Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8666
Peer-review started: April 14, 2021
First decision: June 15, 2021
Revised: June 19, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: October 16, 2021
Processing time: 184 Days and 3.4 Hours
Diabetes mellitus affects people worldwide, and management of its acute complications or treatment-related adverse events is particularly important in critically ill patients. Previous reports have confirmed that hyperglycemia can increase the risk of mortality in patients cared in the intensive care unit (ICU). In addition, severe and multiple hypoglycemia increases the risk of mortality when using insulin or intensive antidiabetic therapy. The innovation of continuous glucose monitoring (CGM) may help to alert medical caregivers with regard to the development of hyperglycemia and hypoglycemia, which may decrease the potential complications in patients in the ICU. The major limitation of CGM is the measurement of interstitial glucose levels rather than real-time blood glucose levels; thus, there will be a delay in the treatment of hyperglycemia and hypoglycemia in patients. Recently, the European Union approved a state-of-art artificial intelligence directed loop system coordinated by CGM and a continuous insulin pump for diabetes control, which may provide a practical way to prevent acute adverse glycemic events related to antidiabetic therapy in critically ill patients. In this mini-review paper, we describe the application of CGM to patients in the ICU and summarize the pros and cons of CGM.
Core Tip: Management of diabetes mellitus induced acute complications or treatment-related adverse events is particularly important in critically ill patients. The innovation of continuous glucose monitoring (CGM) helps medical staffs to alert the emergence hyperglycemia and hypoglycemia, which may decrease potential complications in patients in intensive care unit. The major limitation of CGM is the measurement of interstitial glucose levels rather than the real-time blood glucose levels; thus, there will be a time gap in the appropriate treatment of hyperglycemia and hypoglycemia in patients. The European Union approved a state-of-art artificial intelligence directed loop system coordinated by CGM and a continuous insulin pump for diabetes control, providing a practical way to prevent acute adverse glycemic events related to antidiabetic therapy in critically ill patients.
- Citation: Sun MT, Li IC, Lin WS, Lin GM. Pros and cons of continuous glucose monitoring in the intensive care unit. World J Clin Cases 2021; 9(29): 8666-8670
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8666.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8666
Diabetes mellitus (DM) affected 451 million people in 2017 and will increase to 693 million by 2045[1]. Chronic hyperglycemia can lead to a high risk of DM complications. In contrast, interventions for DM may result in an episode of severe hypoglycemia, particularly with intensive treatments[2]. In addition, glucose deviations are related to oxidative stress, which increases the risk of complications[3] especially in critically ill patients[4-6], highlighting the issue of balancing blood glucose. The best way to control DM is to know the immediate level of blood glucose in an affected individual to prevent sustained hyperglycemia, incident hypoglycemia, or glucose changes. Currently, measurements of fingerstick blood glucose and HbA1c are widely applied. HbA1C is mainly used to detect the mean blood glucose in the past 3 to 4 mo. However, there were disadvantages when using these two methods. Fingerstick blood glucose can detect only one instant blood glucose; therefore, it does not represent long term day-to-week blood glucose levels. Although the HbA1c level represents the mean blood glucose in the past 3 mo, it does not reflect the fluctuations of blood glucose. To solve these shortcomings, a continuous glucose monitor (CGM) is a device developed to monitor interstitial glucose levels by a mini-invasive subcutaneous sensor[7].
CGM has two styles: Professional CGM (retrospective) and real-time CGM (RT-CGM). The main manufacturers, including Medtronic Guardian Connect, Dexcom G5/G6, and Abbott Freestyle Libre, have developed their CGM products for the market. Medtronic 630G and 670G can be integrated with an insulin pump, which requires at least two fingerstick calibrations per day. In contrast, the Dexcom and Abbott products are factory settings and do not need self-calibration. The Abbott Freestyle Libre is a flash glucose monitoring system belonging to a kind of retrospective CGM device that cannot provide real-time glucose levels. The mean absolute relative difference (MARD) defines the average of the absolute error between all CGM values and matched reference values[8], and a lower number means better accuracy. The MARDs of all of these devices are less than 10%. Since 2013, most professional CGMs have been used in outpatients, aimed at monitoring the glucose level without an alert below or beyond the cautious limits (blinded). A comprehensive reading of the interstitial glucose concentration data from the diabetic examinees was retrospectively performed in the hospital. According to the retrospective data from the professional CGM, the physicians would prescribe or make adjustments for the dosage or times that antidiabetic medications are given and lifestyle modifications, including diet and exercise, for the examinees. Thanks to an improvement in biotechnology of artificial intelligence and wearable devices in the last 4 years, RT-CGM has been developed and widely used in outpatients or inpatients with instant alerts (nonblinded).
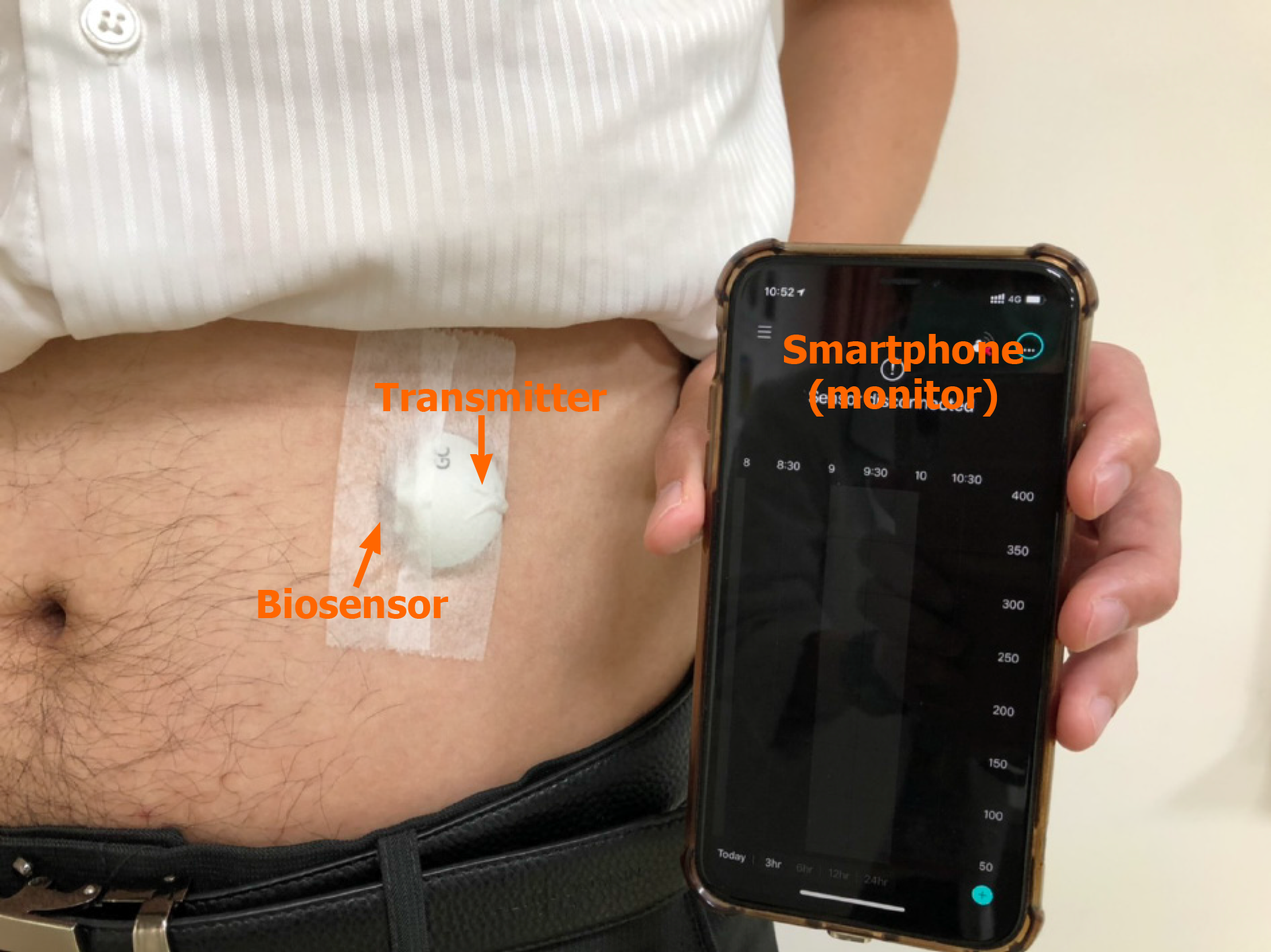
The RT-CGM system is mainly comprised of 3 components: The biosensor, transmitter and monitor (Figure 1). The biosensor is a tiny cannula penetrating the skin to obtain glucose levels in interstitial fluid. The biosensor must be changed every 7-14 d, and some biosensors can be used for a maximum of 90 d (Senseonics Eversense). The transmitter of RT-CGM is a small, coin-like, and reusable device that is connected to the biosensor to send the measured data of interstitial glucose levels wirelessly. Finally, the monitor receives the wireless real-time interstitial glucose signal. Some monitors can be applied to a smartphone so that patients can bring their own smartphone every time. The monitor can show real-time glucose levels and provide feedback in time when the interstitial glucose level is too high or too low. The smartphone can also send glucose readings to the cloud, and the medical staff can access the data and give advice to the outpatient clinic instantly. Finally, the large amount of data of glucose levels can be analyzed to produce an output that combines the glucose readings and suggested medications, diet and exercise amount through the cloud system.
RT-CGM was first applied in the intensive care unit (ICU) in 2003[9], and an error grid analysis showed that 60%-70% of the interstitial glucose levels obtained from the RT-CGM were clinically accurate and defined by a deviation of arterial blood glucose level < 20% using the glucometer[9]. According to the current evidence, tight glucose control can lower the risk of postoperative infection rates, short-term mortality and length of ICU stay in patients undergoing a major operation in the surgical ICU; however, it may also increase the risk of hypoglycemic episodes[10,11]. In addition, a previous review study reported that 22.4% of patients on tight glucose control experienced at least one hypoglycemic episode, defined as a blood glucose level less than 82 mg/dL, and more severe hypoglycemia events were associated with a higher risk of in-hospital mortality[12]. Another study also confirmed that the consequences of multiple hypoglycemic episodes were associated with a higher 90 d mortality in patients in the ICU[13]. Hypoglycemic events are commonly observed in the ICU, not merely in patients on antidiabetic therapy but also in those with a fasting status, severe sepsis or hepatic failure. The RT-CGM was found to reduce the absolute risk of severe hypoglycemia by 9.9% in critically ill patients under mechanical ventilation and continuous insulin infusion therapy[14]. Moreover, several studies have uncovered that having a greater glycemic variability of patients in the ICU was associated with a higher risk of in-hospital mortality independent of the mean level of blood glucose and incident hypoglycemia[6,15]. Therefore, frequent monitoring of blood glucose levels is very important in critically ill patients in the ICU; however, it is time-consuming and labor-intensive, especially in those with acute severe complications related to diabetes, such as diabetic ketoacidosis or hyperglycemic hyperosmotic syndrome. In these situations, the frequency of testing for blood glucose level is performed every 1-2 h. CGM provides an alternative method to estimate the subcutaneous interstitial glucose levels, which correlate well with the blood glucose levels and decrease the workload of ICU members due to the calibration only obtained 2-3 times per day. Another advantage of using a CGM is that if patients who need frequent monitoring of blood glucose levels are highly contagious, the risk of infectious microbial transmission to medical staff in the ICU might increase. Using CGM can decrease the caregiver’s time of contact with the patient and thus reduce the risk of infection, particularly in the COVID-19 pandemic[16].
Although severe hypoglycemic episodes can be reduced by using RT-CGM, it may not improve glycemic control (time above range or time in range) compared with intensive insulin therapy following the guidance of an algorithm[14,16,17]. Additionally, artificial intelligence can analyze the glucose level readings, medications and food automatically and predict mortality[18]. Recently, many closed-loop systems of integrated CGM and automated insulin delivery have been investigated and approved for specific groups of people[19]. Few major disadvantages of applying CGM to patients in the ICU are noted. First, the transportation of glucose from the blood to the subcutaneous interstitium takes 15-20 min[7]. The lag time should be taken into account if the levels of glucose highly fluctuate. Second, the lifespan of the biosensors is relatively short, approximately 7 d. Third, CGM needs calibration by fingerstick glucose, which is usually 2-3 times per day. Fourth, the timing of calibrations should be avoided after eating as the blood glucose level increases sharply and might not be increased in parallel to the glucose level in the interstitial fluid at the same time. Fifth, the readings for glucose levels are limited. For instance, the range of glucose levels can be merely within 40-400 mg/dL in Medtronic devices, which limits the surveillance range in critically ill patients. Finally, there is still a lack of adequate evidence for the correlation of glucose levels between the blood and interstitial fluids in patients with severe generalized edema, such as those with hypoalbuminemia and hepatic failure.
According to the latest evidence, using RT-CGM to monitor the interstitial glucose levels can reduce severe hypoglycemic events and may improve glycemic variability for patients cared for in the ICU. Whether these advantages of RT-CGM can decrease the risk of overall mortality in critically ill patients requires further investigation. In the COVID-19 pandemic, RT-CGM provides an alternative way to monitor the blood glucose levels of patients in need of care and reduce the ICU caregivers’ risk of infections due to the reduction in frequent contact with the affected patient merely for blood glucose testing. In the future, noninvasive and low-cost CGM may become more convenient in the ICU or outpatient use[20].
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Verma AK S-Editor: Wang LL L-Editor: A P-Editor: Xing YX
| 1. | Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan PF. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 695] [Cited by in RCA: 915] [Article Influence: 183.0] [Reference Citation Analysis (1)] |
| 2. | in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes. 1997;46:271-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 550] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 3. | Monnier L, Colette C. Glycemic variability: should we and can we prevent it? Diabetes Care. 2008;31 Suppl 2:S150-S154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Meynaar IA, Eslami S, Abu-Hanna A, van der Voort P, de Lange DW, de Keizer N. Blood glucose amplitude variability as predictor for mortality in surgical and medical intensive care unit patients: a multicenter cohort study. J Crit Care. 2012;27:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Ali NA, O'Brien JM Jr, Dungan K, Phillips G, Marsh CB, Lemeshow S, Connors AF Jr, Preiser JC. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36:2316-2321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 6. | Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 592] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 7. | Chen C, Zhao XL, Li ZH, Zhu ZG, Qian SH, Flewitt AJ. Current and Emerging Technology for Continuous Glucose Monitoring. Sensors (Basel). 2017;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 8. | Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, Garg S, Heinemann L, Hirsch I, Amiel SA, Beck R, Bosi E, Buckingham B, Cobelli C, Dassau E, Doyle FJ 3rd, Heller S, Hovorka R, Jia W, Jones T, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Maahs D, Murphy HR, Nørgaard K, Parkin CG, Renard E, Saboo B, Scharf M, Tamborlane WV, Weinzimer SA, Phillip M. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care. 2017;40:1631-1640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1375] [Cited by in RCA: 1383] [Article Influence: 172.9] [Reference Citation Analysis (0)] |
| 9. | Chee F, Fernando T, van Heerden PV. Closed-loop glucose control in critically ill patients using continuous glucose monitoring system (CGMS) in real time. IEEE Trans Inf Technol Biomed. 2003;7:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Wang YY, Hu SF, Ying HM, Chen L, Li HL, Tian F, Zhou ZF. Postoperative tight glycemic control significantly reduces postoperative infection rates in patients undergoing surgery: a meta-analysis. BMC Endocr Disord. 2018;18:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Clain J, Ramar K, Surani SR. Glucose control in critical care. World J Diabetes. 2015;6:1082-1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, Bailey M. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 13. | Kalfon P, Le Manach Y, Ichai C, Bréchot N, Cinotti R, Dequin PF, Riu-Poulenc B, Montravers P, Annane D, Dupont H, Sorine M, Riou B; CGAO-REA Study Group. Severe and multiple hypoglycemic episodes are associated with increased risk of death in ICU patients. Crit Care. 2015;19:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Holzinger U, Warszawska J, Kitzberger R, Wewalka M, Miehsler W, Herkner H, Madl C. Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care. 2010;33:467-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008-3013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 520] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 16. | Reutrakul S, Genco M, Salinas H, Sargis RM, Paul C, Eisenberg Y, Fang J, Caskey RN, Henkle S, Fatoorehchi S, Osta A, Srivastava P, Johnson A, Messmer SE, Barnes M, Pratuangtham S, Layden BT. Feasibility of Inpatient Continuous Glucose Monitoring During the COVID-19 Pandemic: Early Experience. Diabetes Care. 2020;43:e137-e138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | De Block CE, Gios J, Verheyen N, Manuel-y-Keenoy B, Rogiers P, Jorens PG, Scuffi C, Van Gaal LF. Randomized Evaluation of Glycemic Control in the Medical Intensive Care Unit Using Real-Time Continuous Glucose Monitoring (REGIMEN Trial). Diabetes Technol Ther. 2015;17:889-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Vettoretti M, Cappon G, Facchinetti A, Sparacino G. Advanced Diabetes Management Using Artificial Intelligence and Continuous Glucose Monitoring Sensors. Sensors (Basel). 2020;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Freckmann G, Link M, Kamecke U, Haug C, Baumgartner B, Weitgasser R. Performance and Usability of Three Systems for Continuous Glucose Monitoring in Direct Comparison. J Diabetes Sci Technol. 2019;13:890-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Noninvasive, Low Cost CGM for Type 2 Diabetes Coming in US and EU [Internet]. [cited 5 December 2020]. Available from: https://www.medscape.com/viewarticle/941851?nlid=138558_4961&src=WNL_mdplsnews_201204_mscpedit_card&uac=252983MK&spon=2&impID=2716352&faf=1#vp_2. |