Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8831
Peer-review started: June 8, 2021
First decision: June 25, 2021
Revised: June 28, 2021
Accepted: August 17, 2021
Article in press: August 17, 2021
Published online: October 16, 2021
Processing time: 129 Days and 2.6 Hours
Eosinophilic fasciitis (EF) is a rare disease characterized by inflammation of the fascia with immune system involvement. Failure to promptly diagnose and treat this disease can seriously affect the quality of life of patients. However, no clear and uniform criteria for diagnosis and treatment exist.
In this paper, we report two cases of EF, both of which showed symmetrical limb swelling and rigidity, increased eosinophils in the peripheral blood and bone marrow, increased red blood cell sedimentation rate, increased antinuclear antibody titer, and pathological changes in the tissues such as eosinophil and lymphocyte infiltration. Both patients were treated with hormones and cyclosporine, and showed significant improvements in their conditions.
EF is an autoimmune disease causing swelling and sclerosis of the fascia and eosinophilia. It is diagnosable by magnetic resonance imaging, positron emission tomography-computed tomography, blood routine tests, and bone marrow puncture. Glucocorticoids and immunosuppressants are effective treatments.
Core Tip: Eosinophilic fasciitis is a rare disease that can affect the immune system. Currently, there are no clear diagnostic criteria for this entity. Because of the rarity of eosinophilic fasciitis, patients may go to orthopedics department, dermatology department, etc., for dyskinesias, skin lesions, etc., so the disease is diagnosis and treatment will be restricted in many ways. This article records two eosinophilic fasciitis patients, in order to provide more detailed and comprehensive information for the clinical diagnosis and treatment of this rare disease.
- Citation: Song Y, Zhang N, Yu Y. Diagnosis and treatment of eosinophilic fasciitis: Report of two cases. World J Clin Cases 2021; 9(29): 8831-8838
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8831.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8831
Eosinophilic fasciitis (EF) is a rare disease characterized by diffuse swelling and sclerosis of the fascia, also known as diffuse fasciitis. The etiology of EF is unclear. Currently, the disease is generally believed to be associated with abnormalities of the immune system, and no clear diagnosis and classification criteria exist. Among the existing reported cases, an increase in eosinophils precedes the development of the characteristic skin lesions[1]. The disease can occur among people of all age groups and it most commonly affects those aged between 20 and 60 years, with a male-to-female ratio of 2:1[2]. In this study, we report the clinical characteristics and related data of two patients with EF to improve the understanding of this disease among clinicians.
Case 1: A 62-year-old woman was admitted to Shengjing Hospital of China Medical University on December 24, 2020, with a chief complaint of subcutaneous tissue induration for 6 mo and aggravation for over 10 d.
Case 2: A 36-year-old man was admitted to Shengjing Hospital of China Medical University on October 19, 2020, with a chief complaint of subcutaneous tissue induration that had lasted for 5 mo and had worsened for 1 mo.
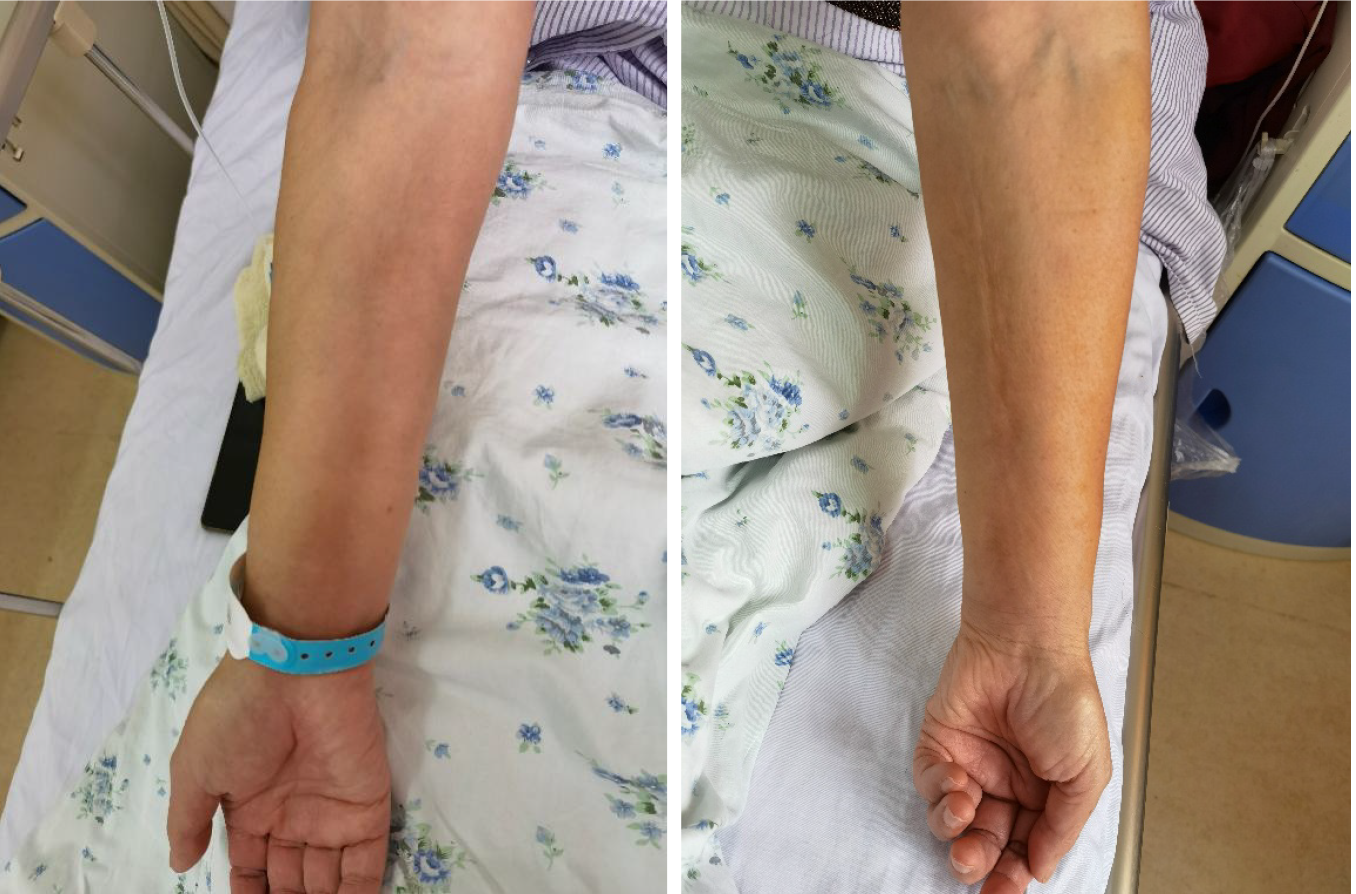
Case 1: Six months before presentation, the patient developed hyperpigmentation and induration of the anterior tibial skin of the left leg with no obvious cause, and the affected area gradually increased in size, but without swelling, itching, or pain. Later, swelling and induration gradually developed in the face, both arms, both lumbar and hypochondrial regions, the left anterior thorax, and the skin of the upper back (Figure 1). The patient’s forearms were tender and felt heavy when waking up in the morning, but this improved after activity. There was restriction of movement of the elbows and knees and local stretching pain during activity, but there was no local elevation of skin temperature.
Case 2: Five months before initial presentation, the patient developed swelling, induration, and pain in the subcutaneous tissue of the right sole of the foot with no obvious cause, which gradually developed into swelling and induration of the subcutaneous tissues of the right leg and both forearms. The local skin temperature was 1.2 ºC higher than that of normal tissue, as measured using an infrared thermometer. There was no systematic diagnosis or treatment. Two months prior, he had discovered hyperpigmentation on the skin of the right sole. One month prior, the patient felt that the symptoms had worsened, with swelling and induration of the subcutaneous tissue of the left leg; restriction of movement of the wrists (limited flexion, extension and abduction of the wrists), ankles, and knees; and local contracture pain during activity.
Case 1: Investigation of family history and past medical history did not indicate any significant abnormalities.
Case 2: The patient had a history of congenital scoliosis. He had had hypothyroidism for 6 mo before presentation, which is currently controlled with oral levothyroxine (125 µg once a day). The patient had no clear family history of genetic disease and chronic systemic inflammation.
Cases 1 and 2: Both patients denied a clear family history of rheumatic immune diseases and had no history of smoking or alcoholism.
Case 1: The major abnormalities found on physical examination were mild pitting edema in both legs; palpable induration in the subcutaneous tissues of the limbs, lumbar and hypochondrial regions, left anterior thorax, and upper back with unclear margins; slight tenderness of both forearms; mild restriction of movement of the elbows and knees; but no joint tenderness. Skin hyperpigmentation with an irregular shape was found on the anterior tibia of the left calf, with no itching, desquamation, or pain. The muscle strength was measured as level IV using the Lovett grading method and there was no muscle grip pain.
Case 2: The major abnormalities found on physical examination were local skin pitting and hyperpigmentation in the right sole and the back of the thigh. The skin of both forearms and both calves was red, the local skin temperature was 0.8°C higher than that of normal tissue, and the skin was tense. There was palpable swelling and induration in the subcutaneous tissue of the limbs with unclear margins; slight tenderness and limited mobility of the wrists, knees, and ankles; but no joint tenderness.
Case 1: Anti-nuclear antibodies (ANA) were detected using flow cytometry testing under laboratory standards, yielding a positive result for IgG (1:80). The erythrocyte sedimentation rate was 42 mm/h. C-reactive protein (CRP) was 11.8 mg/L. The complete blood count yielded the following values: WBC count, 10.7 × 109/L; eosinophil percentage, 21.3%; eosinophil count, 2.3 × 109/L. Electromyography showed that motor nerve conduction velocities of the right median nerve and the left ulnar nerve were normal, and compound muscle action potential amplitudes were decreased. Bone biopsy revealed bone marrow hyperplasia, a normal myeloid /erythroid ratio, and elevated eosinophil percentage. Qualitative polymerase chain reaction (PCR) results for bone marrow fusion gene were negative (-).
Case 2: Examination for ANA IgG was positive. The erythrocyte sedimentation rate was 60 mm/h and CRP was 33.4 mg/L. The complete blood count was as follows: Lymphocyte percentage, 15.0%; eosinophil percentage, 15.2%; eosinophil count, 1.4 × 109/L. EMG was normal. The bone marrow biopsy suggested eosinophilia. Qualitative PCR results for the bone marrow fusion gene were negative (-).
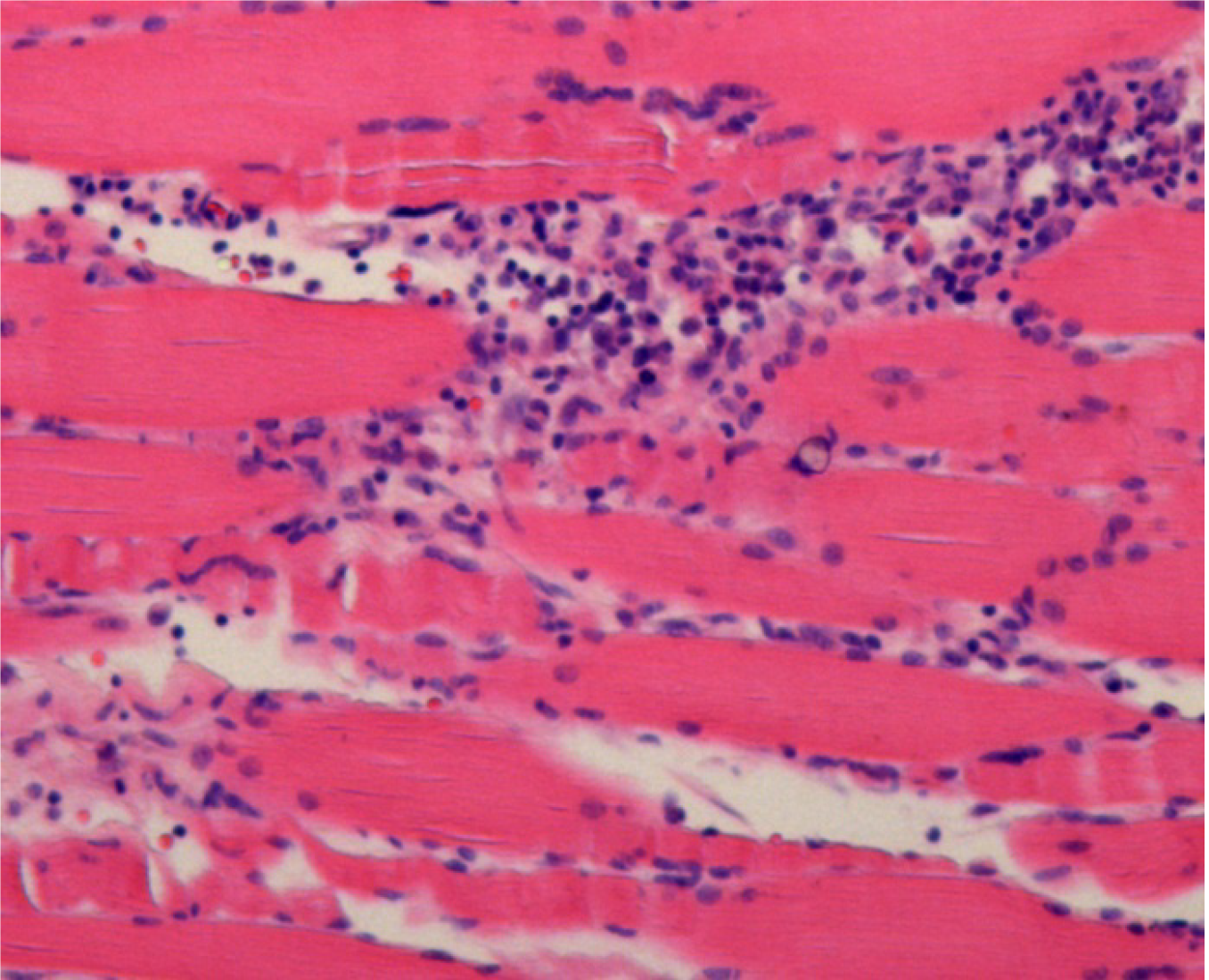
Case 1: The pathology examination revealed epidermal atrophy and thinning, loss of the stratum corneum, hyperpigmentation of the basal layer, slight hyperplasia of dermal fibers, hyaline degeneration, and normal skin appendages. There was a small amount of focal lymphocyte infiltration in the dermis and subcutaneous fat lobules, which was primarily located around small blood vessels. No obvious eosinophils were observed. The deep subcutaneous fascia fibers had noticeably proliferated, with increased membranous hyalinosis and a large amount of chronic inflammatory cell infiltration, mainly consisting of lymphocytes, with several monocytes, some plasma cells, occasional eosinophils, and patches of bleeding. Striated muscle cells had a regular morphology and clear striations. There was proliferation of the perimysium and intermuscular membranes and a large amount of chronic inflammatory cell infiltration, mainly consisting of lymphocytes, with several monocytes, some plasma cells, and occasional eosinophils. Striated muscle cells at the sarcolemma junctions were destroyed, and there was infiltration of inflammatory cells (Figure 2). Positron emission tomography–computed tomography (PET-CT) yielded the following findings: (1) Scanning subcutaneous fat and muscle indicated mostly diffuse exudation with slightly increased metabolism. Connective tissue disease and dermatomyositis were suspected; and (2) There were multiple foci of high fluorodeoxyglucose (FDG) metabolism in the bilateral axillary regions and slight enlargement of lymph nodes, which suggested a high probability of reactive hyperplasia.
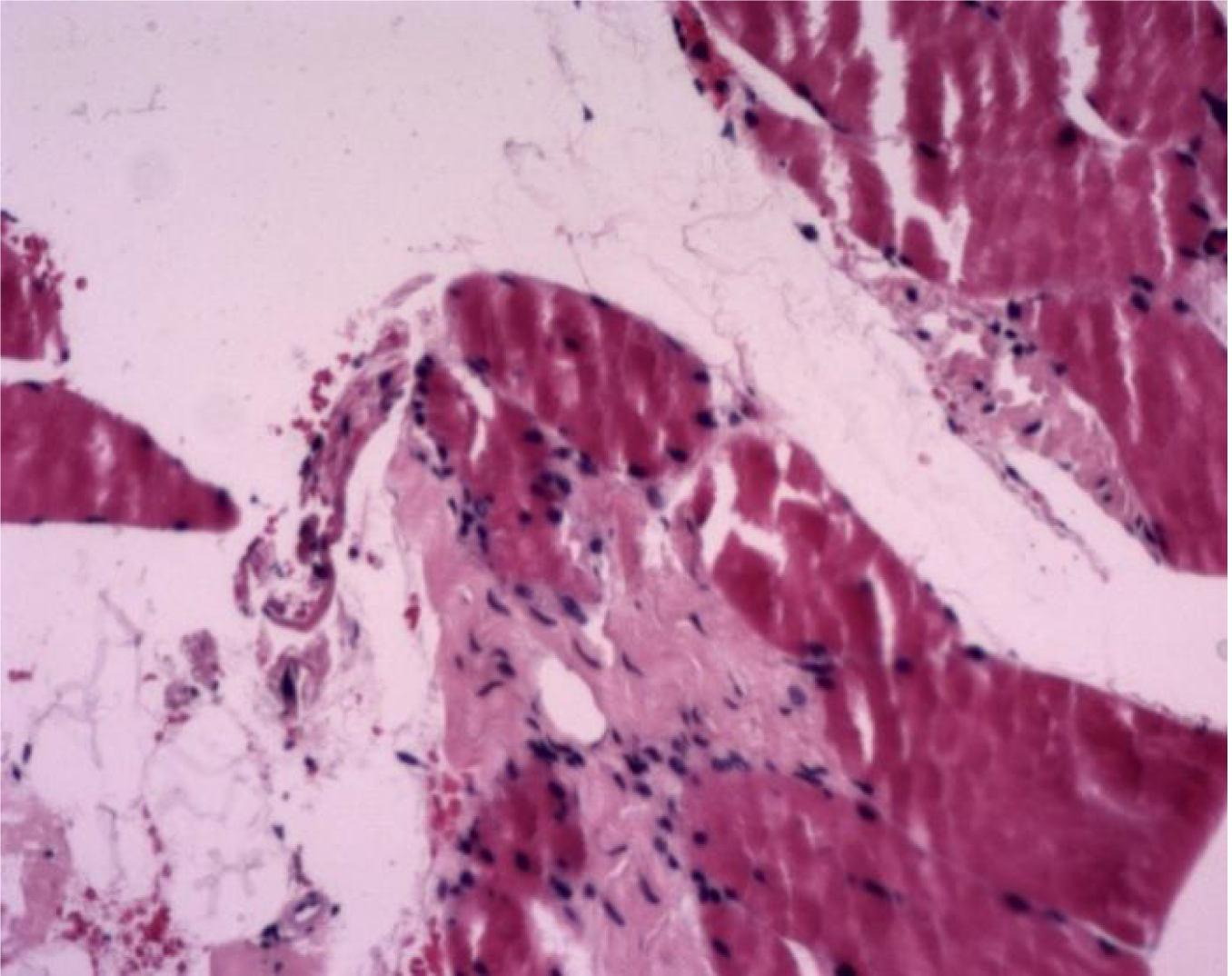
Case 2: Some unclear striations were found in fragmented muscle tissue, and there was proliferation of fibrous tissue and small blood vessels (Figure 3). Ultrasound showed thickening of subcutaneous soft tissue in multiple sites in the arms and legs.
In both patients, there was a significant increase of eosinophils in routine blood examination. Local histopathological examination showed eosinophil infiltration and fibrous tissue hyperplasia but no cell proliferation. Analysis of bone marrow biopsy showed a high ratio of eosinophils and the qualitative PCR of the bone marrow fusion gene was negative, excluding hematological malignant neoplasms. Both patients were diagnosed with EF.
The patient was administered with 20 mg intravenous methylprednisolone (once a day) for 6 d, which was later changed to 24 mg oral methylprednisolone q.d. plus oral cyclosporine 25 mg twice a day to manage the condition.
The patient was administered with 40 mg of methylprednisolone q.d. for 4 d, which was changed to 40 mg of prednisone q.d. plus oral cyclosporine 50 mg twice a day to manage the condition.
The patient’s induration, limited joint mobility, and other symptoms were significantly improved. The patient began to experience relief of her symptoms after 3 d of medication during hospitalization, and reported that her condition had significantly improved on telephonic follow-up 1 mo after discharge.
The patient’s skin swelling and induration, limited joint mobility, and other symptoms were significantly improved. The patient began to experience relief of his symptoms after 7 d of medication during hospitalization, and reported that his condition had significantly improved on telephonic follow-up 1 mo after discharge.
EF is a rare disease characterized by diffuse swelling and sclerosis of the fascia. It is often accompanied by eosinophilia and may present in a manner similar to that of systemic sclerosis, with possible involvement of the dermis. Systemic sclerosis is an autoimmune disease characterized by localized or diffuse skin thickening and fibrosis, which may affect the internal organs (e.g., lungs, heart, kidneys, and gastrointestinal tract). It is a common disorder of the connective tissue. The etiology and pathogenesis of EF are currently unclear, but they are believed to be associated with abnormal immune system responses[3]. For example, the two cases described in this article were positive for ANA. Many patients may also have stressors such as overwork, trauma, or infection before onset, but there was no obvious precipitating cause before onset in the two cases described in this study.
The existing criteria for diagnosis of EF are incomplete. The EF diagnostic criteria proposed by Pinal-Fernandez et al[1] in 2014 and the Japanese classification criteria proposed in 2017[4] are the most commonly used, but typical clinical symptoms, imaging examinations, laboratory indicators, and pathological changes are still required for accurate determination. Patients with EF often show increased peripheral blood eosinophil counts, which can be decreased after effective treatment. In Case 1, the peripheral blood eosinophil percentage was 21.3%, and eosinophil count was 2.3 × 109/L, and in Case 2, the values were 15.2% and 1.4 × 109/L, respectively. To establish a diagnosis of EF, both peripheral blood eosinophil percentage and eosinophil count are required to be significantly higher than the normal ranges of 0.7%-7.8% and 0.04-0.49 × 109/L, respectively. The eosinophil count and percentage in the peripheral blood of the two patients in this study were both significantly increased, and they showed a downward trend after hormone combined with immunosuppressive therapy.
Most patients with EF have a chronic onset, but a small number of patients may have an acute onset. The clinical symptoms of EF usually appear symmetrically but can also present unilaterally. However, in a case series reported by Berianu et al[2], the clinical symptoms unilaterally involved the left limb, but magnetic resonance imaging examination showed that both limbs were affected. EF generally affects the limbs, but other parts may also be involved, such as the chest, back, abdomen, and face. In the present case, the limbs, chest, back, and abdomen of Case 1 were all affected, whereas Case 2 primarily exhibited severe involvement of the limbs. Patients with EF often have skin induration in the limbs with or without pitting edema at early stages, which gradually progresses to “peau d’orange” and induration of the skin. Some patients may have significant furrows in underlying veins, known as the “groove sign,” when the affected limb is raised, due to exacerbation of fibrosis in the fascia of the skin[5]. Symptoms of myxedema can appear in hypothyroidism; myxedema is caused by infiltration of fluids rich in hyaluronic acid, mucin, and mucopolysaccharides into tissues. This is classified as non-pitting edema and usually occurs in the anterior tibial area. A small number of patients have pitting edema, and they generally have no obvious hyperpigmentation. In the present case, Case 2 had hypothyroidism but took medication regularly, and the results of thyroid function tests were within the normal range, indicating that the patient’s hypothyroidism was stable. The patient mostly had pitting edema accompanied by skin and muscle induration. There was partial skin pitting and hyperpigmentation on the sole of the right foot and the back of the thigh. Thus, the patient’s edema symptoms were not considered to be significantly correlated with hypothyroidism.
MRI and PET-CT examinations can help in the diagnosis of EF. Additionally, MRI and PET-CT examinations can guide pathological examination. Typical changes seen on MRI include a high fasciitis signal in the lesion. High signal can also be seen in the fat-suppressed T2W1 sequence, whereas normal fascia tissue presents as a normal low signal[6]. PET-CT is valuable for excluding potentially malignant tumors, warranting further study for early diagnosis of EF[7]. In Case 1, PET-CT examination of sub
Early detection, diagnosis, and treatment are the most effective measures for improving outcomes in patients with EF. A very small number of patients can obtain relief without intervention, and most patients require drug intervention[11]. The first choice for treatment for EF is glucocorticoids, and most patients with EF respond well to hormone therapy[12]. However, hormone therapy alone results in a longer course of treatment and greater side effects, and recurrence can occur after the hormone dose is reduced. Therefore, adding immunosuppressants at early stages at the same time as multifocal and multitargeted therapy is recommended. Early combined use of immunosuppressants allows the prompt reduction of the hormone dose, shortening of the course of hormone administration, and reduction in the development of adverse reactions[12]. In some patients with refractory EF, specific monoclonal antibody biologics can be selected for treatment. However, because EF is still rare, the efficacy of such biologics in the treatment of EF requires further study. In this study, both patients were treated with corticosteroids and cyclosporine. At follow-up, it was found that the symptoms of skin induration and joint contracture in both patients were significantly relieved.
EF is a rare rheumatic immunological disease with diffuse sclerosis of the fascia. Generally, the prognosis is good, but some patients may gradually develop joint contractures due to skin induration and tightness if treatment is delayed, which significantly impacts their quality of life. Therefore, early detection, diagnosis, and treatment are the most effective measures for improving outcomes in patients with EF. MRI, PET-CT, and histopathological examinations are recommended for diagnosis and monitoring for changes. For treatment, hormones and immunosuppressant therapy with glucocorticoids and cyclosporine, respectively, are recommended for multifocal and multitargeted therapy, as these drugs are effective for most patients with EF. When hormones and related immunosuppressive agents are used, it is crucial to adjust the drug administration according to the patient's condition and markers indicating their systemic state. However, due to its rarity, knowledge of EF is still limited, and more samples are still required for systematic studies to improve its diagnosis and treatment.
We thank the Department of Rheumatology of Shengjing Hospital of China Medical University for technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Rheumatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hasan A S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Isaacson G. Comprehensive management of infected preauricular sinuses/cysts. Int J Pediatr Otorhinolaryngol. 2019;127:109682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 2. | Choo OS, Kim T, Jang JH, Choung YH. The clinical efficacy of early intervention for infected preauricular sinus. Int J Pediatr Otorhinolaryngol. 2017;95:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Tan T, Constantinides H, Mitchell TE. The preauricular sinus: A review of its aetiology, clinical presentation and management. Int J Pediatr Otorhinolaryngol. 2005;69:1469-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Fourman P, Fourman J. Hereditary deafness in family with ear-pits (fistula auris congenita). Br Med J. 1955;2:1354-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Tan B, Lee TS, Loh I. Reconstruction of preauricular soft tissue defects using a superiorly based rotation advancement scalp flap - A novel approach to the surgical treatment of preauricular sinuses. Am J Otolaryngol. 2018;39:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Chami RG, Apesos J. Treatment of asymptomatic preauricular sinuses: challenging conventional wisdom. Ann Plast Surg. 1989;23:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Rataiczak H, Lavin J, Levy M, Bedwell J, Preciado D, Reilly BK. Association of Recurrence of Infected Congenital Preauricular Cysts Following Incision and Drainage vs Fine-Needle Aspiration or Antibiotic Treatment: A Retrospective Review of Treatment Options. JAMA Otolaryngol Head Neck Surg. 2017;143:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Gur E, Yeung A, Al-Azzawi M, Thomson H. The excised preauricular sinus in 14 years of experience: is there a problem? Plast Reconstr Surg. 1998;102:1405-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Tang IP, Shashinder S, Kuljit S, Gopala KG. Outcome of patients presenting with preauricular sinus in a tertiary centre--a five year experience. Med J Malaysia. 2007;62:53-55. [PubMed] |
| 10. | Bruijnzeel H, van den Aardweg MT, Grolman W, Stegeman I, van der Veen EL. A systematic review on the surgical outcome of preauricular sinus excision techniques. Laryngoscope. 2016;126:1535-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |