Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8627
Peer-review started: February 20, 2021
First decision: May 3, 2021
Revised: May 19, 2021
Accepted: August 24, 2021
Article in press: August 24, 2021
Published online: October 16, 2021
Processing time: 236 Days and 21.9 Hours
Neuroendocrine tumors (NETs) are a rare and heterogeneous disease group and constitute 0.5% of all malignancies. The annual incidence of NETs is increasing worldwide. The reason for the increase in the incidence of NETs is the detection of benign lesions, incidental detection due to the highest use of endoscopic and imaging procedures, and higher recognition rates of pathologists. There have been exciting developments regarding NET biology in recent years. Among these, first of all, somatostatin receptors and downstream pathways in neuroendocrine cells have been found to be important regulatory mechanisms for protein synthesis, hormone secretion, and proliferation. Subsequently, activation of the mammalian target of rapamycin pathway was found to be an important mechanism in angiogenesis and tumor survival and cell metabolism. Finally, the importance of proangiogenic factors (platelet-derived growth factor, vascular endothelial growth factor, fibroblastic growth factor, angiopoietin, and semaphorins) in the progression of NET has been determined. Using the combination of biomarkers and imaging methods allows early evaluation of the appropriateness of treatment and response to treatment.
Core Tip: Neuroendocrine tumors (NETs) originate from cells of the diffuse neuroendocrine system that can show both nerve and endocrine cell features and can be found in many organs in the body. NETs show different clinical and biological characteristics according to the regions where they develop. In recent years, there have been changes in the distribution of NETs within themselves, especially due to the more frequent use of screening colonoscopy and imaging methods. Using the combination of biomarkers and imaging methods allows early evaluation of the appropriateness of treatment and response to treatment.
- Citation: Yozgat A, Kekilli M, Altay M. Time to give up traditional methods for the management of gastrointestinal neuroendocrine tumours. World J Clin Cases 2021; 9(29): 8627-8646
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8627.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8627
Neuroendocrine tumors (NETs) originate from cells of the diffuse neuroendocrine system that can show both nerve and endocrine cell features and can be found in many organs in the body[1]. NETs are called functional (approximately 40%)[2] or non-functional according to the hormone secretion that causes clinical syndrome[3]. GI NETs originate from enterochromaffin cells, a member of the diffuse neuroendocrine system, while pancreatic NETs originate from Langerhans islets[4,5].
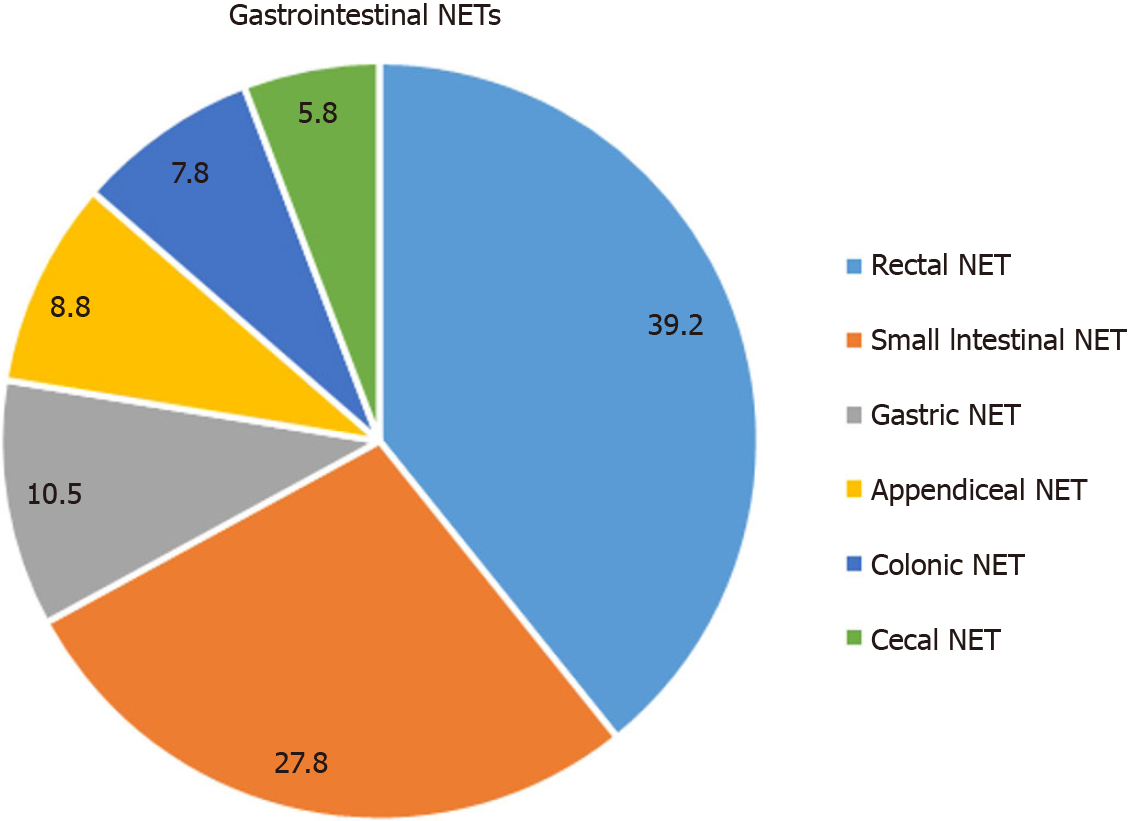
NETs show different clinical and biological characteristics according to the regions where they develop. In recent years, there have been changes in the distribution of NETs within themselves, especially due to the more frequent use of screening colonoscopy and imaging methods[6]. Although it can be seen in many organs, it is most commonly seen in the gastrointestinal system (44.6%), lungs (24.6%), primary of unknown origin (21%), pancreas (8.9%), liver, and thymus. According to the 20-year Surveillance, Epidemiology and End Results (SEER) registry data between 1995 and 2014, 39.2% of GI NETs originated from the rectum and 27.8% of the small intestine (SI)[7] (Figure 1). The distribution of GI NETs varies in different geographic regions; rectal NETs are more common in the Asia/Pacific region, while stomach and ileum NETs are more common in Europe[8,9].
While the term carcinoid has historically been used to describe well-differentiated NETs of the GI, AC, kidney, and ovary, World Health Organization (WHO) proposed the term NET for those of GI origin; in modern usage the term carcinoid is now used only for lung tumors.
PubMed database was used to search publications on neuroendocrine tumors. "Neuroendocrine tumor", "NET", "somatostatin receptor", "gastrointestinal NET" were used as keywords. Articles published in the English language were retrieved. Reference lists were manually verified.
NETs are a rare and heterogeneous disease group and constitute 0.5% of all malig
A carcinoid tumor histology was first described by Langerhans in 1869 and was used by WHO in 1980 to describe gastrointestinal NETs (GI-NETs)[16]. In 2000, NETs were classified as well-differentiated NETs and well-poorly differentiated neuroendocrine carcinomas (NECs) by WHO[17]. Until the 2010 classification, different classifications were used for NETs in different sites of the body, which was confusing. In this classification, a uniform classification is proposed for all NETs. The latest NET classification was published by WHO in 2017. According to this classification, NETs are histologically graded as Grade 1 (G1), Grade 2 (G2), and Grade 3 (G3) based on proliferation activity by using the mitotic index and/or Ki-67 proliferation index[18]. Many studies have shown that proliferative activity is important in determining the prognosis in NETs. Although studies are showing that the Ki-67 proliferative index is more important, it has been determined that its use with the mitotic index is more important in predicting survival[19,20]. Mitotic index is assessed by counting the number of mitoses at least 50 fields of 0.2 mm2 (high power fields; HPFs) in mitotically active areas[21]. To determine the Ki-67 proliferation index, at least 500 cell counts are required in the “hotspot areas” with the validated method[17]. It is stated in this classification that grade 3, which was previously thought to be only in NECs, can also be seen in well-differentiated tumors (NETs), and grading is no longer used in NECs and all are considered high grade[22]. NECs are poorly differentiated high grade (> 20 mitoses/2 mm and > 20 proliferative index) and highly aggressive tumors[23]. Tumors containing a neuroendocrine component and a morphologically and immunologically detectable non-neuroendocrine component are defined as mixed tumors (MINENs). Each of these components should account for at least 30% of the tumor[23]. WHO 2019 classification of GI-NETs is shown in Table 1.
| Type of NET | Differentiation | Grade | Mitotic Index | Proliferative index: Ki-67 |
| NET, grade 1 | Well-differantiated | Low | < 2 | < 3 |
| NET, grade 2 | Intermediate | 2-20 | 3-20 | |
| NET, grade 3 | High | > 20 | > 20 | |
| NEC, grade 3 | Poorly-differantiated | High | > 20 | > 20 |
| Small cell type; Large cell type | ||||
| MINENs | Well or poorly differentiated | Variable | ||
Neuroendocrine cells are epithelioid cells that develop from gastrointestinal (GI) stem cells rather than neurocrest[24]. There have been exciting developments regarding NET biology in recent years. Among these, first of all; somatostatin receptors and downstream pathways in neuroendocrine cells have been found to be important regulatory mechanisms for protein synthesis, hormone secretion, and proliferation. Subsequently, activation of the mammalian target of rapamycin (mTOR) pathway was found to be an important mechanism in angiogenesis and tumor survival and cell metabolism. Finally, the importance of proangiogenic factors (platelet-derived growth factor, vascular endothelial growth factor, fibroblastic growth factor, angiopoietin, and semaphorins) in the progression of NET has been determined[25]. The tumor microenvironment (TME) consists of the extracellular matrix and stromal, inflammatory, and endothelial cells; it is important in terms of tumor behavior, growth, response to treatment, and development of fibrotic complications[26]. Extracellular matrix degradation contributes to the development, aggressiveness, and progression of NETs. NET cells stimulate the activation and proliferation of fibroblasts with mediators such as transforming growth factor-β, platelet derived growth factor, and serotonin. Fibroblasts are a major element of TME and their activation causes fibrosis. Due to the hyperactivation of hypoxia-inducible factor 1-α, numerous proangiogenic factors are secreted, which explains why NETs have more vessel density than other tumors[27]. Different immune system cells infiltrate NET, forming an immunosuppressed TME suitable for tumor progression[28]. Immune checkpoint molecule programmed death-ligand 1 is expressed in a minority of NETs, and few of these patients are candidates for targeted therapy to immune checkpoint molecules. Studies targeting angiogenesis, tyrosine kinases, and other potential mechanisms related to TME are ongoing[25].
Neuroendocrine cells often have electron-dense granules containing peptide hormones and/or biological amines such as chromogranin, synaptophysin, and neuron-specific enolase according to the cell type. Since NETs are usually indolent and non-functional tumors, their diagnosis is delayed and they are diagnosed at a late stage. There are many biomarkers used for NETs, such as chromogranin a (CgA), neurokinin, serotonin, pancreastatin, pancreatic polypeptide, 5-hydroxyindoleacetic acid (5-HIAA), neurokinin A, and neuron-specific enolase, these are often insufficient in determining the location of the disease and are not necessary for the diagnosis of the disease. While it is still unclear whether a biomarker will show tumor burden in NETs, it does not indicate tumor grade or behavior[29]. The identification of somatostatin receptors immunohistochemically or by somatostatin receptor imaging is important in evaluating the response of NETs to somatostatin analogs or targeted peptide receptor radiotherapy. Well-differentiated NETs express approximately 80% somatostatin receptor subtype 2[30]. Using the combination of biomarkers and imaging methods allows early evaluation of the appropriateness of treatment and response to treatment[31,32].
The most commonly used biomarker is CgA, and there are several limitations to its use. CgA is an acidic glycoprotein that can be cleaved into smaller peptides such as pancreastatin. While the elevated CgA level in a defined NET is approximately 60%-90% sensitive and significant, its use is ineffective for the first-line diagnosis[33]. CgA levels are detected at higher levels in patients with higher tumor burden[34]. Besides, it should be kept in mind that CgA levels may increase in many neoplastic events other than NET and non-neoplastic diseases, and basal levels may change in situations such as food intake and protein pump inhibitor (PPI) use[35]. It is recommended to use a higher cut-off level to overcome these limitations[36]. There is insufficient data available for the general use of pancreastatin and other granins (CgB and CgC) so they are not widely used. Serotonin and its metabolite 5-HIAA were initially used widely as a NET marker. Serotonin increases, especially in metastatic small intestinal NETs. 5-HIAA can be used in patients with signs of carcinoid syndrome and should be measured before surgery to predict the risk of carcinoid crisis in patients with midgut NETs[37]. Gastrinomas cause Zollinger-Ellison syndrome, and an elevated fasting gastrin level is expected in these patients[38]. Although the gastrin level may increase physiologically due to atrophic gastritis, Heliobacter pylori infection, or PPI use, a level of > 1000 pg/mL is diagnostic for gastrinoma, and it is recommended to use the secretin test at lower levels[39].
The regulation of the release of many peptides and hormones secreted from neuroendocrine cells is regulated by somatostatin. This hormone acts by binding to somatostatin receptors (SSTR), and there are five different types in human cells. SSTR2 is overexpressed in the majority (80%) of NETs[40]. Visualization of NETs using SSTRs has revolutionized the diagnosis and treatment of NETs. Somatostatin receptor imaging (SRI) performed with 111In-pentetreotide (Octreoscan) SPECT, which was used extensively in cases where positron emission tomography (PET) could not be available, yielded successful results at a rate of 60%-80%. In recent years, 68Ga-labelled PET-SRI has become more prominent than other methods in functional imaging using SSTR with a success rate of 95%[41]. Among these, the most commonly used PET tracers are 68Ga- DOTATOC, 68Ga- DOTANOC, and 68Ga-DOTATATE[42]. Besides, the use of Cu-DOTATATE has recently been shown to have higher detection rates than Ga-labeled agents[43]; it is safe and provides high-quality images[44]. Numerous studies are ongoing with different PET SRI tracers.
Many well-differentiated GI NETs are closely associated with inherited syndromes; among these, the most common is multiple endocrine neoplasia type 1 (MEN1) and neurofibromatosis type 1 (NF1). No association was found between hereditary syndromes and poorly differentiated NECs[24]. The most common GI NETs are the duodenal gastrinoma and gastric carcinoids in MEN1, and carcinoids in the periampullary region in NF1[45].
Common tumor suppressor genes (e.g., p53, rb) and oncogene mutations (e.g., ras, myc, fos) in well-differentiated NETs are rare[46]. Because fewer analyses are done in GI NETs, molecular mechanisms are less understood than in pancreatic NETs. In a whole-exome sequencing study with SI NETs including 48 patients; most of them were along the mTOR pathway, and 14 mutations were identified including pho
Esophageal NETs are extremely rare and usually located in the middle and distal esophagus[49]. They are usually associated with Barret's esophagus or rarely with the heterotropic gastric mucosa[18]. The majority of esophageal NENs are NECs (approximately 90%), which are bulky, infiltrative, and large lesions, while NETs are smaller submucosal lesions[50]. CgA and synaptophysin are generally used to show neuroendocrine differentiation in esophageal NENs. Besides, rarely the combination of NEC with esophageal squamous cell carcinoma or adenocarcinoma is seen in esophageal MINENs. The progenitor cells of NENs in this region are thought to be Merkel and APUD cells[51].
Most of the esophageal NENs are poorly differentiated NEC, with more than 50% stage III at diagnosis, many have lymph nodes and distant metastases[52]. Pathologically, most of the NECs are small cell NECs. Since most of the patients are diagnosed at an advanced stage, surgery is performed (partial or total esophagectomy) and then adjuvant chemotherapy is given. Overall survival was 24 mo in a cohort of stage III esophageal NEC who underwent perioperative chemoradiotherapy with surgery[53].
Similar to other NETs, the incidence of gastric NETs (G-NET) has increased signi
| Type 1 | Type 2 | Type 3 | |
| Distribution, % | 70-80 | 5-6 | 15-30 |
| Gender predominance | F > M | F = M | M > F |
| Associated disease | Atrophic gastritis, pernicious anemia | ZES/MEN1 | None |
| Location: gastric | Body, fundus | Body, fundus | Anywhere |
| Tumor | |||
| Number | > 1 | >1 | 1 |
| Size: Generally | < 1 cm | < 1 cm, > 1 cm | > 2 cm |
| Mucosa | Atrophic | Hypertrophic | Normal |
| Gastrin level | Hypergastrinemia | Hypergastrinemia | Normal |
| Gastric pH | High | Low | Normal |
| Treatment | Endoscopic, Surgery | Endoscopic, Surgery | Surgery, LND |
| Prognosis, survival % | 100 | 60-90 | 50 |
Type 1: Type 1 NETs, which are the common type of G-NETs (70-80% of G-NETs), are usually associated with pernicious anemia, chronic atrophic gastritis, and Helicobacter pylori-associated atrophic gastritis[57,58]. Achlorhydria, which develops due to autoimmune chronic atrophic gastritis, causes D cell suppression and antral G cell hyperplasia, and gastrin hypersecretion occurs[59]. Hypergastrinemia also causes hyperplasia of ECL cells. It has been demonstrated that the use of PPIs in animal models may be associated with NETs, but this has not been demonstrated in humans[60]. These types of NETs are more common in women.
Type 1 NETs are small, multicentric, and non-functional, are located in the mucosa or submucosa of antrum and fundus, and are usually benign[61]. Generally, diagnosis is made in the 6th-7th decade by endoscopy performed due to non-specific symptoms. Although the serum CgA level increases, it is not specific for Type 1 G-NETs and can be used as a response to treatment and a surveillance marker[62]. The diagnosis is usually made by endoscopy. Endoscopically, they are usually < 1 cm, round, polypoid, and a depressed lesion with an ulcer located in the center. EUS can be performed to determine the invasion depth especially for a lesion larger than 1-2 cm. They are seen as a hypoechoic/isoechoic smooth, well-circumscribed lesion arising from the second (lamina propria) or third (submucosa) echo layer in EUS[63]. If there is a suspicion of metastasis or lymph node involvement, computed tomography (CT) and magnetic resonance imaging (MRI) should be performed. While metastasis is less common (~2%-5%) in lesions smaller than 2 cm, it can be up to 20% in large lesions[64]. The use of SRI with 68Ga-DOTATATE or new tracers is limited in type 1 NETs and limited to metastatic disease[65].
There is no consensus on the treatment of Type 1 G-NETs smaller than 1 cm; some clinicians recommend endoscopic resection [simple resection or endoscopic mucosal resection (EMR)], while others recommend conservative management[60]. In such lesions, treatment should be individualized. If the lesion is confined to mucosa or submucosa in EUS, endoscopic treatments [EMR, endoscopic submucosal dissection (ESD)] should be performed on patients with lesions larger than 1 cm. Surgery resection should be performed if there is a single lesion larger than 2 cm or if 3-4 lesions are larger than 1 cm in a person with six lesions[66]. Local excision or partial gastrectomy should be considered in patients with a positive margin or extension beyond the submucosa. Antrectomy is a controversial approach and can be reserved to prevent hypergastrinemia in patients with resistant disease to endoscopic treatments[67]. The use of somatostatin analogs is not recommended in early disease. It is effective in patients with multiple lesions that are not endoscopically eradicated[68] and may also be considered in metastatic disease. Netazepide is a gastrin/cholecystokinin-2 receptor antagonist, providing complete remission during treatment in Type 1 NETs[69]. Remission does not persist when treatment was discontinued, because the gastrin level does not change during treatment. Therefore, randomized studies are needed for its widespread recommendation[65].
Patients who undergo conservative management are recommended to be followed up with clinical and laboratory findings every 6-12 mo and endoscopically every 12-24 mo[65]. Although there is no consensus on how the surveillance will be in patients undergoing endoscopic resection, endoscopic surveillance is recommended at 1-3 years[67]. Patients benefit from endoscopic surveillance and resection strategy when necessary[70]. Five-year disease-related survival in type 1 G-NET is excellent and 100%[62].
Type 2: Type 2 accounts for about 5% of G-NETs and is the least common type. Its invasion and malignant potential are higher than Type 1[66]. There is submucosal involvement in 60% and lamina propria involvement in 10%, and 10%-30% had metastasis at the time of diagnosis in patients. Hypergastrinemia (greater than 1000 pg/mL) is also present in this type and unlike Type 1, it is usually related to MEN1 associated ectopic gastrinoma [Zollinger Ellison Syndrome (ZES)]. They occur in < 1% of patients with sporadic gastrinoma and 22%-33% of patients with MEN1/ZES[71,72]. Patients with ZES due to MEN1 usually have small duodenal gastrinomas, while sporadic gastrinomas are usually larger and located in the pancreas[73]. Hypergastrinemia causes ECL cell hypertrophy and hyperplasia. Small, multiple, and polypoid NETs are usually observed in the fundus and sometimes in the antrum and also gastric and duodenal ulcers on endoscopy. They are larger than Type 1 G-NET; only 35% are < 1 cm and 20% are > 2 cm. Type 2 G-NET is usually treated by removing the detected gastrinoma surgically[74]. Surgical removal of the gastrinoma leads to involution in NETs. If surgical removal is not possible, long-term and high-dose PPI treatment should be given. For NETs, similar to Type 1, endoscopic or surgical treatment should be performed based on size[75]. Annual post-treatment endoscopy surveillance is recommended after endoscopic treatment. Its prognosis is worse than that of Type 1, and the 5-year disease-related survival is between 60%-90%.
Type 3: Type 3 G-NET is not associated with hypergastrinemia or ZES, occurs sporadically, and accounts for 20% of G-NETs[76]. They have the highest malignant potential, and more than 50% of patients have local or liver metastases at the time of diagnosis[66]. While ECL cell hyperplasia is not seen in type 3 G-NET, a large number of endocrine cells associated with atypical carcinoid syndrome can be seen. Despite the large lesion, patients may be asymptomatic or present with abdominal pain, iron deficiency anemia, weight loss, and gastrointestinal bleeding. Generally, fasting serum gastrin level, and gastric acid production are at normal levels[77]. They are seen endoscopically as large, single, infiltrating, and sometimes ulcerated. These tumors are histologically well-differentiated, and a small number of patients may develop carcinoid syndrome due to the production of 5-hydroxytryptamine, and rarely, bronchospasm due to histamine production may develop in some patients[76]. Because the risk of metastasis is high, all patients should be staged with imaging methods (CT or MRI) and systemic treatment should be applied to metastatic disease. Surgery should be performed in non-metastatic patients[65]. A partial-subtotal-total gastrectomy and lymph node dissection are performed surgically. EMR or ESD may be considered in small (< 1 cm) Type 3 G-NETs without metastases[78]. Disease-related survival is 75% at 3 years and 50% at 5 years[76].
Neuroendocrine carcinoma: A poorly differentiated type, defined by some authors as Type 4 G-NET, neuroendocrine carcinoma accounts for 3%-8% of G-NETs. This type of G-NEC has a high malignant potential and is mostly present with metastasis. At the time of diagnosis, more than 80% of metastases are present, and the disease-related mortality rate is more than 50%[76]. It is a tumor that has an endocrine phenotype and behaves like adenocarcinoma[79]. Surgery is rarely required in these patients, except for palliative surgery.
Small intestine NETs: Duodenal and jejunoileal NETs will be discussed under separate headings as they differ from biological and clinical behavior.
Duodenal NETs (D-NETs) are rare tumors, most commonly located in the first and second part of the duodenum (> 90%), approximately 20% in the periampullary region[80]. Its annual incidence is 0.19 per 100000. It has been reported that its incidence has increased rapidly in recent years. It constitutes 2%-3% of duodenal tumors and 5% of GI-NETs. D-NETs are mostly < 2 cm in size and are located mucosally or sub
Gastrinoma: ZES and gastrinoma are terms that are often used synonymously. Gastrinomas are the most common duodenal NET (44%-66%)[85] with an annual incidence between 0.5-2 per million[86]. ZES occurs in 58% of duodenal gastrinomas. ZES develops due to ectopic gastrin secretion from duodenal or pancreatic NETs (gastrinoma). While the majority of gastrinomas are sporadic (75%-80%), lesions are generally solitary in these patients; gastrinomas associated with MEN1 are less common and are usually multiple and multicentric[87]. Hypergastrinemia causes parietal cell and gastric mucosa hypertrophy as well as ECL cell hypertrophy. This causes a 4-10 fold increase in basal and stimulated acid secretion[88]. Gastrin hypersecretion causes peptic ulcer disease (PUD), gastroesophageal reflux disease (GERD), and chronic diarrhea. Causes of diarrhea in gastrinoma are maldigestion and malabsorption due to the inhibition of pancreatic enzymes and the development of intestinal epithelial damage by the excessive gastric acid secretion that neutralizes pancreatic bicarbonate. Another reason is the excessive secretion of gastrin that reduces the absorption of water and sodium[39].
Gastrinomas are also the most common functional NETs in the pancreas, but only 25% are located in the pancreas, while more than half are located in the duodenum[39]. Most of the gastrinomas (60%-90%) are located in the gastrinoma triangle (the junction of the duodenum second and third part inferiorly, the junction of the cystic and main bile duct posteriorly, the junction of pancreatic neck and body)[89]. Gastrinomas may be non-duodenal and non-pancreatic localized at a lesser rate (5%-15%)[84]. Approximately 90% of duodenal gastrinomas are located in the first (56%) and second (32%) part of the duodenum[80]. Gastrinomas are malignant in 60%-90% of patients, with lymph node involvement and/or hepatic metastasis. Besides, 31% of patients have bone metastases, most of which are in the axial skeleton[90]. Duodenal gastrinomas are smaller (< 1 cm), multiple, and less likely to have liver metastasis than pancreatic ones. Duodenal gastrinomas usually develop in the submucosa layer and frequently infiltrate the mucosa, and large ones infiltrate the muscular layer[91].
Clinical findings in patients with gastrinoma are generally associated with gastric acid hypersecretion. Most of the patients have refractory and atypical PUD (> 90%), chronic diarrhea (50%), and GERD (55%) findings. PUD complications such as stricture, perforation, and bleeding have significantly decreased (30%) in recent years due to the widespread use of PPI[88]. Although there are many laboratory and imaging methods, the diagnosis of gastrinoma is usually delayed for 4-7 years[92]. The most important reasons for this are that gastrinoma is very rare, PUD disease is very common, gastrinoma findings are not different from PUD findings, and PPIs, which are effective antisecretory agents that can suppress even gastrinoma findings, are widely used.
The diagnosis of gastrinoma is made by detecting elevated fasting serum gastrin (FSG) concentration (> 99%) and inappropriate gastric acid secretion[92]. FSG concentration detected normally in repeated measurements probably excludes the diagnosis of gastrinoma. While an elevation of more than 10 times in the FSG concentration is significant for the diagnosis of gastrinoma, it can also be seen in conditions such as PPI use and achlorhydria. In this case, the FSG concentration should be measured again by discontinuing PPI therapy for at least 1 wk or gradually tapering it[93]. Approximately two-thirds of patients with gastrinoma have less than 10-fold higher FSG concentration, which can be seen in conditions such as gastric outlet obstruction, Helicobacter pylori infection, retained gastric antrum, and renal failure. In these patients, a secretin stimulation test should be performed to distinguish the cause of hypergastrinemia. An increase of > 120 pg/mL in serum gastrin level after administration of secretin indicates that the patient has gastrinoma with 94% sensitivity[39] and 100% specificity. Serum CgA level correlates with tumor burden, but FSG level is more specific for diagnosis than CgA level[33]. Because gastrinomas are usually small, it can sometimes be difficult to locate the tumor. Tumor localization should start with endoscopy first and then continue with triphasic CT and MRI examinations, and when necessary, 68Ga-labelled PET-SRI and EUS, respectively. Occasionally, angiography and venous sampling with secretin stimulation may be required. Rarely, when the tumor cannot be localized with these methods, duodenal transillumination and intraoperative ultrasound may be required during surgery[94].
Localized disease treatment (nonmalignant disease) consists of suppression of gastric acid secretion with any high-dose PPI and surgical treatment. While 60 mg omeprazole is sufficient in most patients, 60 mg twice a day should be used in MEN1/ZES patients and patients with severe GERD. Long-term disease-free survival can be achieved by removing the tumor in non-metastatic sporadic gastrinomas. Since MEN1/ZES is usually associated with multiple lesions and the risk of lymph node involvement is high, routine surgical practice is controversial[84]. Some experts recommend antisecretory treatment alone in this situation, while others recommend pancreatoduodenectomy, but it is not routinely recommended[95,96]. When making a treatment decision, the treatment should be individualized according to the patient's clinical characteristics. Survival associated with gastrinoma is usually associated with the malignant or benign behavior of the disease. While 10-year survival was 34% in patients with liver metastasis at the time of diagnosis, the 15-year survival was 83% in patients without liver metastasis[97]. In patients with MEN/ZES, the risk of metastasis is very low, and survival is significantly higher in these patients[98].
Somatostatinoma: Somatostatinoma is the second most common functional duodenal NET and a rare tumor with an incidence of 1 in 40 million. Somatostatinomas can be seen sporadically or with syndromes such as MEN1 (40%-50% cases), NF1, and Von Hippel Lindau[99]. Somatostatinomas are most commonly seen in the pancreas (68%), duodenum (19%), ampulla vater (3%), and small bowel (3%)[100]. Most of the patients are asymptomatic, and less than 10% present with symptoms due to excessive release of somatostatin. Duodenal somatostatinomas are generally well-differentiated, solitary, large, and non-functional tumors and present with abdominal pain, nausea, weight loss, and signs of obstruction in the biliary system in the late course of the disease, whereas pancreatic somatostatinomas are usually functional and present with findings related to somatostatinoma syndrome[101]. Somatostatinoma syndrome, which is defined by the triad of biliary lithiasis, diarrhea, and diabetes mellitus, develops due to the inhibitory effects of somatostatin on the gastrointestinal system and is rarely seen in the duodenal somatostatinoma. Somatostatinoma is malignant in 75% of patients, and most of them have lymph node and liver metastases at the time of diagnosis, but the malignant potential is less in duodenal tumors than pancreatic ones[102].
Since somatostatinomas are generally present with large lesions, and they can be detected by conventional methods such as CR and MRI. Those that cannot be detected by these methods can be detected by EUS and other endoscopic methods[103]. In patients presenting with symptoms of somatostatinoma syndrome, a serum fasting somatostatin level of > 30 pg/mL is diagnostic. Although endoscopic resection is an option for small and non-metastatic tumors (1-2 cm), most cases are advanced, requiring surgical resections, including excision of the primary tumor and lymph node dissection[104].
Gangliocytic paraganglioma: Gangliocytic paraganglioma (GP) is an extremely rare NET that is usually located in the second part of the duodenum near the ampulla of Vater[105]. Histopathologically, it consists of three components; ganglion-like cells, spindle-shaped cells, and epithelioid. It is mostly a benign tumor, the mean age at diagnosis is 53, and the mean size is 2.5 cm. At the time of diagnosis, lymph node metastasis is detected in approximately 10% of patients, and pancreatic involvement and liver metastasis are detected in 1% of patients. The most common symptoms are gastrointestinal bleeding (40%), abdominal pain (40%), anemia (17%), nausea (6%), and weight loss (4%)[106]. The diagnosis is usually established incidentally by endoscopic or conventional imaging methods. Endoscopically, it is submucosally located and well circumscribed. The treatment is performed by endoscopic methods in patients with localized disease in preoperative evaluation, while pancreaticoduodenectomy is performed in patients with advanced disease[107].
Non-functional D-NETs: Approximately 90% of D-NETs are non-functional. It is usually detected incidentally by endoscopic procedures and imaging methods such as CT and MRI, like other non-functional NETs, and in some cases, it is detected while being examined for symptoms such as nausea and vomiting. They are mucosal and submucosal tumors usually located in the first part of the duodenum. These D-NETs have 20%-54% lymph node metastasis[81]. While the size of the tumor does not predict lymph node involvement, the grade of the tumor is predictive for prognosis. In these lesions, invasion of the muscularis propria (MP) is important and is used together with the lesion size in making the treatment decision. In the treatment, first of all, the depth of invasion (presence of invasion to the MP) should be determined by CT, MRI, and EUS[108]. Endoscopic resection with ESD and EMR should be considered for lesions smaller than 1 cm without MP invasion. Endoscopic methods or surgical excision and lymph node dissection should be considered in patients without MP invasive lesions smaller than 2 cm. Surgical excision and lymph node dissection should be considered in all patients with lesions larger than 2 cm and with MP invasion[109].
Duodenal NEC: Duodenal NECs are poorly differentiated and aggressive, large tumors that account for about 3% of D-NETs and are usually located in the periampullary region[84]. In differentiating between a NET-G3 and NEC, the Ki-67 index in NEC is generally extremely high (> 75%), and the mitotic index is above 20. In some cases, immunohistochemical studies are needed[110]. Lymph node and liver metastases are usually present at the time of diagnosis. Since these tumors are generally non-functional, they do not cause symptoms in the early stage; symptoms depend on the compression or clinical effects of the tumor in the advanced stage. The most common symptoms at the time of diagnosis are jaundice, abdominal pain, nausea, vomiting, gastrointestinal bleeding, and weight loss[82]. Fluorodeoxyglucose-PET/CT is used as imaging modalities for NEC in conjunction with conventional examinations such as CT and MRI. SRI with 68Ga DOTATATE in NEC is usually negative in more than 50% of patients and is not diagnostic[111]. Besides, serum CgA level is high in approximately half of the patients, and its routine use is not recommended.
Adjuvant etoposide and cisplatin treatment are recommended after surgery in resectable lesions, while multimodal therapy including chemotherapy, radiotherapy, and surgery in appropriate cases is recommended for locally advanced lesions. In metastatic lesions, palliative chemotherapy is recommended with a combination of etoposide and cisplatin or agents containing irinotecan[112]. In studies evaluating immune checkpoint inhibitors (e.g., pembrolizumab) targeting PD-1 in patients with NEC and high-grade NEN, no sufficient response was obtained; additional studies are needed for its widespread use[113]. The prognosis of NEC is generally poor, with a median survival of 38 mo in localized disease and 5-14 mo in metastatic disease[111].
Jejunoileal NETs (SI-NETs): The incidence of SI-NETs has been increasing in recent years with an annual incidence of 1.05 per 100000. SI-NETs accounts for 27.8% of all GI-NETs[7]. The majority of SI-NETs are located in the ileum (70%-87%), and most of them are located in the terminal ileum (40%-70%). SI-NETs are usually small lesions (two-thirds of lesions are < 2 cm); in only 8% of patients, the lesion is > 5 cm[114]. It has been reported that 25%-56% of SI-NETs are multiple in different studies[115,116]. The majority of SI-NETs (90%) are well-differentiated G1 tumors and develop from ECL cells. They are often invasive, with approximately 50% of them have an invasion of the muscularis propria. They typically induce a mesenteric fibrotic reaction and tend to form a mesenteric mass with lymph nodes in about 50% of patients[117]. Consequently, patients may present with crampy abdominal pain, mesenteric ischemia, gastrointestinal bleeding, and obstructive symptoms. Emergency surgical intervention may be required in up to 25% of patients[118]. Although it progresses slowly, lymph node, liver, lung, mesentery, and bone metastases are found in most patients. Classical carcinoid syndrome findings in SI-NETs are not expected to be seen except for severe liver metastasis, since the hormones secreted from the tumor are inactivated in the liver[119].
SI-NETs and their metastases can be anatomically visualized with CT and MRI. SSTR is highly expressed in 80%-100% of SI-NETs. Eighty-eight percent to 93% of lesions that cannot be detected by conventional imaging methods can be detected with 68Ga DOTATATE SRI[120]. If the lesion cannot be detected by anatomical and functional imaging since it is mostly located in the terminal ileum, it can be detected by colonoscopy; rarely capsule endoscopy and double-balloon enteroscopy are required.
In the treatment of local and loco-regional disease, surgery and lymph node dissection is the recommended treatment. Prophylactic cholecystectomy is recommended in case of future gallstones due to SAs during surgery[121]. Unlike other NETs, since it has been shown to increase survival in SI-NETs, hepatic cytoreductive or debulking surgery (resection of the primary tumor) is recommended despite high recurrence rates[117,122].
Appendiceal NETs: Appendiceal NETs (A-NETs) are usually benign behavioral lesions that are detected coincidentally after an appendectomy, and in some cases, right hemicolectomy is performed together with appendectomy, since malignancy is suspected. The annual incidence is 0.1-0.6 per 100000 persons[123]. It was determined that the majority of appendiceal tumors (from 32% to 88%) were NETs in different series. The proportion of A-NETs in all GI-NETs has been found to vary widely (from 5%-10% to 38%) in different populations[11]. According to the data obtained from SEER in recent years, this rate was found to be 8.7%[7]. It is slightly more common in women than in men. The mean age of A-NETs at diagnosis is between 38-51-years-old. A-NET is detected in 3-5 out of 1000 appendectomies.
A-NETs develop from subepithelial neuroendocrine cells located in the mucosal and submucosal layers of the appendix. Approximately 90% of A-NETs are well-differentiated G1, and less than 1% are poorly-differentiated G3 tumors[124]. Most of the A-NETs are located in the appendix tip, and approximately 10% are located at the base. Appendectomy is sufficient except for those with deep meso-appendiceal invasion or margin positivity[123]. A-NETs located at the tip, which is the most common area, are not expected to cause appendicitis. Since most of them are detected incidentally, they are asymptomatic and rarely may cause abdominal pain and obstruction symptoms due to local invasion or metastasis. Carcinoid syndrome can be seen very rarely[123]. It is important to evaluate the patient in terms of local recurrence and metastasis risk after A-NET diagnosis. The most important negative prognostic factors are size, proliferation index (> 20), location of the tumor (base of the appendix), and presence of > 3 mm mesoappendiceal invasion. Survival is 100% after appendectomy in lesions smaller than 1 cm. In lesions larger than 2 cm, there is a risk of systemic dissemination of up to 40%[125]. If preoperative imaging has not been performed and the lesion is < 1 cm, additional imaging is not required, whereas imaging should be performed in patients with lesions > 2 cm, have negative prognostic factors, and suspected metastasis or locoregional invasion[123]. 68Ga DOTATATE SRI may be considered in the presence of metastasis or positive surgical margin. Measurement of serum and urine biomarkers (CgA, 5-HIAA) is generally not useful and routine use is not recommended except for distant metastases[126].
A-NETs smaller than 2 cm are usually cured by appendectomy. Those between 1-2 cm with negative prognostic factors and those > 2 cm should be treated with right hemicolectomy[123]. Size and the presence of metastases are the most important criteria in survival. While survival is 100% for tumors < 1 cm, 5-year survival is 70% for tumors > 2 cm. In patients with distant metastases, 5-year survival is less than 25%[127].
Colonic NETs: Colonic NETs (C-NETs) accounts for 3.5% of all NETs and 7.8% of GI-NETs[7]. Its annual incidence is about 0.2 per 100000 persons[128]. In recent years, the incidence among NETs has decreased relatively, probably due to the rapid increase in the frequency of rectal carcinoids. They are less common than rectal NETs but are more aggressive and poorly-differentiated tumors. C-NETs are diagnosed at a mean age of 65 years. They are usually detected incidentally on colonoscopy. Although 50% of them are asymptomatic at the time of diagnosis, they are usually large and metastasize to lymph nodes, liver, mesentery, and lung. More than half of the lesions are > 5 cm, and 45% are localized at the time of diagnosis[129]. Most of the C-NETs occurs in the cecum (68%) and have transmural invasion or invasion to adjacent organs (76%). Carcinoid syndrome is often caused by metastatic cecal NETs[130]. Colonic NETs smaller than 2 cm can be treated endoscopically (EMR or ESD), while those larger than 2 cm should be treated with segmental colon resection and lymph node dissection[131]. The tumor stage is the most important prognostic factor in prognosis. Five-year survival is 33% in metastatic disease and 70% in localized disease[132].
Rectal NETs: The incidence of rectal NETs (R-NETs) has been increasing in recent years. The most important reasons for this situation are the widespread use of colon cancer screening programs and diagnostic endoscopy. R-NETs make up 17% of all NETs and 39% of GI-NETs. The annual incidence of R-NETs is 1.04 per 100000[7,12]. Unlike C-NETs, R-NETs have an indolent behavior and more than 80% of them are smaller than 1 cm. R-NETs are diagnosed at a mean age of 56 years, and the majority (80%) of R-NETs are localized at the time of diagnosis. Most of them are pathologically well-differentiated (98% is G1-2).
Most of them are asymptomatic; uncommon symptoms are hematochezia, changes in bowel habit, and extremely rare carcinoid syndrome (0.7%). Most R-NETs are detected incidentally at colonoscopy[132]. Metastasis is less than 5% in tumors smaller than 1 cm, while this rate is approximately 20% in all R-NETs[133]. In tumors larger than 2 cm, the risk of metastasis is significantly higher (up to 80%). The most important prognostic risk factors in terms of metastasis and survival are size larger than 1 cm, high Ki-67 proliferation index, lymphovascular invasion, and atypical findings in endoscopy[134]. The most common metastasis sites are lymph node, bone, liver, and mesentery.
Total colonoscopy should be performed in all patients to assess different NETs. Since the risk of metastasis is very low in lesions smaller than 2 cm and located submucosally, there is no need for additional cross-sectional imaging like CT or MRI. If the lesion is larger than 2 cm or there is submucosal invasion, cross-sectional examinations and, if necessary, SRI should be performed to evaluate the extent of the disease. Endoscopic rectal ultrasonography (ERUS) may be considered to assess the depth of invasion and lymph node involvement[131].
Endoscopic polypectomy is considered sufficient for NETs that are confined to the submucosa, smaller than 1 cm, and have no poor prognostic factor. NETs between 1-2 cm in size, limited to the submucosa, and without poor prognostic factors should be evaluated for invasion by ERUS, and those without deep invasion should be treated with EMR or ESD. NETs larger than 2 cm should be treated with total mesorectal excision or low anterior resection such as adenocarcinoma. Surveillance is not necessary for lesions smaller than 2 cm and limited to the submucosa. Long term surveillance with cross-sectional imaging, colonoscopy, and ERUS should be warranted for patients with metastasis, lymph node involvement, and muscularis propria invasion[132,134].
Management of metastatic disease: Metastatic liver disease should be treated with a multidisciplinary approach. In these patients, loco-regional, systemic therapies, and surgery can be used individually or in combination[60]. Patients with potentially resectable (> 90% removable) liver metastases should undergo hepatic cytoreductive surgery, which provides reduction of tumor growth, also symptom control in patients with functional NET[135]. Only 5%-15% of patients are potentially resectable. Debulking surgery may be considered in patients with carcinoid syndrome and uncontrolled functional tumors. SAs should be initiated before debulking surgery to prevent carcinoid crisis in patients with carcinoid syndrome findings. Debulking surgery may also be considered in patients who do not progress for 6 mo and have local symptoms related to the tumor[136].
Local ablative treatments, especially radiofrequency ablation, can be used. Although the use of radiofrequency ablation in patients with large and multiple lesions is contraindicated, response rates of up to 95% have been reported[137]. Another treatment method that can be used for unresectable metastatic G-NETs is transarterial embolization and transarterial chemoembolization. Chemotherapeutics such as 5-fluorouracil (5-FU), mitomycin, cisplatin, and doxorubicin are used in transarterial chemoembolization. Its use is contraindicated in advanced liver disease and portal vein thrombosis. Five-year survival rates have been reported between 30%-50%[138]. Radioembolization using 90Yttrium (90Y) microspheres is another liver-directed treatment method, with a response rate of 32% and improvement in symptoms in 50% of patients[139,140].
In systemic treatment, SAs that inhibit the growth of tumor cells and show antiproliferative activity on TME is used. SAs also show antisecretory activity, especially in patients with carcinoid syndrome. In SRI performed using the aforementioned PET tracers, the SSTR status of the patient is determined and whether the patient will respond to his/her SAs is determined. The most commonly used SAs are octreotide and lanreotide. Improved progression-free survival was detected in patients[141,142]. Everolimus is an mTOR inhibitor that increases progression-free survival in non-functional GI-NETs and can be used in cases that progress despite other treatments[143]. Although numerous antiangiogenic agents have been studied in GI-NETs, none has been shown to have a significant effect. Peptide receptor radionuclide therapy is a treatment that can be used in SSTR-positive (especially SSTR2 and SSTR5) patients with metastatic GI-NETs[144]. The most commonly used radioisotope in peptide receptor radionuclide therapy is 177Lu-DOTATATE, and four cycles of treatment are recommended as standard. It should be considered in GI-NETs that progress under SA treatments[145].
In many studies on the effectiveness of cytotoxic chemotherapy in metastatic GI-NETs, only small responses were found. Some of the chemotherapeutics that have been investigated for effectiveness include capecitabine, dacarbazine, 5-FU, temozolomide, FOLFOX (oxaliplatin, folinic acid, and 5-FU) combination chemotherapy. These treatments can be considered in patients with progressive metastatic GI-NETs who have no other treatment options[146].
NETs are most frequently seen in the gastrointestinal system (44.6%); among GI NETs, most frequently originated from the rectum with 39.2% and the small intestine with 27.8%. The annual incidence has increased 6.4 times in the last 40 years. In this increase, incidental detection due to the highest use of endoscopic and imaging procedures has an important place. For many years, different classifications were used for NETs in different sites of the body, which was confusing. Since 2017, a uniform classification defined by WHO has been used for all NETs.
Somatostatin receptors and downstream pathways in neuroendocrine cells are important regulatory mechanisms for protein synthesis, hormone secretion, and proliferation. Well-differentiated NETs express approximately 80% somatostatin receptor subtype 2. The identification of SSTRs immunohistochemically or by SRI is important in evaluating the response of NETs to somatostatin analogs or targeted peptide receptor radiotherapy. In recent years, 68Ga-labelled PET-SRI has become more prominent than other methods in functional imaging using SSTR with a success rate of 95%[41]. With the use of new imaging techniques and new treatment modalities such as endoscopic (EMR-ESD), and local ablative treatments, radioembolization, SAs, and mTOR inhibitors, life expectancy in these patients is increasing.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Liu JH
| 1. | Modlin IM, Champaneria MC, Bornschein J, Kidd M. Evolution of the diffuse neuroendocrine system--clear cells and cloudy origins. Neuroendocrinology. 2006;84:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (11)] |
| 3. | Cives M, Strosberg J. An update on gastroenteropancreatic neuroendocrine tumors. Oncology (Williston Park). 2014;28:749-756, 758. [PubMed] |
| 4. | Grande E, Capdevila J, Barriuso J, Antón-Aparicio L, Castellano D. Gastroenteropancreatic neuroendocrine tumor cancer stem cells: do they exist? Cancer Metastasis Rev. 2012;31:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Klöppel G, Anlauf M, Perren A. Endocrine precursor lesions of gastroenteropancreatic neuroendocrine tumors. Endocr Pathol. 2007;18:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Taghavi S, Jayarajan SN, Powers BD, Davey A, Willis AI. Examining rectal carcinoids in the era of screening colonoscopy: a surveillance, epidemiology, and end results analysis. Dis Colon Rectum. 2013;56:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 7. | Sackstein PE, O'Neil DS, Neugut AI, Chabot J, Fojo T. Epidemiologic trends in neuroendocrine tumors: An examination of incidence rates and survival of specific patient subgroups over the past 20 years. Semin Oncol. 2018;45:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K, Doi R, Shimatsu A, Nishida T, Komoto I, Hirata Y, Nakamura K, Igarashi H, Jensen RT, Wiedenmann B, Imamura M. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 261] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 9. | Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 10. | Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia. 2017;19:991-1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 482] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 11. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3247] [Article Influence: 191.0] [Reference Citation Analysis (0)] |
| 12. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2491] [Article Influence: 311.4] [Reference Citation Analysis (4)] |
| 13. | Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran's Gastrointestinal and Liver Disease, Neuroendocrin Tumors. 11th ed Elsevier; 2021. |
| 14. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, Moss SF, Nilsson O, Rindi G, Salazar R, Ruszniewski P, Sundin A. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1183] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 15. | Siddiqa A, Adel H, Khan SA, Huda F, Sattar A. Gastrointestinal and pancreatic neuroendocrine tumours and carcinomas; a review of rare tumour type. J Pak Med Assoc. 2019;69:533-540. [PubMed] |
| 16. | de Herder WW, Rehfeld JF, Kidd M, Modlin IM. A short history of neuroendocrine tumours and their peptide hormones. Best Pract Res Clin Endocrinol Metab. 2016;30:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Bosman FT CF, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. World Health Organization; 2010. |
| 18. | Assarzadegan N, Montgomery E. What is New in the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System: Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch Pathol Lab Med. 2021;145:664-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 19. | Kemmerling R, Weyland D, Kiesslich T, Illig R, Klieser E, Jäger T, Dietze O, Neureiter D. Robust linear regression model of Ki-67 for mitotic rate in gastrointestinal stromal tumors. Oncol Lett. 2014;7:745-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | van Velthuysen ML, Groen EJ, van der Noort V, van de Pol A, Tesselaar ME, Korse CM. Grading of neuroendocrine neoplasms: mitoses and Ki-67 are both essential. Neuroendocrinology. 2014;100:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 22. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2442] [Article Influence: 488.4] [Reference Citation Analysis (3)] |
| 23. | Lokuhetty D WV, Watanabe R, Cree I. WHO classification of tumours of the digestive system. 5th ed. International Agency for Research on Cancer; 2018. |
| 24. | Patel N, Barbieri A, Gibson J. Neuroendocrine Tumors of the Gastrointestinal Tract and Pancreas. Surg Pathol Clin. 2019;12:1021-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Cives M, Pelle' E, Quaresmini D, Rizzo FM, Tucci M, Silvestris F. The Tumor Microenvironment in Neuroendocrine Tumors: Biology and Therapeutic Implications. Neuroendocrinology. 2019;109:83-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Laskaratos FM, Rombouts K, Caplin M, Toumpanakis C, Thirlwell C, Mandair D. Neuroendocrine tumors and fibrosis: An unsolved mystery? Cancer. 2017;123:4770-4790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Svejda B, Kidd M, Giovinazzo F, Eltawil K, Gustafsson BI, Pfragner R, Modlin IM. The 5-HT(2B) receptor plays a key regulatory role in both neuroendocrine tumor cell proliferation and the modulation of the fibroblast component of the neoplastic microenvironment. Cancer. 2010;116:2902-2912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | da Silva A, Bowden M, Zhang S, Masugi Y, Thorner AR, Herbert ZT, Zhou CW, Brais L, Chan JA, Hodi FS, Rodig S, Ogino S, Kulke MH. Characterization of the Neuroendocrine Tumor Immune Microenvironment. Pancreas. 2018;47:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 29. | Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, Metz DC, Heaney A, Kwekkeboom D, Strosberg J, Meyer T, Moss SF, Washington K, Wolin E, Liu E, Goldenring J. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015;16:e435-e446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 30. | Oberg KE, Reubi JC, Kwekkeboom DJ, Krenning EP. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology. 2010;139:742-753, 753.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Bodei L, Sundin A, Kidd M, Prasad V, Modlin IM. The status of neuroendocrine tumor imaging: from darkness to light? Neuroendocrinology. 2015;101:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Capdevila J, Meeker A, García-Carbonero R, Pietras K, Astudillo A, Casanovas O, Scarpa A. Molecular biology of neuroendocrine tumors: from pathways to biomarkers and targets. Cancer Metastasis Rev. 2014;33:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A--biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 34. | Zatelli MC, Torta M, Leon A, Ambrosio MR, Gion M, Tomassetti P, De Braud F, Delle Fave G, Dogliotti L, degli Uberti EC; Italian CromaNet Working Group. Chromogranin A as a marker of neuroendocrine neoplasia: an Italian Multicenter Study. Endocr Relat Cancer. 2007;14:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Modlin IM, Bodei L, Kidd M. Neuroendocrine tumor biomarkers: From monoanalytes to transcripts and algorithms. Best Pract Res Clin Endocrinol Metab. 2016;30:59-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 36. | Campana D, Nori F, Piscitelli L, Morselli-Labate AM, Pezzilli R, Corinaldesi R, Tomassetti P. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol. 2007;25:1967-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Woltering EA, Wright AE, Stevens MA, Wang YZ, Boudreaux JP, Mamikunian G, Riopelle JM, Kaye AD. Development of effective prophylaxis against intraoperative carcinoid crisis. J Clin Anesth. 2016;32:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Oberg K, Couvelard A, Delle Fave G, Gross D, Grossman A, Jensen RT, Pape UF, Perren A, Rindi G, Ruszniewski P, Scoazec JY, Welin S, Wiedenmann B, Ferone D; Antibes Consensus Conference participants. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Biochemical Markers. Neuroendocrinology. 2017;105:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 39. | Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006;85:295-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Chin RI, Wu FS, Menda Y, Kim H. Radiopharmaceuticals for Neuroendocrine Tumors. Semin Radiat Oncol. 2021;31:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Sadowski SM, Neychev V, Millo C, Shih J, Nilubol N, Herscovitch P, Pacak K, Marx SJ, Kebebew E. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J Clin Oncol. 2016;34:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 266] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 42. | Hofland J, Zandee WT, de Herder WW. Role of biomarker tests for diagnosis of neuroendocrine tumours. Nat Rev Endocrinol. 2018;14:656-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 43. | Johnbeck CB, Knigge U, Loft A, Berthelsen AK, Mortensen J, Oturai P, Langer SW, Elema DR, Kjaer A. Head-to-Head Comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. J Nucl Med. 2017;58:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 44. | Delpassand ES, Ranganathan D, Wagh N, Shafie A, Gaber A, Abbasi A, Kjaer A, Tworowska I, Núñez R. 64Cu-DOTATATE PET/CT for Imaging Patients with Known or Suspected Somatostatin Receptor-Positive Neuroendocrine Tumors: Results of the First U.S. Prospective, Reader-Masked Clinical Trial. J Nucl Med. 2020;61:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 45. | Jensen RT, Berna MJ, Bingham DB, Norton JA. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113:1807-1843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 46. | Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469-1492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 637] [Cited by in RCA: 543] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 47. | Banck MS, Kanwar R, Kulkarni AA, Boora GK, Metge F, Kipp BR, Zhang L, Thorland EC, Minn KT, Tentu R, Eckloff BW, Wieben ED, Wu Y, Cunningham JM, Nagorney DM, Gilbert JA, Ames MM, Beutler AS. The genomic landscape of small intestine neuroendocrine tumors. J Clin Invest. 2013;123:2502-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 48. | Verdugo AD, Crona J, Starker L, Stålberg P, Åkerström G, Westin G, Hellman P, Björklund P. Global DNA methylation patterns through an array-based approach in small intestinal neuroendocrine tumors. Endocr Relat Cancer. 2014;21:L5-L7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Ye L, Lu H, Wu L, Zhang L, Shi H, Wu HM, Tu P, Li M, Wang FY. The clinicopathologic features and prognosis of esophageal neuroendocrine carcinomas: a single-center study of 53 resection cases. BMC Cancer. 2019;19:1234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Liu M, Popa EC, Finnerty BM, Fahey TJ 3rd, Zarnegar R. Clinicopathological Features of Gastroesophageal Neuroendocrine Neoplasms. Curr Gastroenterol Rep. 2020;22:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Deng HY, Ni PZ, Wang YC, Wang WP, Chen LQ. Neuroendocrine carcinoma of the esophagus: clinical characteristics and prognostic evaluation of 49 cases with surgical resection. J Thorac Dis. 2016;8:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Erdem S, Troxler E, Warschkow R, Tsai C, Yerokun B, Schmied B, Stettler C, Blazer DG 3rd, Hartwig M, Worni M, Gloor B. Is There a Role for Surgery in Patients with Neuroendocrine Tumors of the Esophagus? Ann Surg Oncol. 2020;27:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Pellat A, Walter T, Augustin J, Hautefeuille V, Hentic O, Do Cao C, Lievre A, Coriat R, Hammel P, Dubreuil O, Cohen R, Couvelard A, André T, Svrcek M, Baudin E, Afchain P. Chemotherapy in Resected Neuroendocrine Carcinomas of the Digestive Tract: A National Study from the French Group of Endocrine Tumours. Neuroendocrinology. 2020;110:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Yang Z, Wang W, Lu J, Pan G, Pan Z, Chen Q, Liu W, Zhao Y. Gastric Neuroendocrine Tumors (G-Nets): Incidence, Prognosis and Recent Trend Toward Improved Survival. Cell Physiol Biochem. 2018;45:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 55. | Fraenkel M, Faggiano A, Valk GD. Epidemiology of Neuroendocrine Tumors. Front Horm Res. 2015;44:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 56. | Corey B, Chen H. Neuroendocrine Tumors of the Stomach. Surg Clin North Am. 2017;97:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 57. | Borch K, Ahrén B, Ahlman H, Falkmer S, Granérus G, Grimelius L. Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann Surg. 2005;242:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 58. | Sculco D, Bilgrami S. Pernicious anemia and gastric carcinoid tumor: case report and review. Am J Gastroenterol. 1997;92:1378-1380. [PubMed] |
| 59. | Burkitt MD, Pritchard DM. Review article: Pathogenesis and management of gastric carcinoid tumours. Aliment Pharmacol Ther. 2006;24:1305-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Laird AM, Libutti SK. Management of Other Gastric and Duodenal Neuroendocrine Tumors. Surg Oncol Clin N Am. 2020;29:253-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Dakin GF, Warner RR, Pomp A, Salky B, Inabnet WB. Presentation, treatment, and outcome of type 1 gastric carcinoid tumors. J Surg Oncol. 2006;93:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Ravizza D, Fiori G, Trovato C, Fazio N, Bonomo G, Luca F, Bodei L, Pelosi G, Tamayo D, Crosta C. Long-term endoscopic and clinical follow-up of untreated type 1 gastric neuroendocrine tumours. Dig Liver Dis. 2007;39:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Sato Y. Endoscopic diagnosis and management of type I neuroendocrine tumors. World J Gastrointest Endosc. 2015;7:346-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 64. | Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005;103:1587-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 65. | Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, De Herder WW, Pascher A, Ruszniewski P; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 66. | Basuroy R, Srirajaskanthan R, Prachalias A, Quaglia A, Ramage JK. Review article: the investigation and management of gastric neuroendocrine tumours. Aliment Pharmacol Ther. 2014;39:1071-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 67. | Gluckman CR, Metz DC. Gastric Neuroendocrine Tumors (Carcinoids). Curr Gastroenterol Rep. 2019;21:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 68. | Massironi S, Zilli A, Fanetti I, Ciafardini C, Conte D, Peracchi M. Intermittent treatment of recurrent type-1 gastric carcinoids with somatostatin analogues in patients with chronic autoimmune atrophic gastritis. Dig Liver Dis. 2015;47:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 69. | Boyce M, Moore AR, Sagatun L, Parsons BN, Varro A, Campbell F, Fossmark R, Waldum HL, Pritchard DM. Netazepide, a gastrin/cholecystokinin-2 receptor antagonist, can eradicate gastric neuroendocrine tumours in patients with autoimmune chronic atrophic gastritis. Br J Clin Pharmacol. 2017;83:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 70. | Chin JL, O'Connell J, Muldoon C, Swan N, Reynolds JV, Ravi N, Geoghegan J, Conlon KC, O'Shea D, O'Toole D. Selective Resection of Type 1 Gastric Neuroendocrine Neoplasms and the Risk of Progression in an Endoscopic Surveillance Programme. Dig Surg. 2021;38:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore). 2004;83:43-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 72. | Berna MJ, Annibale B, Marignani M, Luong TV, Corleto V, Pace A, Ito T, Liewehr D, Venzon DJ, Delle Fave G, Bordi C, Jensen RT. A prospective study of gastric carcinoids and enterochromaffin-like cell changes in multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: identification of risk factors. J Clin Endocrinol Metab. 2008;93:1582-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 73. | Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist. 2014;19:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Dias AR, Azevedo BC, Alban LBV, Yagi OK, Ramos MFKP, Jacob CE, Barchi LC, Cecconello I, Ribeiro U Jr, Zilberstein B. Gastric neuroendocrine tumor: review and update. Arq Bras Cir Dig. 2017;30:150-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 75. | Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, Kulke MH, Liu EH, Metz DC, Phan AT, Sippel RS, Strosberg JR, Yao JC; North American Neuroendocrine Tumor Society. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 444] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 76. | Lawrence B, Kidd M, Svejda B, Modlin I. A clinical perspective on gastric neuroendocrine neoplasia. Curr Gastroenterol Rep. 2011;13:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Kwon YH, Jeon SW, Kim GH, Kim JI, Chung IK, Jee SR, Kim HU, Seo GS, Baik GH, Choi KD, Moon JS. Long-term follow up of endoscopic resection for type 3 gastric NET. World J Gastroenterol. 2013;19:8703-8708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Modlin IM, Kidd M, Lye KD. Biology and management of gastric carcinoid tumours: a review. Eur J Surg. 2002;168:669-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Hoffmann KM, Furukawa M, Jensen RT. Duodenal neuroendocrine tumors: Classification, functional syndromes, diagnosis and medical treatment. Best Pract Res Clin Gastroenterol. 2005;19:675-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 81. | Soga J. Endocrinocarcinomas (carcinoids and their variants) of the duodenum. An evaluation of 927 cases. J Exp Clin Cancer Res. 2003;22:349-363. [PubMed] |
| 82. | Rossi RE, Rausa E, Cavalcoli F, Conte D, Massironi S. Duodenal neuroendocrine neoplasms: a still poorly recognized clinical entity. Scand J Gastroenterol. 2018;53:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 83. | Scherübl H, Cadiot G, Jensen RT, Rösch T, Stölzel U, Klöppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy. 2010;42:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 84. | Delle Fave G, Kwekkeboom DJ, Van Cutsem E, Rindi G, Kos-Kudla B, Knigge U, Sasano H, Tomassetti P, Salazar R, Ruszniewski P; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology. 2012;95:74-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 85. | Rosentraeger MJ, Garbrecht N, Anlauf M, Raffel A, Knoefel WT, Wiedenmann B, Klöppel G. Syndromic vs non-syndromic sporadic gastrin-producing neuroendocrine tumors of the duodenum: comparison of pathological features and biological behavior. Virchows Arch. 2016;468:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 86. | Oberg K. Pancreatic endocrine tumors. Semin Oncol. 2010;37:594-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 87. | Jensen RT, Niederle B, Mitry E, Ramage JK, Steinmuller T, Lewington V, Scarpa A, Sundin A, Perren A, Gross D, O'Connor JM, Pauwels S, Kloppel G; Frascati Consensus Conference; European Neuroendocrine Tumor Society. Gastrinoma (duodenal and pancreatic). Neuroendocrinology. 2006;84:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 88. | Norton JA, Foster DS, Ito T, Jensen RT. Gastrinomas: Medical or Surgical Treatment. Endocrinol Metab Clin North Am. 2018;47:577-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 89. | Sugg SL, Norton JA, Fraker DL, Metz DC, Pisegna JR, Fishbeyn V, Benya RV, Shawker TH, Doppman JL, Jensen RT. A prospective study of intraoperative methods to diagnose and resect duodenal gastrinomas. Ann Surg. 1993;218:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 110] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 90. | Leboulleux S, Dromain C, Vataire AL, Malka D, Aupérin A, Lumbroso J, Duvillard P, Elias D, Hartl DM, De Baere T, Guigay J, Schlumberger M, Ducreux M, Baudin E. Prediction and diagnosis of bone metastases in well-differentiated gastro-entero-pancreatic endocrine cancer: a prospective comparison of whole body magnetic resonance imaging and somatostatin receptor scintigraphy. J Clin Endocrinol Metab. 2008;93:3021-3028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Klöppel G, Anlauf M. Gastrinoma--morphological aspects. Wien Klin Wochenschr. 2007;119:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J Gastroenterol. 2012;18:5495-5503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 93. | Metz DC, Cadiot G, Poitras P, Ito T, Jensen RT. Diagnosis of Zollinger-Ellison syndrome in the era of PPIs, faulty gastrin assays, sensitive imaging and limited access to acid secretory testing. Int J Endocr Oncol. 2017;4:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 94. | Jensen RT, Norton JA. Treatment of Pancreatic Neuroendocrine Tumors in Multiple Endocrine Neoplasia Type 1: Some Clarity But Continued Controversy. Pancreas. 2017;46:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 95. | Jensen RT, Ito T. Gastrinoma. 2020 Nov 21. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Grossman A, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000 . [PubMed] |
| 96. | Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML; Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97:2990-3011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 882] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 97. | Norton JA, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, Goebel SU, Peghini PL, Roy PK, Gibril F, Jensen RT. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 312] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 98. | Weber HC, Venzon DJ, Lin JT, Fishbein VA, Orbuch M, Strader DB, Gibril F, Metz DC, Fraker DL, Norton JA. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 302] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 99. | Nesi G, Marcucci T, Rubio CA, Brandi ML, Tonelli F. Somatostatinoma: clinico-pathological features of three cases and literature reviewed. J Gastroenterol Hepatol. 2008;23:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 100. | Mansour JC, Chen H. Pancreatic endocrine tumors. J Surg Res. 2004;120:139-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 101. | Tanaka S, Yamasaki S, Matsushita H, Ozawa Y, Kurosaki A, Takeuchi K, Hoshihara Y, Doi T, Watanabe G, Kawaminami K. Duodenal somatostatinoma: a case report and review of 31 cases with special reference to the relationship between tumor size and metastasis. Pathol Int. 2000;50:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Doherty GM. Rare endocrine tumours of the GI tract. Best Pract Res Clin Gastroenterol. 2005;19:807-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Elangovan A, Zulfiqar H. Somatostatinoma. StatPearls. 2020. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430685/. |
| 104. | Singh S, Moody L, Chan DL, Metz DC, Strosberg J, Asmis T, Bailey DL, Bergsland E, Brendtro K, Carroll R, Cleary S, Kim M, Kong G, Law C, Lawrence B, McEwan A, McGregor C, Michael M, Pasieka J, Pavlakis N, Pommier R, Soulen M, Wyld D, Segelov E; Commonwealth Neuroendocrine Tumour Collaboration (CommNETS) Follow-up Working Group. Follow-up Recommendations for Completely Resected Gastroenteropancreatic Neuroendocrine Tumors. JAMA Oncol. 2018;4:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 105. | Okubo Y, Yokose T, Tuchiya M, Mituda A, Wakayama M, Hasegawa C, Sasai D, Nemoto T, Shibuya K. Duodenal gangliocytic paraganglioma showing lymph node metastasis: a rare case report. Diagn Pathol. 2010;5:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 106. | Okubo Y, Yokose T, Motohashi O, Miyagi Y, Yoshioka E, Suzuki M, Washimi K, Kawachi K, Nito M, Nemoto T, Shibuya K, Kameda Y. Duodenal Rare Neuroendocrine Tumor: Clinicopathological Characteristics of Patients with Gangliocytic Paraganglioma. Gastroenterol Res Pract. 2016;2016:5257312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 107. | Kwon J, Lee SE, Kang MJ, Jang JY, Kim SW. A case of gangliocytic paraganglioma in the ampulla of Vater. World J Surg Oncol. 2010;8:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 108. | Min BH, Kim ER, Lee JH, Kim KM, Min YW, Rhee PL, Kim JJ, Rhee JC. Management strategy for small duodenal carcinoid tumors: does conservative management with close follow-up represent an alternative to endoscopic treatment? Digestion. 2013;87:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Weatherall T, Denbo J, Sharpe J, Martin M, O'Brien T, Gupta R, Groshart K, Behrman S, Dickson P. Well-Differentiated, Non-Functional, Non-Ampullary Duodenal Neuroendocrine Tumors: Toward Defining Evaluation and Management. World J Surg. 2017;41:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L, Basturk O, Allen PJ, Klimstra DS. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin Cancer Res. 2016;22:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 111. | Walter T, Tougeron D, Baudin E, Le Malicot K, Lecomte T, Malka D, Hentic O, Manfredi S, Bonnet I, Guimbaud R, Coriat R, Lepère C, Desauw C, Thirot-Bidault A, Dahan L, Roquin G, Aparicio T, Legoux JL, Lombard-Bohas C, Scoazec JY, Lepage C, Cadiot G; CEPD investigators. Poorly differentiated gastro-entero-pancreatic neuroendocrine carcinomas: Are they really heterogeneous? Eur J Cancer. 2017;79:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 112. | Yamaguchi T, Machida N, Morizane C, Kasuga A, Takahashi H, Sudo K, Nishina T, Tobimatsu K, Ishido K, Furuse J, Boku N, Okusaka T. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014;105:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 113. | Vijayvergia N, Dasari A, Deng M, Litwin S, Al-Toubah T, Alpaugh RK, Dotan E, Hall MJ, Ross NM, Runyen MM, Denlinger CS, Halperin DM, Cohen SJ, Engstrom PF, Strosberg JR. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials. Br J Cancer. 2020;122:1309-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 114. | Soga J. Carcinoids of the small intestine: a statistical evaluation of 1102 cases collected from the literature. J Exp Clin Cancer Res. 1997;16:353-363. [PubMed] |
| 115. | Yantiss RK, Odze RD, Farraye FA, Rosenberg AE. Solitary vs multiple carcinoid tumors of the ileum: a clinical and pathologic review of 68 cases. Am J Surg Pathol. 2003;27:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 116. | Keck KJ, Maxwell JE, Utria AF, Bellizzi AM, Dillon JS, O'Dorisio TM, Howe JR. The Distal Predilection of Small Bowel Neuroendocrine Tumors. Ann Surg Oncol. 2018;25:3207-3213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 117. | Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang YZ, Law CH, Liu EH, Kim MK, Menda Y, Morse BG, Bergsland EK, Strosberg JR, Nakakura EK, Pommier RF. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46:715-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 268] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 118. | Deguelte S, Perrier M, Hammoutene C, Cadiot G, Kianmanesh R. Surgery and Perioperative Management in Small Intestinal Neuroendocrine Tumors. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 119. | Singh S, Asa SL, Dey C, Kennecke H, Laidley D, Law C, Asmis T, Chan D, Ezzat S, Goodwin R, Mete O, Pasieka J, Rivera J, Wong R, Segelov E, Rayson D. Diagnosis and management of gastrointestinal neuroendocrine tumors: An evidence-based Canadian consensus. Cancer Treat Rev. 2016;47:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 120. | Scott AT, Howe JR. Management of Small Bowel Neuroendocrine Tumors. J Oncol Pract. 2018;14:471-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 121. | Jann H, Roll S, Couvelard A, Hentic O, Pavel M, Müller-Nordhorn J, Koch M, Röcken C, Rindi G, Ruszniewski P, Wiedenmann B, Pape UF. Neuroendocrine tumors of midgut and hindgut origin: tumor-node-metastasis classification determines clinical outcome. Cancer. 2011;117:3332-3341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 122. | Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC, Celinksi SA, Kooby DA, Staley CA, Stokes JB, Chu CK, Ferrero A, Schulick RD, Choti MA, Mentha G, Strub J, Bauer TW, Adams RB, Aldrighetti L, Capussotti L, Pawlik TM. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 123. | Pape UF, Niederle B, Costa F, Gross D, Kelestimur F, Kianmanesh R, Knigge U, Öberg K, Pavel M, Perren A, Toumpanakis C, O'Connor J, Krenning E, Reed N, O'Toole D; Vienna Consensus Conference participants. ENETS Consensus Guidelines for Neuroendocrine Neoplasms of the Appendix (Excluding Goblet Cell Carcinomas). Neuroendocrinology. 2016;103:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 124. | Volante M, Daniele L, Asioli S, Cassoni P, Comino A, Coverlizza S, De Giuli P, Fava C, Manini C, Berruti A, Papotti M. Tumor staging but not grading is associated with adverse clinical outcome in neuroendocrine tumors of the appendix: a retrospective clinical pathologic analysis of 138 cases. Am J Surg Pathol. 2013;37:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 125. | Moris D, Tsilimigras DI, Vagios S, Ntanasis-Stathopoulos I, Karachaliou GS, Papalampros A, Alexandrou A, Blazer DG 3RD, Felekouras E. Neuroendocrine Neoplasms of the Appendix: A Review of the Literature. Anticancer Res. 2018;38:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 126. | Toumpanakis C, Fazio N, Tiensuu Janson E, Hörsch D, Pascher A, Reed N, O Apos Toole D, Nieveen van Dijkum E, Partelli S, Rinke A, Kos-Kudla B, Costa F, Pape UF, Grozinsky-Glasberg S, Scoazec JY; The ENETS 2016 Munich Advisory Board Participants; ENETS 2016 Munich Advisory Board Participants. Unmet Needs in Appendiceal Neuroendocrine Neoplasms. Neuroendocrinology. 2019;108:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 127. | Hrabe J. Neuroendocrine Tumors of the Appendix, Colon, and Rectum. Surg Oncol Clin N Am. 2020;29:267-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 128. | Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010;105:2563-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 129. | Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O'Dorisio TM, Warner RR, Wiseman GA, Benson AB 3rd, Pommier RF; North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 130. | Soga J. Carcinoids of the colon and ileocecal region: a statistical evaluation of 363 cases collected from the literature. J Exp Clin Cancer Res. 1998;17:139-148. [PubMed] |
| 131. | Byrne RM, Pommier RF. Small Bowel and Colorectal Carcinoids. Clin Colon Rectal Surg. 2018;31:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 132. | Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, Plöckinger U, Papotti M, Salazar R, Pascher A; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 133. | Li P, Wu F, Zhao H, Dou L, Wang Y, Guo C, Wang G, Zhao D. Analysis of the factors affecting lymph node metastasis and the prognosis of rectal neuroendocrine tumors. Int J Clin Exp Pathol. 2015;8:13331-13338. [PubMed] |
| 134. | Bertani E, Ravizza D, Milione M, Massironi S, Grana CM, Zerini D, Piccioli AN, Spinoglio G, Fazio N. Neuroendocrine neoplasms of rectum: A management update. Cancer Treat Rev. 2018;66:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (2)] |
| 135. | Glazer ES, Tseng JF, Al-Refaie W, Solorzano CC, Liu P, Willborn KA, Abdalla EK, Vauthey JN, Curley SA. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford). 2010;12:427-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 136. | Pavel M, O'Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, Krenning E, Knigge U, Salazar R, Pape UF, Öberg K; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103:172-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 743] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 137. | Akyildiz HY, Mitchell J, Milas M, Siperstein A, Berber E. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: long-term follow-up. Surgery. 2010;148:1288-93; discussion 1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 138. | Maire F, Lombard-Bohas C, O'Toole D, Vullierme MP, Rebours V, Couvelard A, Pelletier AL, Zappa M, Pilleul F, Hentic O, Hammel P, Ruszniewski P. Hepatic arterial embolization vs chemoembolization in the treatment of liver metastases from well-differentiated midgut endocrine tumors: a prospective randomized study. Neuroendocrinology. 2012;96:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 139. | King J, Quinn R, Glenn DM, Janssen J, Tong D, Liaw W, Morris DL. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer. 2008;113:921-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 140. | Lacin S, Oz I, Ozkan E, Kucuk O, Bilgic S. Intra-arterial treatment with 90yttrium microspheres in treatment-refractory and unresectable liver metastases of neuroendocrine tumors and the use of 111in-octreotide scintigraphy in the evaluation of treatment response. Cancer Biother Radiopharm. 2011;26:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 141. | Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R; PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 1742] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 142. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1290] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 143. | Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 903] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 144. | van Essen M, Krenning EP, Kam BL, de Herder WW, Feelders RA, Kwekkeboom DJ. Salvage therapy with (177)Lu-octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2010;51:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 145. | Hope TA, Bodei L, Chan JA, El-Haddad G, Fidelman N, Kunz PL, Mailman J, Menda Y, Metz DC, Mittra ES, Pryma DA, Reidy-Lagunes DL, Singh S, Strosberg JR. NANETS/SNMMI Consensus Statement on Patient Selection and Appropriate Use of 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy. J Nucl Med. 2020;61:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 146. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 698] [Article Influence: 139.6] [Reference Citation Analysis (0)] |