Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8846
Peer-review started: May 12, 2021
First decision: June 15, 2021
Revised: June 18, 2021
Accepted: August 18, 2021
Article in press: August 18, 2021
Published online: October 16, 2021
Processing time: 155 Days and 18.8 Hours
Toxic epidermal necrolysis and Stevens-Johnson syndrome are acute life-threatening skin reactions. AZD9291 has been developed as a third-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) with activity against T790M mutation.
Herein we report a 68-year-old woman who developed a large area of skin necrosis and was diagnosed with toxic epidermal necrolysis after AZD-9291 ingestion. To the best of our knowledge, this is the first case reported in patients with EGFR T790M mutation in non-small cell lung cancer (NSCLC). Cabozantinib combined with erlotinib had clinically meaningful effectiveness, with additional toxicity that was generally manageable.
Treatment with AZD-9261 is effective in regressing the growth of the NSCLC and can bring some hope to despairing patients. We hope that more research will be carried out on the association between severe rashes and EGFR-TKIs, and more safe and effective drugs can be developed.
Core Tip: To the best of our knowledge, this is the first case reported in a patient with epidermal growth factor receptor T790M mutation in non-small cell lung cancer. Cabozantinib combined with erlotinib had clinically meaningful effectiveness, with additional toxicity that was generally manageable.
- Citation: Li W, He X, Liu H, Zhu J, Zhang HM. Successful treatment after toxic epidermal necrolysis induced by AZD-9291 in a patient with non-small cell lung cancer: A case report. World J Clin Cases 2021; 9(29): 8846-8851
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8846.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8846
Toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS) are acute life-threatening skin reactions. Both are rare, with TEN and SJS affecting approximately 1 or 2/1000000 annually. The average reported mortality rate of SJS is 1%-5%, and that of TEN is 25%-35%. The diseases are characterized by mucosal skin blistering and shedding, along with sudden signs of high fever and systemic toxicity[1]. SJS is diagnosed when the epidermal detachment covers 10% of the body surface area, TEN is diagnosed when 30% is affected, and SJS/TEN overlap when 10%-30% is affected[2].
Drugs are assumed or identified as the main cause of SJS/TEN in most cases. Medications most often used for SJS/TEN include B-Lactams, nonsteroidal anti-inflammatory drugs, anticonvulsants, barbiturates, and allopurinol[3]. It is rarely reported with targeted antitumor drugs, especially AZD-9291, which is a representative third-generation epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI). AZD-9291 was shown to have superior efficacy and less toxicity, and to be especially suited for patients with EGFR T790M mutation[4]. To the best of our knowledge, there is only one previously reported case of AZD-9291precipitating SJS/TEN in patients with advanced EGFR mutation-positive non-small cell lung cancer (NSCLC)[5]. We report a 68-year-old woman who developed extensive skin necrosis after taking AZD-9291, and was diagnosed with TEN.
The patient was a 68-year-old woman with a medical history of postoperative treatment of NSCLC with bone metastasis 1 year 8 mo before presentation.
A review of the patient’s medical history revealed nothing significant other than lung cancer and hypertension.
A review of the patient’s medical history revealed nothing significant other than lung cancer and hypertension.
The patient had no significant personal or family history.
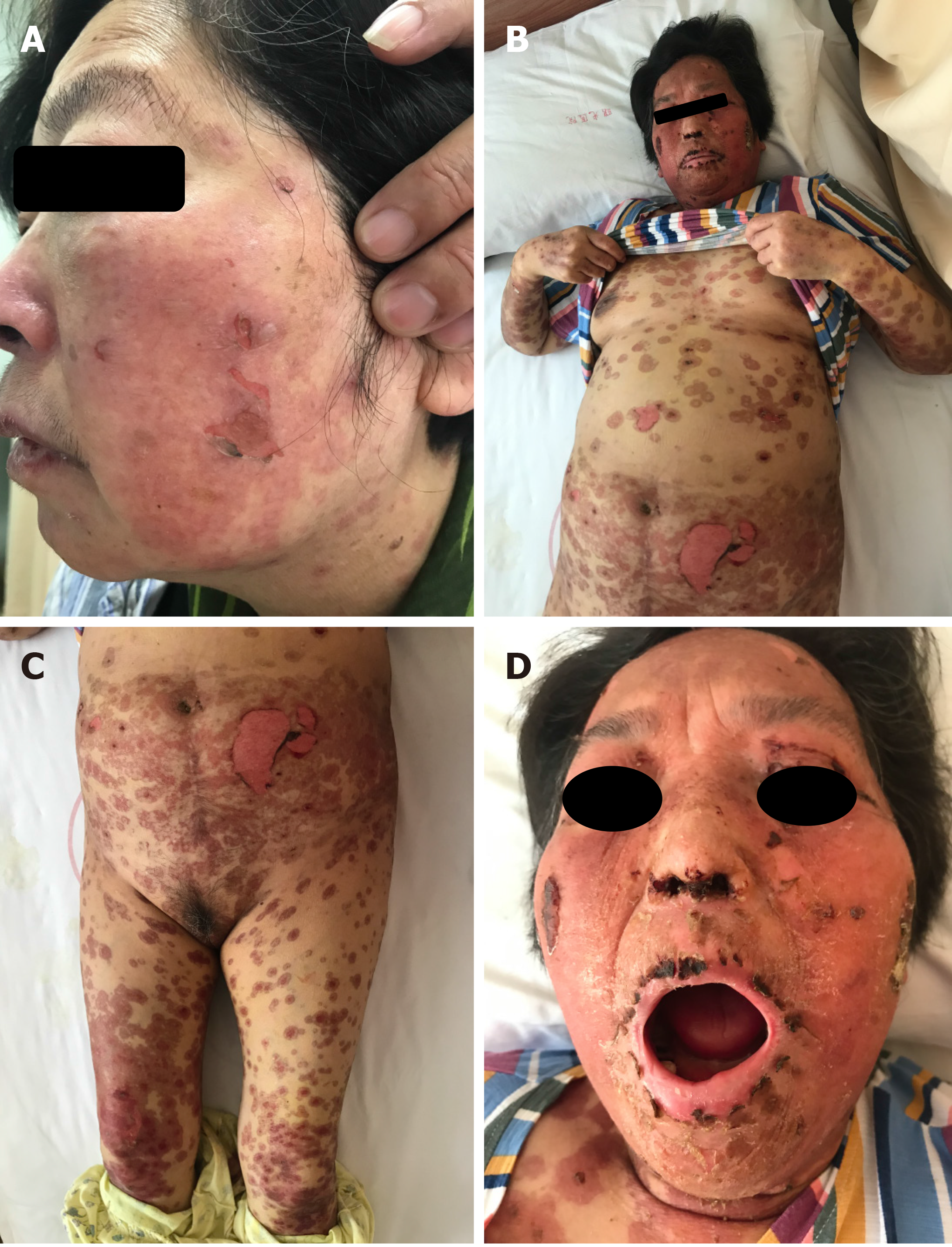
The patient presented with multiple erythematous papules mainly on the face and thigh on day 74. Seventy-eight days later, the patient developed several oral blisters and scattered erythema papules that rapidly developed into mucosal erosion and systemic epidermal abscission including the skin on the face and back (Figure 1). The large area of exfoliation involved 30% of the body surface area. Nikolsky’s sign was positive. Despite the severity of the disease, the patient’s general condition is acceptable.
Routine laboratory examination revealed normal alanine aminotransferase (ALT 22 U/L, normal limits 13-69 U/L). Laboratory markers of systemic involvement indicated anemia, leucopenia, hypoalbuminemia, and hyponatremia. More importantly, there was no short-term drug or diet exposure before the outbreak. In addition, the interval between the first administration of AZD-9291 and the onset of disease was consistent with hypersensitivity latency. Bacterial cultures of blood, urine, and blisters revealed no evidence of bacterial infection. A drug lymphocyte-stimulation test was positive for AZD-9291, with a stimulation index of 2.6. As the above information was indicative enough, and the patient refused to have a pathological examination, biopsy was not performed.
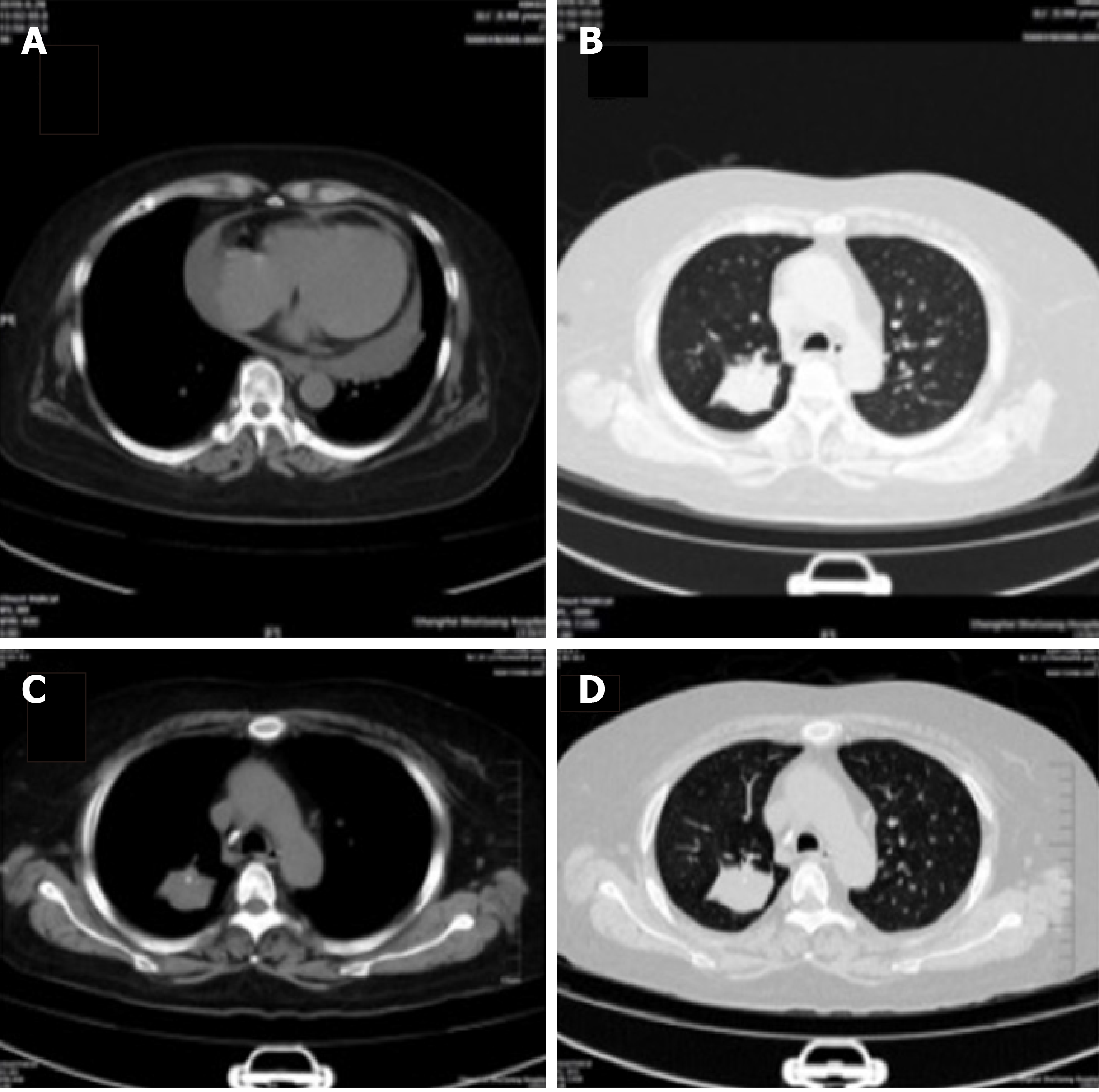
Chest computed tomography (CT) showed pulmonary lesions in posterior segment of right upper lobe, and peripheral lung cancer with multiple pulmonary metastases. Multiple metastases of the thoracic vertebrae, sternum, and ribs were considered (Figure 2A and B), which were similar to previous CT images (Figure 2C and D). Follow-up was recommended.
We concluded that AZD-9291 had caused TEN.
AZD-9291 was immediately discontinued. Emergency routine blood and blood chemistry examination indicated a low leukocyte count, hypoalbuminemia, and electrolyte disorder, suggesting that we needed to maintain the leukocyte level and correct the electrolyte disorder by administering albumin. The patient was treated with 10 g/d human immunoglobulins for 5 d and 60 mg/d of methylprednisolone for 5 d as steroid pulse therapy, followed thereafter by 40 mg/d for 3 d. Blisters and sites with exudate were coated with ethacridine lactate solution once a day. Erosive areas were treated with chloramphenicol dexamethasone ointment and covered with oil gauze once a day. During treatment, secretions were regularly collected to detect infection. Erosive genital mucosa was treated with medical colloidal dispersant several times a day. Blood scabs involving the eyes, nose and oral mucosa were regularly cleaned to prevent adhesion. The patient recovered from TEN after 2 wk of intensive therapy. Prednisolone was tapered off and finally discontinued over 1 mo. No recurrences were observed.
After she stopped taking the AZD-9291, the patient received oral erlotinib 150 mg daily plus oral cabozantinib 60 mg daily. A mild rash on the face was considered an adverse event, and the dose of cabozantinib was reduced to 20 mg on day 10. The patient is still being treated with erlotinib plus cabozantinib without acquired resistance or SJS/TEN recurrence 12 mo after TEN.
Chest CT follow-up after discharge showed that the lung tumor size was similar to before. Until the submission of this report, the tumor was still the same as before. What’s more, no new metastatic lesions have been found.
SJS and TEN are difficult to resolve because of rapid progression. Therefore, early diagnosis and prompt withdrawal of the culprit drugs are of great importance. Histological workup of fresh cryosections and conventional formalin-fixed sections of the skin and direct immune fluorescence staining should be additionally performed. The skin should be treated conservatively to prevent the loss of fluid and occurrence of infection.
Lung cancer is the most common cancer-related mortality worldwide, with about 80%-85% of patients suffering from NSCLC[6]. EGFR mutations are present in approximately 10% of Caucasian NSCLC patients and 30% of Asian NSCLC patients[7]. EGFR-TKIs are key drugs, with clinical benefit to NSCLC patients harboring EGFR mutations. Despite an initial favorable response, the vast majority of patients will experience disease progression and acquire resistance to EGFR-TKIs[8]. T790M as the secondary mutation in EGFR is the most common mechanism of acquired resistance[9]. AZD9291 has been developed as third-generation EGFR-TKI with activity against T790M mutation. AZD-9291, also known as osimertinib, is a selective inhibitor of EGFR-TKI sensitization and EGFR T790M mutation with obvious curative effect and a low incidence of serious side effects.
The incidence of cutaneous adverse events, which are common during treatment with EGFR-TKIs, is less frequent with AZD-9291 compared with first and second generation EGFR-TKIs, afatinib, gefitinib, or erlotinib. Adverse effects consist mainly of diarrhea, rash, dry skin, pruritus, and nausea. It was reported that the severity of rash is positively correlated with the EGFR-TKI titer. A meta-analysis of the relationship between EGFR-TKI-related rash severity and efficacy found that patients with rash had longer progression-free survival than patients without rash[9]. Perhaps, rash can be regarded as a response marker that may be related to prolonged survival time. However, when severe epidermal ablation and necrosis occur, the drug must be discontinued. It is very beneficial to accurately evaluate the severity of drug reactions and promptly take corrective treatment measures.
This is the second report of TEN associated with AZD-9291. The patient discontinued AZD-9291 because of TEN. CT of the lung was basically unchanged from previous images, and there seemed to be less hydrothorax. Compared with the other reported case, this patient has longer survival. The patient in our study received oral erlotinib 150 mg daily plus cabozantinib 20 mg daily. Erlotinib is approved for the treatment of all patients with advanced NSCLC but is most active in the treatment of EGFR with mutant EGFR.
Cabozantinib-based regimens are used as second-line or third-line treatment of advanced NSCLC patients with wild-type EGFR. This case is consistent with the reports that, in NSCLC patients with EGFR T790M mutation, cabozantinib combined with erlotinib has clinically meaningful results, with additional toxicity that was generally manageable. On the other hand, compared with the previously reported case, the woman in our study used AZD-929 longer. Perhaps the patient benefit can be attributed to the longer time of use of AZD-929. In the Asian population, first-line treatment with osimertinib showed clinical improvement of progression-free survival (PFS) compared with standard EGFR-TKIs, with an overall safety profile consistent with that seen in the phase III FLAURA study population[10]. That is to say, AZD-9261 treatment inhibited the growth of NSCLC and can bring some hope to despairing patients. We hope to conduct more research on the relationship between severe rashes and EGFR-TKIs in order to develop safer and more effective drugs.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sugimura H S-Editor: Wang LL L-Editor: Filipodia P-Editor: Xing YX
| 1. | Isvy-Joubert A, Ingen-Housz-Oro S, Vincent R, Haddad C, Valeyrie-Allanore L, Chosidow O, Race JM, Wolkenstein P. Severe cutaneous adverse reactions to drugs: from patients to the national office for compensation of medical accidents. Dermatology. 2014;228:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Chen CB, Wu MY, Ng CY, Lu CW, Wu J, Kao PH, Yang CK, Peng MT, Huang CY, Chang WC, Hui RC, Yang CH, Yang SF, Chung WH, Su SC. Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag Res. 2018;10:1259-1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, Auquier A, Bastuji-Garin S, Correia O, Locati F. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333:1600-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 870] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 4. | Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, Shah R, Cobo M, Lee KH, Cheema P, Tiseo M, John T, Lin MC, Imamura F, Kurata T, Todd A, Hodge R, Saggese M, Rukazenkov Y, Soria JC; FLAURA Investigators. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020;382:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1879] [Article Influence: 375.8] [Reference Citation Analysis (0)] |
| 5. | Wang J, Cheng X, Lu Y, Zhou B. A case report of toxic epidermal necrolysis associated with AZD-9291. Drug Des Devel Ther. 2018;12:2163-2167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Dubey AK, Gupta U, Jain S. Epidemiology of lung cancer and approaches for its prediction: a systematic review and analysis. Chin J Cancer. 2016;35:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3395] [Cited by in RCA: 4292] [Article Influence: 390.2] [Reference Citation Analysis (0)] |
| 8. | Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3081] [Cited by in RCA: 3205] [Article Influence: 160.3] [Reference Citation Analysis (0)] |
| 9. | Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan DM, Song Y. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e55128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Cho BC, Chewaskulyong B, Lee KH, Dechaphunkul A, Sriuranpong V, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cheng Y, Cho EK, Voon PJ, Lee JS, Mann H, Saggese M, Reungwetwattana T, Ramalingam SS, Ohe Y. Osimertinib vs Standard of Care EGFR TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC: FLAURA Asian Subset. J Thorac Oncol. 2019;14:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |