery. Br J Anaesth. 2010;104:487-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Kleopa KA, Rosenberg H, Heiman-Patterson T. Malignant hyperthermia-like episode in Becker muscular dystrophy. Anesthesiology. 2000;93:1535-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Ohkoshi N, Yoshizawa T, Mizusawa H, Shoji S, Toyama M, Iida K, Sugishita Y, Hamano K, Takagi A, Goto K. Malignant hyperthermia in a patient with Becker muscular dystrophy: dystrophin analysis and caffeine contracture study. Neuromuscul Disord. 1995;5:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Bang SU, Kim YS, Kwon WJ, Lee SM, Kim SH. Peripheral nerve blocks as the sole anesthetic technique in a patient with severe Duchenne muscular dystrophy. J Anesth. 2016;30:320-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Shimauchi T, Yamaura K, Sugibe S, Hoka S. Usefulness of sugammadex in a patient with Becker muscular dystrophy and dilated cardiomyopathy. Acta Anaesthesiol Taiwan. 2014;52:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Kawaai H, Tanaka K, Yamazaki S. Continuous infusion propofol general anesthesia for dental treatment in patients with progressive muscular dystrophy. Anesth Prog. 2005;52:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kim TW, Nemergut ME. Preparation of modern anesthesia workstations for malignant hyperthermia-susceptible patients: a review of past and present practice. Anesthesiology. 2011;114:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Jain A. Propofol-induced violent coughing in a patient with Becker's muscular dystrophy. Indian J Pharmacol. 2011;43:476-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Zhou SY, Wang D, Liu C, Zhang S, Shan BL, Ma HC. Laparoscopic gynecological surgery in an adult woman with Becker muscular dystrophy performed with sevoflurane with cisatracurium anesthesia: A case report. Medicine (Baltimore). 2020;99:e19733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |