Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8658
Peer-review started: February 25, 2021
First decision: April 21, 2021
Revised: April 30, 2021
Accepted: August 20, 2021
Article in press: August 20, 2021
Published online: October 16, 2021
Processing time: 232 Days and 1 Hours
The efficacy of traditional treatment for post-traumatic stress disorder (PTSD) is still unsatisfactory. Repetitive transcranial magnetic stimulation (rTMS) has been widely used in the treatment of various types of mental disorders, including PTSD. Although rTMS has been demonstrated to be effective in many cases, there are still arguments regarding its mechanism and protocol. This review aims to summarize the origin, development, principle, and future direction of rTMS and introduce this neuro-stimulation therapy to relevant clinicians.
Core Tip: This review concludes the update clinical development of repetitive transcranial magnetic stimulation (rTMS) in the treatment of post-traumatic stress disorder, providing the detail explanation of this emerging physical therapy. This review aims to summarize the origin, development, principle, and future direction of rTMS and introduce this neuro-stimulation therapy to relevant clinicians.
- Citation: Cheng P, Zhou Y, Xu LZ, Chen YF, Hu RL, Zou YL, Li ZX, Zhang L, Shun Q, Yu X, Li LJ, Li WH. Clinical application of repetitive transcranial magnetic stimulation for post-traumatic stress disorder: A literature review. World J Clin Cases 2021; 9(29): 8658-8665
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8658.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8658
Post-traumatic stress disorder (PTSD) is recognized as a complex psychiatric disorder. Based on the DSM-5 diagnostic criteria for PTSD[1], the four core symptoms of PTSD are repeated recurrence of a traumatic experience, continuous avoidance of stimuli related to the traumatic event, negative cognitive and mood changes, and sustained increased alertness. Many epidemiological studies have shown that the incidence of PTSD is increasing. An American study indicated that PTSD has a 12-mo prevalence of 3.5%[2]. The overall lifetime prevalence of PTSD reported in the World Mental Health studies was 3.9% for people with a randomly selected trauma[3]. In addition, PTSD has been shown to have a high level of comorbidity, especially co-occurring with anxiety disorder, mood disorder, or substance use disorder[4]. Due to the impairment of cognitive function and mental status in PTSD patients and the high rate of comorbidity, the burden of PTSD is potentially substantial and it has become one of the most common mental disorders affecting human health.
As PTSD is a common psychiatric illness affecting various populations, a multitude of pharmacological and neuro-stimulation treatments have been proposed to treat PTSD. However, conventional drugs for PTSD (such as selective serotonin reuptake inhibitors) are not very effective. A systematic review showed that at least one-third of PTSD patients did not achieve symptom relief following conventional therapy[5]. In the past decades, neurostimulation treatments, as a non-invasive and safe physical method, have been studied extensively by many psychiatric researchers. Among various kinds of neurostimulation treatments, transcranial magnetic stimulation (TMS) has been widely applied clinically for mental disorders. TMS delivers electrical stimuli through the scalp in conscious humans. Furthermore, researchers have indicated that repetitive TMS (rTMS) has been used as a treatment for psychiatric disorders, inducing changes in brain activity that can last beyond the stimulation period[6]. rTMS has been proved to be an efficient neuro-stimulation therapy for PTSD; however, there is still some debate regarding the mechanism and optimal parameters of rTMS.
Therefore, we summarize the available literature on the development of rTMS in the treatment of PTSD, the principle of rTMS for PTSD, and the clinical progress of rTMS in the management of PTSD for a better understanding of this neuro-stimulation therapy.
As early as in 1980, Merton and Morton[7] conducted the first experiments of transcranial stimulation in conscious humans. However, rTMS was used for the first time as a treatment for PTSD in 1998[8]. This was a case study that enrolled two patients stimulated with a figure-8 coil in the right frontal area (no specific region reported). The treatment parameters were set at 1 Hz, 1200 pulses/d, 17 sessions, and 30 sessions for each patient. During the course of treatment, the PTSD checklist score used to assess PTSD symptoms significantly improved.
Since then, many studies exploring the efficacy of rTMS in PTSD patients have emerged[9-12]. The main differences between these studies were the anatomic site of stimulation, the figure of the coil, the frequency of rTMS stimulation, the number of excitation pulses, and the type of traumatic event.
Cohen et al[12] conducted a sham-controlled study and demonstrated the beneficial effects of high-frequency rTMS (HF-rTMS) delivered to the right dorsolateral prefrontal cortex (DLPFC), but low frequency rTMS (LF-rTMS) stimulation applied to the DLPFC was not as effective as HF-rTMS. Furthermore, a study used a figure-8 coil, applied HF-rTMS to both the left and right DLPFC, and found a significant decrease in PTSD symptoms after left DLPFC stimulation, but a moderate improvement after right DLPFC stimulation[11]. However, other studies have demonstrated that LF-rTMS applied to the right DLPFC obtained curative benefits in a small patient sample (20 patients)[10]. Isserles et al[9] conducted a double-blind crossover study, using a novel H-coil at high frequency (20 Hz) stimulation to the medial prefrontal cortex in patients with refractory PTSD, and the results showed that the average score of the Clinician-Administered PTSD Scale-5 (CAPS-5), especially the intrusive symptom cluster, improved in patients receiving rTMS treatment. In conclusion, these rTMS-related studies with different parameters and various samples indicated that rTMS may be effective in treating the symptoms of PTSD.
The first systematic review about the efficiency of TMS in treating PTSD was conducted by Karsen et al[13], and this Meta-analysis of eight primary studies suggested that TMS in the treatment of PTSD was an effective and safe treatment. For stimulating areas, right-sided TMS may obtain better efficacy than left-sided TMS in the treatment of PTSD. In terms of frequency of TMS, it was still not clear which was better, high frequency or low frequency. Generally speaking, as a new physical treatment for PTSD, the safety and reliability of TMS have been widely proved. However, another review that focused on the frequency of rTMS for PTSD suggested that LF-rTMS can alleviate both PTSD and depression symptoms, while HF-rTMS mainly improve related symptoms of PTSD[14]. An updated evidence-based guideline released in 2020 concluded that the HF-rTMS applied to the right DLPFC in the treatment of PTSD can reach level B evidence (‘‘probably effective”)[15]. LF-rTMS applied to the same area of the brain can be as a substitutional way for patients with PTSD, since a comparative study[16] did not find obvious differences between the efficacy of LF-rTMS and HF-rTMS in stimulating the right DLPFC.
Many studies have suggested that PTSD is related to a dysregulated response to fear[17]. Neuronal circuits involved in fear are related to the development of PTSD symptoms[18], especially flashback symptoms. Both animal and human studies have suggested that several brain regions, including the medial prefrontal cortex, anterior cingulate cortex, hippocampus, and amygdala, were associated with fear formation and memory recovery[17,19-21]. Aberrant neuronal activities of the structures mentioned above may be potential reasons underlying the development and retention of PTSD-relevant symptoms.
Using TMS to modulate prefrontal structures of the brain has been recognized to have a potential role in the treatment of PTSD. The DLPFC is located in the emotion regulatory network like the amygdala and hippocampus. rTMS of DPLFC has demonstrated potential antidepressant efficacy via changing neuro-activity throughout this emotion regulatory network[22].
rTMS can regulate cortical activity after the period of stimulating activation, which makes rTMS a promising physical therapy for mental disorders. The physiological basis of this after-effect of rTMS has not yet been clarified. Many animal experiments have shown that the after-effect of rTMS in the treatment of mental illness is similar to long-term potentiation (LTP) or long-term depression (LTD)[23]. LTP or LTD means that when the pre-synaptic fibers are stimulated, the response of the post-synaptic cells will increase or decrease for a long period. Generally speaking, this enhancement will last for minutes or even months. Existing research suggests that the molecular biological mechanism of hippocampal neuron loss may be related to LTP.
The mechanism of LTP production must meet specific conditions[24]. First, strong depolarization of the postsynaptic membrane removes the blockade of magnesium ions on the N-methyl-D-aspartate (NMDA) receptor coupling channel[25]. Second, there is the excitatory release of pre-synaptic nerve terminals, mainly glutamate. Third, neurotransmitter binds to the post-synaptic membrane NMDA receptor, and then the ion channel opens for calcium ion influxes. Subsequently, when the endocellular calcium ion concentration is increased, calcium/calmodulin kinase II will be activated to redistribute the non-synaptic α-amino-3-carboxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors in the protruding posterior membrane to the synaptic network, resulting in increased AMPA receptor activity[26]; in addition, calcium ion influx activated protein kinase C increases the sensitivity of AMPA receptors to enhance synaptic response. Physical stimulation can amplify the excitatory post-synaptic potential (EPSP). rTMS stimulation can cause the EPSP to have a superimposing effect. When the post-synaptic membrane depolarization reaches a certain level, it can move the magnesium ions located in the NMDA receptor channel to prevent the influx of calcium ions. In this way, when the transmitter binds to the NMDA receptor, the channel opens and calcium ions flow in, and the intracellular calcium ion concentration increases, which in turn induces the production of LTP.
Therefore, rTMS may regulate the effects of LTP by interfering with the NMDA and AMPA pathways, and then cause neuroplasticity of the brain, thereby remodeling the original nerve structure, and intervene in the endocrine system to produce sustained therapeutic effects[6].
The four core symptoms of PTSD are repeated recurrence of a traumatic experience, continuous avoidance of stimuli related to the traumatic event, negative cognitive and mood changes, and sustained increased alertness[1]. rTMS can relieve all the core symptoms of PTSD, and this has been proved by many researchers, especially the re-experiencing of symptoms as well as continuous avoidance reactions[27]. rTMS of the right DLPFC interferes with episodic memory retrieval, related to the repeated recurrence of traumatic experience. Additionally, rTMS also has a certain therapeutic effect on the common comorbid symptoms, depression, and anxiety in PTSD patients[12,28,29].
rTMS is a non-invasive neurostimulation method altering brain activity through quickly repeated repetitive magnetic pulses of the coil’s electromagnetic fields. The extremely short duration of magnetic pulses is generated by the circular electrical currents. The magnetic field penetrates the brain areas painlessly, neither attenuating the magnetic field strength nor the induced voltage generated in the brain areas. Actually, the stimulation in brain areas is not caused by the magnetic field itself but the induced voltage of the movement of the magnetic field.
Different frequencies of rTMS can cause various influences in the excitability of the brain regions. Frequency lower than 1 Hz is called low frequency, and frequency higher than 5 Hz is called high frequency. Early electrophysiologic studies showed that LF-TMS inhibits and HF-TMS excites neurons in the stimulated brain regions[30]. Besides, the depth of rTMS stimulations also varies. In traditional rTMS, stimulations only can reach 3 cm from the coil surface to the cerebral cortex, while deep rTMS can reach about 5 cm, which is near twice as deep as the traditional rTMS[31].
For the stimulating areas of rTMS, the sites of stimulation are usually the left and right DLPFC and dorsomedial prefrontal cortex. The DLPFC participates in the suppression of trauma memory and related negative emotions by inhibiting the response of the amygdala. At present, most studies use rTMS of the DLPFC to improve PTSD-related symptoms. In the common methods of rTMS of the DLPFC, HF-rTMS stimulations are applied to the left-brain hemisphere, and the right-brain hemisphere is stimulated by LF-rTMS. Some studies showed that the combination of both stimulations mentioned above did not obtain additional benefits, compared to unilateral rTMS stimulation[32].
Most LF-rTMS studies usually set the stimulation frequency at 1 Hz, while the stimulation intensity and pulse number vary based on the specific requirements of studies. Generally speaking, LF-rTMS is widely perceived to have an inhibitory effect. However, at low intensities, which is less than the motor threshold (MT), LF-rTMS often did not obtain significant effects on motor excitability. This is possible because of the influences from the level of motor cortex excitability.
Some studies indicate that the differences in the feedback of LF-rTMS might be due to the level of motor cortex excitability of the targeted muscle. Concerning the motor-evoked-potential (MEP), the depression of MEP could be increased if LF-rTMS is preceded by a high-frequency subthreshold excitation as compared to just a single stimulus. Cortical depression can be increased by at least 60 min that way[33].
On the contrary, HF-rTMS is considered to enhance the excitability of cortical neurons. Berardelli et al[34] showed that HF-rTMS set at 120% of the MT could prolong MEP for 1 s, and their research indicated that rTMS may increase pre-synaptic inhibition of the Ia afferent fibers responsible for the H-reflex at the spinal level. Nevertheless, the duration of the effect induced by HF-rTMS varies depending on the stimulation intensity, the number of pulses, and the stimulation frequency. In some cases, the after-effects of HF-rTMS can last up to 90 min after the initial stimulation. Surprisingly, the after-effects induced by HF-rTMS may be reversed due to changes of stimulation. Modulations induced by HF-rTMS depend on the degree of excitability of motor neurons in the target muscle. If the target muscle underwent a brief isometric contraction before stimulation, MEP facilitation caused by HF-rTMS was longer compared to the control group that did not undergo contraction before stimulation.
Thus, according to the guideline of rTMS for PTSD, the level B evidence (probable efficacy) still applies to the HF-rTMS stimulation of the right DLPFC in PTSD with more evidence-based medical results. Therefore, we still believe that HF-rTMS stimulation will benefit PTSD patients more, but the effect of LF-rTMS in various regions of the brain for PTSD patients also requires further research.
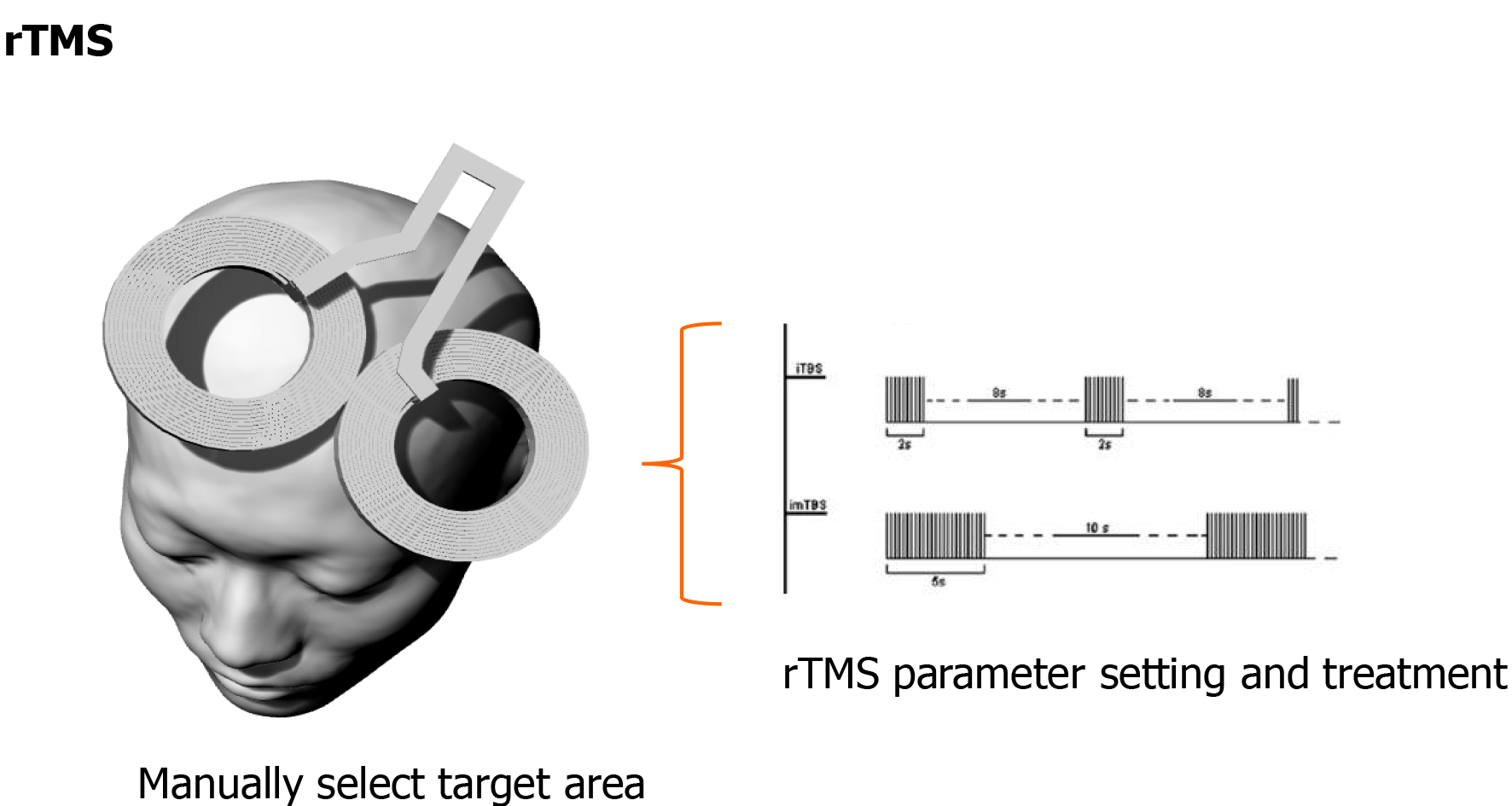
With the advancement of technology, the rTMS treatment protocol is constantly being updated. Theta burst stimulation (TBS) is the most frequently used new type of rTMS protocol, and has been used in animal studies to induce synaptic plasticity. The pattern of TBS is based on the brain’s natural theta rhythm occurring in the hippocampus. TBS consists of bursts of high-frequency stimulation. The intensity is subthreshold, usually set at 80% of the MT. Different patterns of TBS produce different effects on motor cortex excitability. An intermittent TBS (iTBS) protocol, with TBS applied for 2 s and then repeated every 10 s, increases motor cortex excitability[35,36]. The patterned nature of TBS resembles theta oscillations of hippocampal memory systems[37]. PTSD is defined, at its core, by the impact of intrusive traumatic memories, and in translational models TBS can induce hippocampal synaptic connections and activity[38]. A sham-controlled study of iTBS for PTSD indicated that PTSD symptoms, depression, and social and occupational function improved after iTBS treatment[39]. Neuroimaging studies also suggested that stronger connectivity within the default mode network and by anticorrelated cross-network connectivity is related to clinical improvement[40].
Clinically, TMS technology has some limitations. Magnetic stimulation requires long-term continuous stimulation of brain nerves in patients, and requires a high stimulation accuracy. As mentioned in Figure 1, traditional methods rely on doctors to manually move the coil position, which is inefficient and difficult to meet the position, angle, and coil direction criteria at the same time. In order to accurately locate the target of iTBS stimulation, the concept of precise iTBS stimulation was proposed.
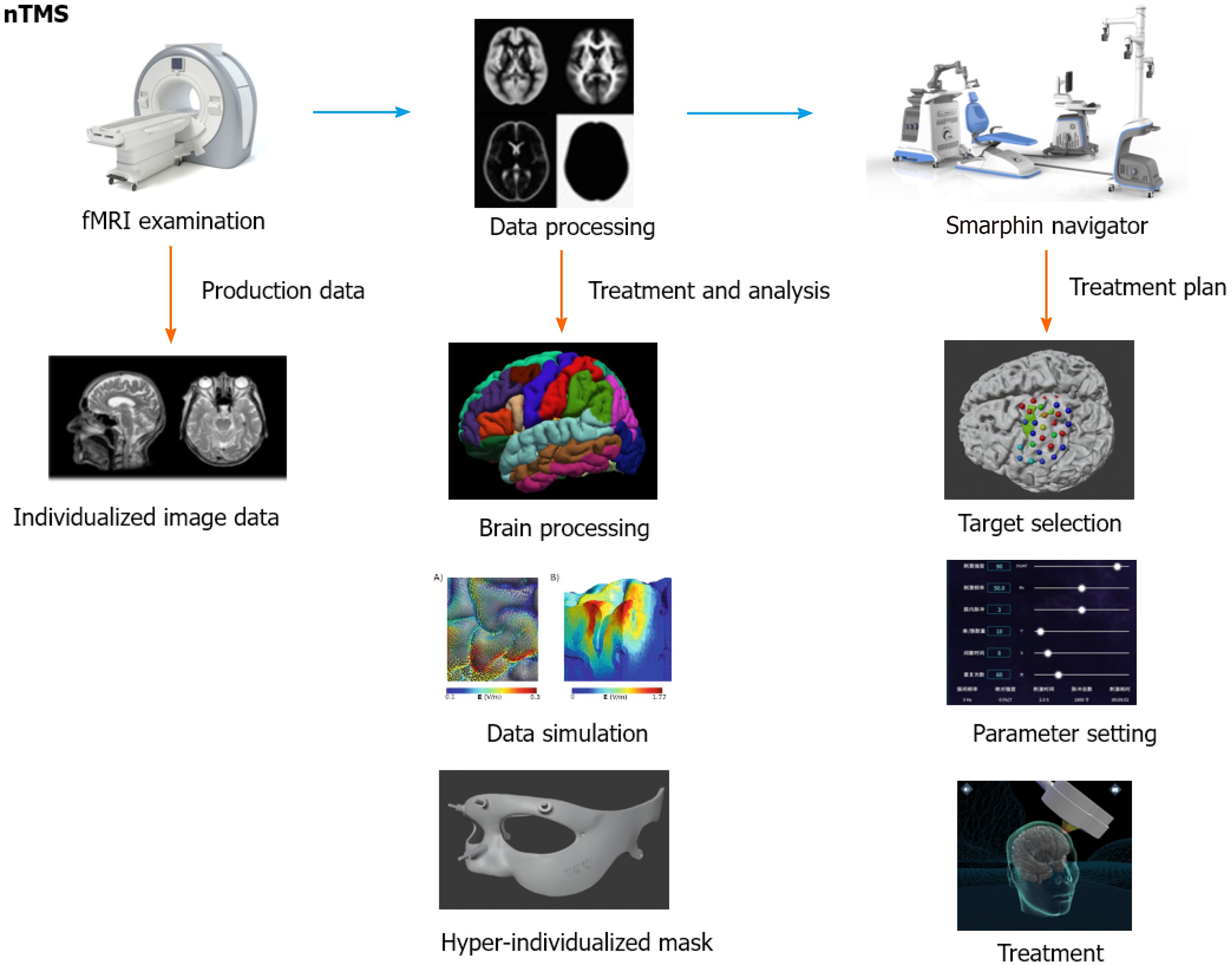
As shown in Figure 2, precise iTBS treatment can be divided into three parts: Brain imaging detection, data cloud analysis, and precise navigation stimulation. It can accurately locate and stimulate the brain area and adjust the Joule energy value of the stimulating magnetic field according to the patient's condition, thereby formulating precise and individualized treatment plans, and ultimately improving the treatment effect. A study using an magnetic resonance imaging-guided precise iTBS treatment plan to treat severe depression showed that 90.5% of participants met remission criteria, and this physical therapy was well tolerated and safe[41]. Although there is a certain theoretical basis, the therapeutic effect of precise iTBS therapy for PTSD requires further research.
This review suggests that rTMS is a promising physical treatment for PTSD with its safety and efficacy. The following inferences can be drawn from previous studies about rTMS for PTSD. Right-sided stimulation may be more effective than left-sided excitation; the optimal frequency of rTMS is still unclear since there is no evidence proving that which is better: rTMS or LF-rTMS. The optimal parameters of rTMS still require further research. In addition, rTMS used in the treatment of PTSD is generally well tolerated. As a new rTMS protocol, iTBS has broad application prospects in the treatment of PTSD. In the future treatment of PTSD, rTMS combined with traditional drug therapy and psychotherapy may achieve promising efficacy.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang WY S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Li JH
| 1. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). American Psychiatric, 2013. [DOI] [Full Text] |
| 2. | Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627. [PubMed] [DOI] [Full Text] |
| 3. | Watson P. PTSD as a Public Mental Health Priority. Curr Psychiatry Rep. 2019;21:61. [PubMed] [DOI] [Full Text] |
| 4. | Shalev A, Liberzon I, Marmar C. Post-Traumatic Stress Disorder. N Engl J Med. 2017;376:2459-2469. [PubMed] [DOI] [Full Text] |
| 5. | Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2007;CD003388. [PubMed] [DOI] [Full Text] |
| 6. | Klomjai W, Katz R, Lackmy-Vallée A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med. 2015;58:208-213. [PubMed] [DOI] [Full Text] |
| 7. | Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227. [PubMed] [DOI] [Full Text] |
| 8. | McCann UD, Kimbrell TA, Morgan CM, Anderson T, Geraci M, Benson BE, Wassermann EM, Willis MW, Post RM. Repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Arch Gen Psychiatry. 1998;55:276-279. [PubMed] [DOI] [Full Text] |
| 9. | Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, Zangen A. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder--a pilot study. Brain Stimul. 2013;6:377-383. [PubMed] [DOI] [Full Text] |
| 10. | Watts BV, Landon B, Groft A, Young-Xu Y. A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul. 2012;5:38-43. [PubMed] [DOI] [Full Text] |
| 11. | Boggio PS, Rocha M, Oliveira MO, Fecteau S, Cohen RB, Campanhã C, Ferreira-Santos E, Meleiro A, Corchs F, Zaghi S, Pascual-Leone A, Fregni F. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J Clin Psychiatry. 2010;71:992-999. [PubMed] [DOI] [Full Text] |
| 12. | Cohen H, Kaplan Z, Kotler M, Kouperman I, Moisa R, Grisaru N. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2004;161:515-524. [PubMed] [DOI] [Full Text] |
| 13. | Karsen EF, Watts BV, Holtzheimer PE. Review of the effectiveness of transcranial magnetic stimulation for post-traumatic stress disorder. Brain Stimul. 2014;7:151-157. [PubMed] [DOI] [Full Text] |
| 14. | Yan T, Xie Q, Zheng Z, Zou K, Wang L. Different frequency repetitive transcranial magnetic stimulation (rTMS) for posttraumatic stress disorder (PTSD): A systematic review and meta-analysis. J Psychiatr Res. 2017;89:125-135. [PubMed] [DOI] [Full Text] |
| 15. | Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, Filipović SR, Grefkes C, Hasan A, Hummel FC, Jääskeläinen SK, Langguth B, Leocani L, Londero A, Nardone R, Nguyen JP, Nyffeler T, Oliveira-Maia AJ, Oliviero A, Padberg F, Palm U, Paulus W, Poulet E, Quartarone A, Rachid F, Rektorová I, Rossi S, Sahlsten H, Schecklmann M, Szekely D, Ziemann U. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin Neurophysiol. 2020;131:474-528. [PubMed] [DOI] [Full Text] |
| 16. | Kozel FA, Van Trees K, Larson V, Phillips S, Hashimie J, Gadbois B, Johnson S, Gallinati J, Barrett B, Toyinbo P, Weisman M, Centorino M, Gibson CA, Catalano G. One hertz vs ten hertz repetitive TMS treatment of PTSD: A randomized clinical trial. Psychiatry Res. 2019;273:153-162. [PubMed] [DOI] [Full Text] |
| 17. | Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci. 2013;16:146-153. [PubMed] [DOI] [Full Text] |
| 18. | Moser DA, Aue T, Suardi F, Kutlikova H, Cordero MI, Rossignol AS, Favez N, Rusconi Serpa S, Schechter DS. Violence-related PTSD and neural activation when seeing emotionally charged male-female interactions. Soc Cogn Affect Neurosci. 2015;10:645-653. [PubMed] [DOI] [Full Text] |
| 19. | Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, Densmore M, Haswell CC, Ipser J, Koch SBJ, Korgaonkar M, Lebois LAM, Peverill M, Baker JT, Boedhoe PSW, Frijling JL, Gruber SA, Harpaz-Rotem I, Jahanshad N, Koopowitz S, Levy I, Nawijn L, O'Connor L, Olff M, Salat DH, Sheridan MA, Spielberg JM, van Zuiden M, Winternitz SR, Wolff JD, Wolf EJ, Wang X, Wrocklage K, Abdallah CG, Bryant RA, Geuze E, Jovanovic T, Kaufman ML, King AP, Krystal JH, Lagopoulos J, Bennett M, Lanius R, Liberzon I, McGlinchey RE, McLaughlin KA, Milberg WP, Miller MW, Ressler KJ, Veltman DJ, Stein DJ, Thomaes K, Thompson PM, Morey RA. Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biol Psychiatry. 2018;83:244-253. [PubMed] [DOI] [Full Text] |
| 20. | O'Doherty DC, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. 2015;232:1-33. [PubMed] [DOI] [Full Text] |
| 21. | Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387-12396. [PubMed] [DOI] [Full Text] |
| 22. | George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, Holtzheimer PE 3rd, Schwartz T, Sackeim HA. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507-516. [PubMed] [DOI] [Full Text] |
| 23. | Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:357-374. [PubMed] [DOI] [Full Text] |
| 24. | Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95-118. [PubMed] [DOI] [Full Text] |
| 25. | Fleming JJ, England PM. AMPA receptors and synaptic plasticity: a chemist's perspective. Nat Chem Biol. 2010;6:89-97. [PubMed] [DOI] [Full Text] |
| 26. | Cirillo G, Di Pino G, Capone F, Ranieri F, Florio L, Todisco V, Tedeschi G, Funke K, Di Lazzaro V. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017;10:1-18. [PubMed] [DOI] [Full Text] |
| 27. | Rossi S, Cappa SF, Ulivelli M, De Capua A, Bartalini S, Rossini PM. rTMS for PTSD: induced merciful oblivion or elimination of abnormal hypermnesia? Behav Neurol. 2006;17:195-199. [PubMed] [DOI] [Full Text] |
| 28. | Vernon LL, Dillon JM, Steiner AR. Proactive coping, gratitude, and posttraumatic stress disorder in college women. Anxiety Stress Coping. 2009;22:117-127. [PubMed] [DOI] [Full Text] |
| 29. | Herrold AA, Kletzel SL, Harton BC, Chambers RA, Jordan N, Pape TL. Transcranial magnetic stimulation: potential treatment for co-occurring alcohol, traumatic brain injury and posttraumatic stress disorders. Neural Regen Res. 2014;9:1712-1730. [PubMed] [DOI] [Full Text] |
| 30. | Speer AM, Kimbrell TA, Wassermann EM, D Repella J, Willis MW, Herscovitch P, Post RM. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry. 2000;48:1133-1141. [PubMed] [DOI] [Full Text] |
| 31. | Freire RC, Cabrera-Abreu C, Milev R. Neurostimulation in Anxiety Disorders, Post-traumatic Stress Disorder, and Obsessive-Compulsive Disorder. Adv Exp Med Biol. 2020;1191:331-346. [PubMed] [DOI] [Full Text] |
| 32. | Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015;11:1549-1560. [PubMed] [DOI] [Full Text] |
| 33. | Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:10867-10872. [PubMed] [DOI] [Full Text] |
| 34. | Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Currà A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122:79-84. [PubMed] [DOI] [Full Text] |
| 35. | Simonetta-Moreau M. Non-invasive brain stimulation (NIBS) and motor recovery after stroke. Ann Phys Rehabil Med. 2014;57:530-542. [PubMed] [DOI] [Full Text] |
| 36. | Di Lazzaro V, Profice P, Pilato F, Dileone M, Oliviero A, Ziemann U. The effects of motor cortex rTMS on corticospinal descending activity. Clin Neurophysiol. 2010;121:464-473. [PubMed] [DOI] [Full Text] |
| 38. | Capocchi G, Zampolini M, Larson J. Theta burst stimulation is optimal for induction of LTP at both apical and basal dendritic synapses on hippocampal CA1 neurons. Brain Res. 1992;591:332-336. [PubMed] [DOI] [Full Text] |
| 39. | Philip NS, Barredo J, Aiken E, Larson V, Jones RN, Shea MT, Greenberg BD, van 't Wout-Frank M. Theta-Burst Transcranial Magnetic Stimulation for Posttraumatic Stress Disorder. Am J Psychiatry. 2019;176:939-948. [PubMed] [DOI] [Full Text] |
| 40. | Akiki TJ, Averill CL, Abdallah CG. A Network-Based Neurobiological Model of PTSD: Evidence From Structural and Functional Neuroimaging Studies. Curr Psychiatry Rep. 2017;19:81. [PubMed] [DOI] [Full Text] |
| 41. | Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, Nejad R, Pankow H, Choi E, Aaron H, Espil FM, Pannu J, Xiao X, Duvio D, Solvason HB, Hawkins J, Guerra A, Jo B, Raj KS, Phillips AL, Barmak F, Bishop JH, Coetzee JP, DeBattista C, Keller J, Schatzberg AF, Sudheimer KD, Williams NR. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am J Psychiatry. 2020;177:716-726. [PubMed] [DOI] [Full Text] |