Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8804
Peer-review started: December 19, 2020
First decision: July 18, 2021
Revised: July 30, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: October 16, 2021
Processing time: 300 Days and 3.8 Hours
Mannitol is a hyperosmolar agent and the combination of mannitol and furosemide is a widely used treatment for intracranial pressure control. Considering the hypertonic properties of mannitol to move water out of intracellular spaces, we hypothesized that mannitol combined with furosemide could relieve focal tissue swelling in refractory lymphedema.
A 90-year-old female had been diagnosed with intracranial hemorrhage and received a combination of mannitol and furosemide for intracranial pressure control. Independent of the intracranial hemorrhage, she had refractory lymphedema of the left lower extremity since 1998. Remarkably, after receiving the mannitol and furosemide, the patient’s lower extremity lymphedema improved dramatically. After the mannitol and furosemide were discontinued, the lymphedema worsened in spite of complete decongestive therapy (CDT) and intermittent pneumatic compression treatment (IPC). To identify the presumed effect of mannitol and furosemide on the lymphedema, these agents were resumed, and the lymphedema improved again.
The present case raises the possibility that a combination of mannitol and furosemide might be considered another effective therapeutic option for refractory lymphedema when CDT and IPC are ineffective.
Core Tip: Mannitol is a hyperosmolar agent and the combination of mannitol and furosemide is a widely used treatment for intracranial pressure control. We found dramatic improvement of refractory lymphedema after administration of mannitol and furosemide. After the mannitol and furosemide were discontinued, the lymphedema worsened in spite of complete decongestive therapy (CDT) and intermittent pneumatic compression (IPC). To identify the presumed effect of mannitol and furosemide on lymphedema, these agents were resumed, and the lymphedema improved again. The present case suggests that the combination of mannitol and furosemide could be considered as another effective therapeutic option for refractory lymphedema when CDT and IPC are ineffective.
- Citation: Kim HS, Lee JY, Jung JW, Lee KH, Kim MJ, Park SB. Is mannitol combined with furosemide a new treatment for refractory lymphedema? A case report. World J Clin Cases 2021; 9(29): 8804-8811
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8804.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8804
Lymphedema is a collection of fluid that is localized to a part of the body. Secondary lymphedema, which is much more prevalent than primary lymphedema, can be caused by obstruction of the lymphatic system, such as in recurrent infection, trauma, surgery and malignancy[1]. Treatment varies from manual manipulation to surgical treatment and is prescribed according to the cause. Despite various treatments, lymphedema tends to progress slowly and is refractory. Herein, we report a case of improved refractory lymphedema that was incidentally observed in a patient who was treated with a combination of mannitol and furosemide for the purpose of intracranial pressure control.
A 90-year-old female presented to the emergency department with acute mental changes. She also had swelling and reddish skin color change on the left lower extremity.
Three hours prior to acute mental change, the patient reported a severe headache and right hemiplegia. Her mental state was stupor when she arrived at the hospital. Tracheal intubation was performed due to stuporous mentality.
The patient had a history of refractory lymphedema of the left lower extremity since 1998 after total abdominal hysterectomy for cervical cancer in 1987, and she had been treated with a pneumatic compression device and short-stretch bandaging at home. She was repeatedly admitted into the rehabilitation department to receive complete decongestive therapy (CDT) with intermittent pneumatic compression treatment (IPC), and her family stated that the lymphedema had become aggravated while she was living at home.
She also had a history of hospitalization for chronic kidney disease in 2017, and was managed with candesartan 4 mg/day through the outpatient department. Baseline estimated glomerular filtration rate (eGFR) before admission was 77 mL/min per 1.73 m2 and serum creatinine level was 0.7 mg/dL.
On physical examination, she was in a stuporous state, and her motor strength was grossly 2/5 grade on the right extremities. Swelling and reddish skin color change were noted in the left lower extremity.
In the laboratory work-up, the leukocyte count was elevated (14.9 × 109/L). Leukocyte differential count indicated 85.7% neutrophils, 7.3% lymphocytes, and 6.8% monocytes. C-reactive protein, ammonia, troponin-I, lactate dehydrogenase, serum osmolarity, creatinine and blood urea nitrogen (BUN) levels were within the normal ranges.
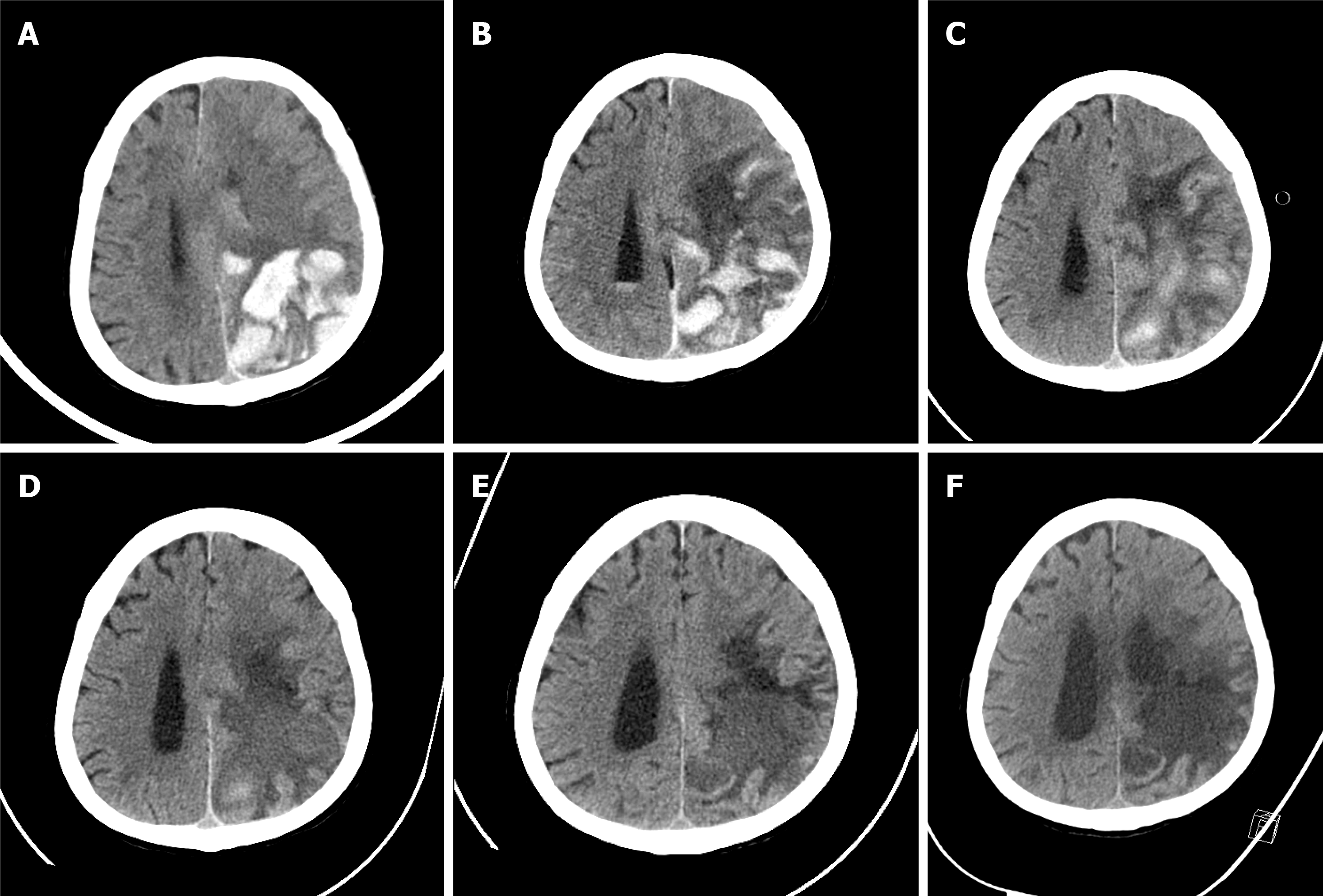
Computed tomography (CT) of the brain revealed an intracranial hemorrhage and brain edema in the left parieto-occipital lobe (Figure 1A).
The patient was diagnosed with intracranial hemorrhage, as revealed by brain CT. With her past history and physical examination, she was also diagnosed with refractory secondary lymphedema of the left lower extremity.
Tracheostomy was performed on the 8th hospital day for prevention of aspiration pneumonia and removal from intubation. The patient’s family refused a surgical procedure for intracranial hemorrhage, so she was admitted to the intensive care unit for conservative treatment. Considering the brain CT and physical examination findings, her neurologic symptoms were attributed to increased intracranial pressure (ICP). Upon admission, she immediately received mannitol (0.2 g/mL, 50 mL every 6 h) and furosemide (5 mg every 6 h) for ICP control. Sedation was not required due to stuporous mentality. Since no invasive procedures were performed, direct ICP monitoring was not possible. However, persistent hemorrhage was confirmed on follow-up brain CT on the 8th hospital day. Thus, ongoing ICP elevation was suspected and ICP control agents were continued.
The effects of mannitol and furosemide on ICP were assessed indirectly through brain CT and changes in neurologic symptoms. In addition, due to concerns about side effects of osmolarity variation, laboratory evaluation of blood gas, electrolyte, osmolarity, and kidney function was performed during hospitalization (Table 1). On the 21st hospital day, the patient’s vital signs had stabilized, and she was transferred to the general ward. Mannitol and furosemide were applied until the 27th hospital day.
| Baseline | HD 1 | HD 2 | HD 3 | HD 4 | HD 5 | HD 7 | HD 9 | HD 11 | HD 13 | HD 15 | HD 17 | HD 19 | HD 21 | HD 48 | HD 58 | HD 65 | HD 77 | HD 85 | HD 95 | HD 108 | HD 115 | HD 129 | |
| Na (mEq/L) | - | 140 | 140 | 139 | 145 | 150 | 144 | 142 | 144 | 145 | 140 | 135 | 138 | 138 | 142 | 145 | 148 | 139 | 142 | 136 | 139 | 135 | 133 |
| K (mEq/L) | - | 3.0 | 3.0 | 2.9 | 3.1 | 2.8 | 2.9 | 2.8 | 3.4 | 3.5 | 3.6 | 3.6 | 3.9 | 3.8 | 3.8 | 3.3 | 3.9 | 3.0 | 4.1 | 4.3 | 4.1 | 4.5 | 4.4 |
| Cl (mEq/L) | 105 | 105 | 106 | 111 | 114 | 111 | 105 | 107 | 110 | 108 | 106 | 108 | 104 | 110 | 107 | 117 | 110 | 114 | 108 | 107 | 102 | 100 | |
| Cr (mg/dL) | 0.7 | 0.82 | 0.83 | 0.84 | 0.77 | 0.68 | 0.68 | 0.67 | 0.74 | 0.64 | 0.57 | 0.54 | 0.53 | 0.52 | 0.51 | 0.58 | 0.59 | 0.57 | 0.55 | 0.78 | 0.65 | 0.68 | 0.66 |
| eGFR (mL/min per 1.73 m2) | 77 | 63 | 62 | 61 | 68 | 77 | 77 | 78 | 72 | 79 | 82 | 83 | 84 | 84 | 85 | 81 | 81 | 82 | 83 | 67 | 78 | 77 | 78 |
| BUN (mg/dL) | - | 25.2 | 21.2 | 40.5 | 45.1 | 38 | 26.1 | 24 | 27.8 | 24.9 | 31.3 | 27.1 | 30 | 30.4 | 23.8 | 16.1 | 25.9 | 15.3 | 17.5 | 29.5 | 20.3 | 19.3 | 24.6 |
| sOsm (mOsm) | - | 302 | 302 | 309 | 323 | 325 | 305 | 308 | 308 | 313 | 305 | 295 | 294 | 296 | |||||||||
| pH | - | - | 7.356 | 7.492 | 7.465 | 7.491 | 7.508 | 7.505 | 7.492 | 7.513 | 7.477 | 7.473 | 7.456 | 7.457 | |||||||||
| pCO2 (mmHg) | - | - | 33.3 | 30.5 | 35.6 | 36.3 | 37.3 | 38.2 | 39.0 | 35.1 | 35.3 | 34.8 | 36.6 | 38.2 | |||||||||
| pO2 (mmHg) | - | - | 117 | 108 | 84.6 | 109 | 71.1 | 137 | 122 | 97.1 | 129 | 146 | 100 | 102 | |||||||||
| HCO3- (mmol/L) | - | - | 18.1 | 23.1 | 25.2 | 27.5 | 29.3 | 29.9 | 29.6 | 28.0 | 25.8 | 25.2 | 25.4 | 26.6 | |||||||||
| BE (mmol/L) | - | - | -6.1 | 0.7 | 2.1 | 4.4 | 6.3 | 6.7 | 6.2 | 5.3 | 2.8 | 2.1 | 2.0 | 3 | |||||||||
| SaO2 (%) | - | - | 98.5 | 98.8 | 96.9 | 98.7 | 95.7 | 99.0 | 98.8 | 97.6 | 98.7 | 99.2 | 97.9 | 98.2 |
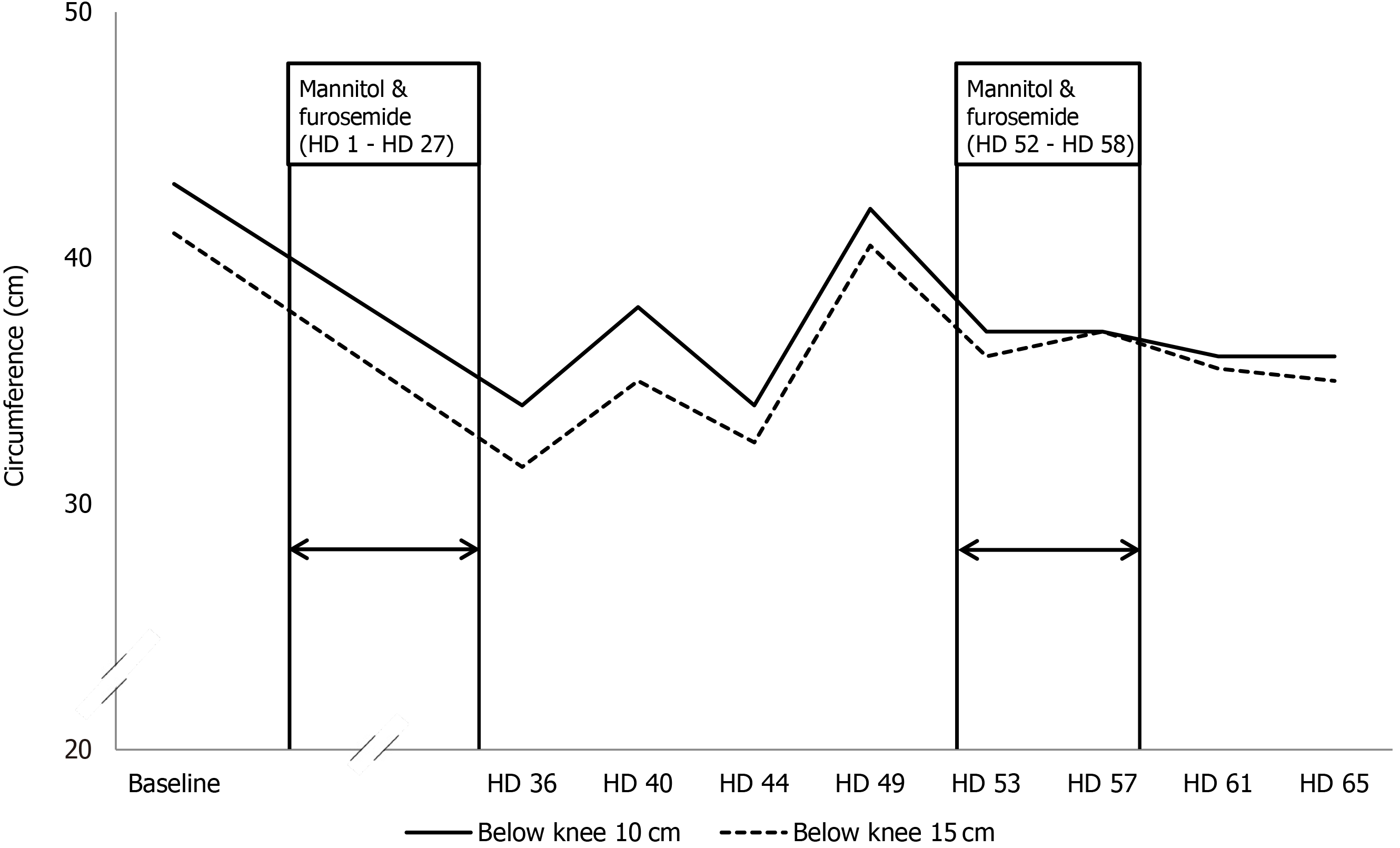
Remarkably, the lower extremity lymphedema improved dramatically after she received mannitol and furosemide. The baseline circumferences 10 cm/15 cm below the left knee were 43 cm/41 cm, respectively. However, after administration of mannitol and furosemide for 27 d, the circumferences 10 cm/15 cm below the left knee had decreased to 34 cm/31.5 cm respectively, on the 36th hospital day (Figure 2).
During her hospital course, the patient received neurodevelopmental therapy and occupational therapy for right hemiplegia. CDT and IPC were performed for lymphedema treatment from the 26th hospital day.
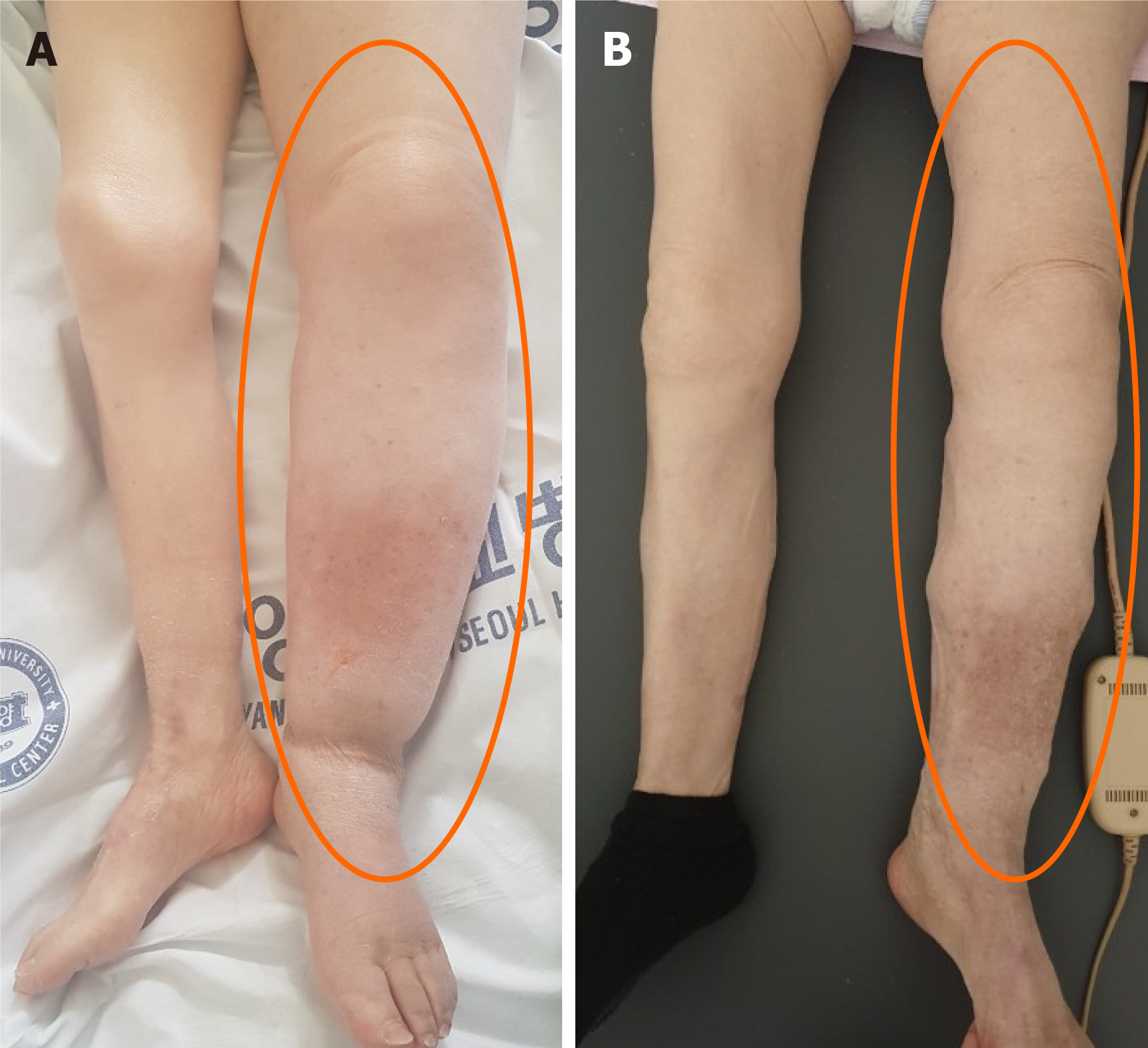
However, despite the interventions, her lower extremity lymphedema worsened after discontinuation of the mannitol and furosemide. On the 49th hospital day, the circumferences 10 cm/15 cm below the left knee increased to 42 cm/40.5 cm, respectively (Figure 3A). To identify the presumed effect of mannitol and furosemide on lymphedema, mannitol (0.2 g/mL, 50 mL every 6 h) and furosemide (5 mg every 6 h) were reinstated from the 52th to the 58th hospital day. As a result, her lymphedema improved, and the circumferences 10 cm/15 cm below the left knee decreased to 37 cm/36 cm, respectively, on the 53th hospital day (Figure 2). The improved lymphedema persisted after mannitol and furosemide were tapered and discontinued. On the 61th hospital day, the circumferences 10 cm/15 cm below the left knee were 36 cm/35.5 cm, respectively (Figure 3B).
Intracranial hemorrhage and brain edema were followed using brain CT during hospitalization. Ongoing resolution of hemorrhage and improvement in brain edema were demonstrated (Figure 1).
The patient was discharged after four months of admission, with no aggravation of lymphedema. At the time of discharge, the patient's laboratory values were sodium 133 mEg/L, potassium 4.4 mEg/L, chloride 100 mEg/L, eGFR 78 mL/min/1.73 m2, creatinine 0.66 mg/dL, and BUN 24.6 mg/dL, with no severe electrolyte imbalance or acute renal failure. Tracheostomy performed during the early phase of hospitalization was successfully decannulated. The patient's state of consciousness had not improved significantly at discharge, despite improvement of intracranial hemorrhage and brain edema findings on CT.
Several treatments have been used for refractory lymphedema (Table 2). CDT is generally considered as first-line treatment for lymphedema and combines manual lymphatic drainage, multilayer bandaging, physical therapy, and skin care[2,3]. IPC is one of the most commonly used treatments. It was reported that IPC stimulates lymphatic function and reduces lymphatic backflow[4]. Surgical methods, such as lymphovenous anastomosis or liposuction, could be considered for advanced lymphedema[5,6].
| Current treatments | |
| Conservative treatment | Complete decongestive therapy: Manual lymphatic drainage; Multilayer bandaging; Physical therapy; Skin care |
| Intermittent pneumatic compression | |
| Compression garments | |
| Surgical treatment | Lymphovenous anastomosis |
| Liposuction | |
| Debulking surgery (excision of lymphatic tissue) |
Although there is no currently established pharmacologic treatment for refractory lymphedema, several studies have demonstrated the therapeutic effects of anti-inflammatory pharmacologic agents on lymphedema. Oral administration of ketoprofen decreased skin thickness and improved histopathologic scores compared with placebo by an anti-inflammatory effect[7]. Moreover, it was reported that tacrolimus, a topical anti-inflammatory agent, improved secondary lymphedema with incremental vessel contraction and dermal back flow decrements[8].
Mannitol is a hyperosmolar agent used for intraophthalmic pressure control in glaucoma and prevention of dialysis-disequilibrium syndrome during dialysis. The combination of mannitol and furosemide is a widely used treatment for ICP control. Because of the hyperosmolar effect of mannitol, the increased osmolarity causes intracellular water to move to the extracellular matrix, and furosemide prevents brain cells from retaining water[9]. Furthermore, the diuretic effect of mannitol in the kidney results in inhibition of osmotic water resorption in the proximal tubules, and passive sodium reabsorption in the loop of Henle decreases[10].
Considering the hypertonic properties of mannitol to move water out of intracellular spaces, we hypothesized that mannitol and furosemide relieved focal tissue swelling by the same principle that underlies lowering ICP. That is, mannitol makes cells shrink, and furosemide accelerates fluid extraction in the kidney. According to Mercadante et al[11], a combination of hypertonic saline and high dose furosemide (500 mg/d) improved lower extremity edema by enhancing urine output. Compared to high dose furosemide (500 mg/d), the relatively low dose furosemide (20 mg/d) employed in the present case resulted in improvement of lymphedema. Therefore, it can be carefully speculated that mannitol may be effective for relief of lymphedema when combined with furosemide. Furthermore, because mannitol reduces lymphedema by osmosis, it can be tried in patients for whom other pharmacologic agents that have anti-inflammatory effects have failed.
Mannitol therapy might cause electrolyte imbalance, rebound cerebral edema, and kidney failure[12-14]. Mannitol use for ICP control in acute stroke is generally short-term (1-2 wk), but we used the above agents for longer to identify the effects of mannitol on lymphedema. One report found no significant difference in the fatality or severe disability rate between short-term use (1 wk) and long-term use (1 mo) of mannitol, based on limited data[15]. Since mannitol was applied for a long period in this case, the patients was carefully monitored for complications during hospitalization, including laboratory tests such as blood gas, electrolyte, serum osmolarity, creatinine and BUN (Table 1). Although eGFR decreased to 20% of baseline during the first week after admission, it recovered to the baseline level and was maintained until discharge. Except for this mild, temporary decrease in renal function, no serious complications occurred during hospitalization.
In this case, mannitol was used for 27 d (hospital days 1-27) after admission and then used for an additional 7 d (hospital days 52-58) under close monitoring. Considering that serious side effects are unlikely to occur when used in this way, it is considered appropriate to use within 1 mo. However, since these results represent administration in only on patient, it is necessary to verify the appropriate period of use through additional large-scale studies.
Intravenous hypertonic saline solution was reported to have a similar effect to mannitol in ICP control[16]. However, in this case, it was not possible to compare the effects of mannitol with hypertonic saline, and the superior effect of mannitol over hypertonic saline should be confirmed in large-scale, long-term study. This is a case report of refractory lymphedema after surgery for cervical cancer that was treated with mannitol and furosemide. Currently, there is no routine pharmacologic treatment for refractory lymphedema and this case suggests that the use of mannitol and furosemide may be considered as a treatment for lymphedema.
There are a few limitations to consider in our case report. First, this is a case report describing one patient. There are limitations to generalizing the use of mannitol to other patients with lymphedema. Second, since pharmacologic agents and CDT were co-administered, the results should be compared only to the effect of mannitol and furosemide. Third, high-dose mannitol therapy necessitates close monitoring because of its side effects, such as congestive heart failure, hyperosmolarity, hyponatremia, hypokalemia, and acute renal failure[8]. In the present case, there were no significant complications with mannitol use, but its side effects would need close monitoring in the outpatient setting.
Although our findings cannot be generalized to a larger population, the present case raises the possibility that a combination of mannitol and furosemide might be an effective therapeutic option for refractory lymphedema when CDT and IPC are ineffective. It is a noninvasive treatment option and could be combined with conventional physical therapy. However, further large-scale studies should be performed to clarify the effect of mannitol and furosemide on lymphedema.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dimopoulos S, Ni GX S-Editor: Gao CC L-Editor: A P-Editor: Li JH
| 1. | Grada AA, Phillips TJ. Lymphedema: Pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017;77:1009-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (1)] |
| 2. | O'Donnell TF Jr, Allison GM, Iafrati MD. A systematic review of guidelines for lymphedema and the need for contemporary intersocietal guidelines for the management of lymphedema. J Vasc Surg Venous Lymphat Disord. 2020;8:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Wang D, Lyons D, Skoracki R. Lymphedema: Conventional to Cutting Edge Treatment. Semin Intervent Radiol. 2020;37:295-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Gutierrez C, Karni RJ, Naqvi S, Aldrich MB, Zhu B, Morrow JR, Sevick-Muraca EM, Rasmussen JC. Head and Neck Lymphedema: Treatment Response to Single and Multiple Sessions of Advanced Pneumatic Compression Therapy. Otolaryngol Head Neck Surg. 2019;160:622-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Jørgensen MG, Toyserkani NM, Sørensen JA. The effect of prophylactic lymphovenous anastomosis and shunts for preventing cancer-related lymphedema: a systematic review and meta-analysis. Microsurgery. 2018;38:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Boyages J, Kastanias K, Koelmeyer LA, Winch CJ, Lam TC, Sherman KA, Munnoch DA, Brorson H, Ngo QD, Heydon-White A, Magnussen JS, Mackie H. Liposuction for Advanced Lymphedema: A Multidisciplinary Approach for Complete Reduction of Arm and Leg Swelling. Ann Surg Oncol. 2015;22 Suppl 3:S1263-S1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Rockson SG, Tian W, Jiang X, Kuznetsova T, Haddad F, Zampell J, Mehrara B, Sampson JP, Roche L, Kim J, Nicolls MR. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Gardenier JC, Kataru RP, Hespe GE, Savetsky IL, Torrisi JS, Nores GD, Jowhar DK, Nitti MD, Schofield RC, Carlow DC, Mehrara BJ. Topical tacrolimus for the treatment of secondary lymphedema. Nat Commun. 2017;8:14345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Thenuwara K, Todd MM, Brian JE Jr. Effect of mannitol and furosemide on plasma osmolality and brain water. Anesthesiology. 2002;96:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Dorman HR, Sondheimer JH, Cadnapaphornchai P. Mannitol-induced acute renal failure. Medicine (Baltimore). 1990;69:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 114] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Mercadante S, Villari P, Ferrera P, David F, Intravaia G. High-dose furosemide and small-volume hypertonic saline solution infusion for the treatment of leg edema in advanced cancer patients. J Pain Symptom Manage. 2009;37:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Davis M, Lucatorto M. Mannitol revisited. J Neurosci Nurs. 1994;26:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Troupp H, Valtonen S, Vapalahti M. Intraventricular pressure after administration of dehydrating agents to severely brain-injured patients: is there a rebound phenomenon? Acta Neurochir (Wien). 1971;24:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Oken DE. Renal and extrarenal considerations in high-dose mannitol therapy. Ren Fail. 1994;16:147-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Bereczki D, Fekete I, Prado GF, Liu M. Mannitol for acute stroke. Cochrane Database Syst Rev. 2007;CD001153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 16. | Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of hypertonic (10%) saline in patients with raised intracranial pressure after stroke. Stroke. 2002;33:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |