Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8340
Peer-review started: June 10, 2021
First decision: July 5, 2021
Revised: July 10, 2021
Accepted: August 16, 2021
Article in press: August 16, 2021
Published online: October 6, 2021
Processing time: 110 Days and 8.8 Hours
Nickel (Ni) may accumulate in the human body and has biological toxicity and carcinogenicity. Ni has an extensive impact on the health of pregnant women and fetuses during gestation.
To evaluate Ni exposure in pregnant women in Kunming, Yunnan Province, China; to describe the distribution of Ni in the maternal-fetal system and placental barrier function; and to investigate the effect of Ni exposure on fetal health in mothers with pregnancy complications.
Seventy-two pregnant women were selected using a case-control design. The women were divided into two groups: The control group (no disease; n = 29) and the disease group [gestational diabetes (GDM), hypertensive disorder complicating pregnancy (HDCP), or both; n = 43]. The pregnant women in the disease group were further divided as follows: 14 cases with GDM (GDM group), 13 cases with HDCP (HDCP group) and 16 cases with both GDM and HDCP (disease combination group). Basic information on the pregnant women was collected by questionnaire survey. Maternal blood, placenta blood and cord blood were collected immediately after delivery. The Ni content in paired samples was determined using inductively coupled plasma mass spectrometry.
Compared to the control group, age was higher and body mass index was greater in pregnant women in the disease groups (28.14 ± 2.54 vs 28.42 ± 13.89, P < 0.05; 25.90 ± 3.86 vs 31.49 ± 5.30, P < 0.05). The birth weights of newborns in the HDCP group and the control group were significantly different (2.52 ± 0.74 vs 3.18 ± 0.41, P < 0.05). The content of Ni in umbilical cord blood in the entire disease group was higher than that in the control group (0.10 ± 0.16 vs 0.05 ± 0.07, P < 0.05).
In the maternal-fetal system of women with pregnancy complications, the barrier effect of the placenta against Ni is weakened, thus affecting healthy growth of the fetus in the uterus.
Core Tip: In this study, the distribution of nickel (Ni) in the maternal-fetal system and placental barrier function was described, and the effect of Ni exposure on fetal health in mothers with pregnancy complications was investigated. The results suggest that in the maternal-fetal system of women with pregnancy complications, the barrier effect of the placenta against Ni is weakened, thus affecting healthy growth of the fetus in the uterus. This study indicated that more attention should be focused on reducing Ni environmental exposure during pregnancy and improving the quality of the living environment in order to ensure normal development of the fetus.
- Citation: Ding AL, Hu H, Xu FP, Liu LY, Peng J, Dong XD. Pregnancy complications effect on the nickel content in maternal blood, placenta blood and umbilical cord blood during pregnancy. World J Clin Cases 2021; 9(28): 8340-8348
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8340.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8340
Gestational diabetes mellitus (GDM) and hypertensive disorder complicating pregnancy (HDCP) are common pregnancy complications. In recent years, the incidence of GDM and HDCP has been increasing[1]. GDM manifests mainly as hyperglycemia caused by impaired glucose tolerance during pregnancy[2]. It is associated with adverse pregnancy outcomes such as macrosomia, shoulder dystocia and neonatal hypoglycemia[3]. HDCP is the main factor associated with maternal morbidity and mortality in the perinatal period[4]. It may cause fetal intrauterine dysplasia and cardiovascular disease in adulthood[5]. In addition to the traditional pathogenic factors, new types of environmental exposure have attracted more and more attention.
With the continuous development of emerging technologies, nickel (Ni)-containing products are widely used in production and in life[6]. The presence of Ni is widespread in the environment. People are generally exposed to Ni through the air, their diet, consumer goods and other channels[7]. Ni may accumulate in the human body and has biological toxicity and carcinogenicity[8]. Ni has a more extensive impact on the health of pregnant women and fetuses during gestation. Studies have shown that there is a correlation between Ni exposure during pregnancy and the risk of pregnancy complications (such as GDM)[9]. Long-term exposure to Ni may lead to premature delivery and have certain effects on the respiratory and cardiovascular systems[10,11]. Maternal environmental exposure during pregnancy and lactation is a direct source of heavy metals in the fetus, and it has been proven that Ni transfers to the fetus through the placenta[12]. The embryotoxicity of Ni not only manifests as direct embryo damage to the placenta, but there are also cytotoxic effects[13]. Animal experiments have shown that exposure to Ni during pregnancy can lead to low birth weight or deformity in offspring[12]. The literature also suggests that the placenta can act as a barrier to heavy metals in the maternal-fetal system, which is defined by the ratio of maternal blood to umbilical blood.
However, there are few reports on the relationship between GDM and HDCP, Ni exposure, and the placental barrier. Therefore, the question arises as to whether in the presence of gestational complications (GDM and HDCP) more Ni will pass through the placenta and enter the fetus, thereby impacting fetal health? We will attempt to answer this question in this study.
A case-control design was adopted in this study; the 72 selected subjects were pregnant women who gave birth in the Obstetrics Department of The First People's Hospital of Yunnan Province between January 2019 and December 2019. The basic characteristics of the pregnant women and information regarding their newborns were obtained from hospital records and a questionnaire, which included pregnancy history, working environment, living environment, family history, maternal disease, etc. Women who had lived in the study area for a short period of time, had smoking or drinking habits, or had a history of occupational exposure to heavy metals were excluded. According to the diagnostic criteria of GDM and HDCP[1] and the health status, the pregnant women were divided into the control group (n = 29) and the disease group (n = 43). The control group included healthy women who delivered at term without pregnancy complications. The pregnant women in the disease group were further divided into the following groups: 14 cases of GDM (GDM group), 13 cases of HDCP (HDCP group) and 16 cases of both GDM and HDCP (disease combination group). This study was reviewed and approved by the Ethics Committee of Yunnan First People's Hospital, and the pregnant women provided written informed consent.
After delivery, 10 mL of maternal blood, 10 mL of umbilical cord blood and 10–20 g of placental tissue were immediately collected and stored in an ultra-low temperature refrigerator at -80 °C. The samples were thawed before analysis, and 0.5 g of whole blood, 3 mL HNO3 and 1 mL H2O2 or 1 g of placental tissue, 5 mL HNO3 and 2 mL H2O2 were mixed, and the samples were digested in a microwave digestion tube at low pressure for 30 min. After digestion and cooling, the solution was diluted with 1% HNO3 to 25 mL. The Ni content in samples was measured using an inductively coupled plasma mass spectrometer, and the standard curve was calibrated and verified by a multivariate standard solution. Each batch of 10 samples contained nine sample (blood/placenta) solutions and one blank solution.
IBM SPSS (Windows 17.0 version; IBM Corp., Chicago, IL, United States) was used to analyze the detection data, and the mean value, skewness and standard deviation were used to describe the distribution of Ni in the maternal-fetal system. An independent sample t test was used to evaluate maternal and neonatal information and whether there were significant correlations between the content of Ni in samples and fetal birth weight and body length. P < 0.05 and P < 0.001 were considered statistically significant.
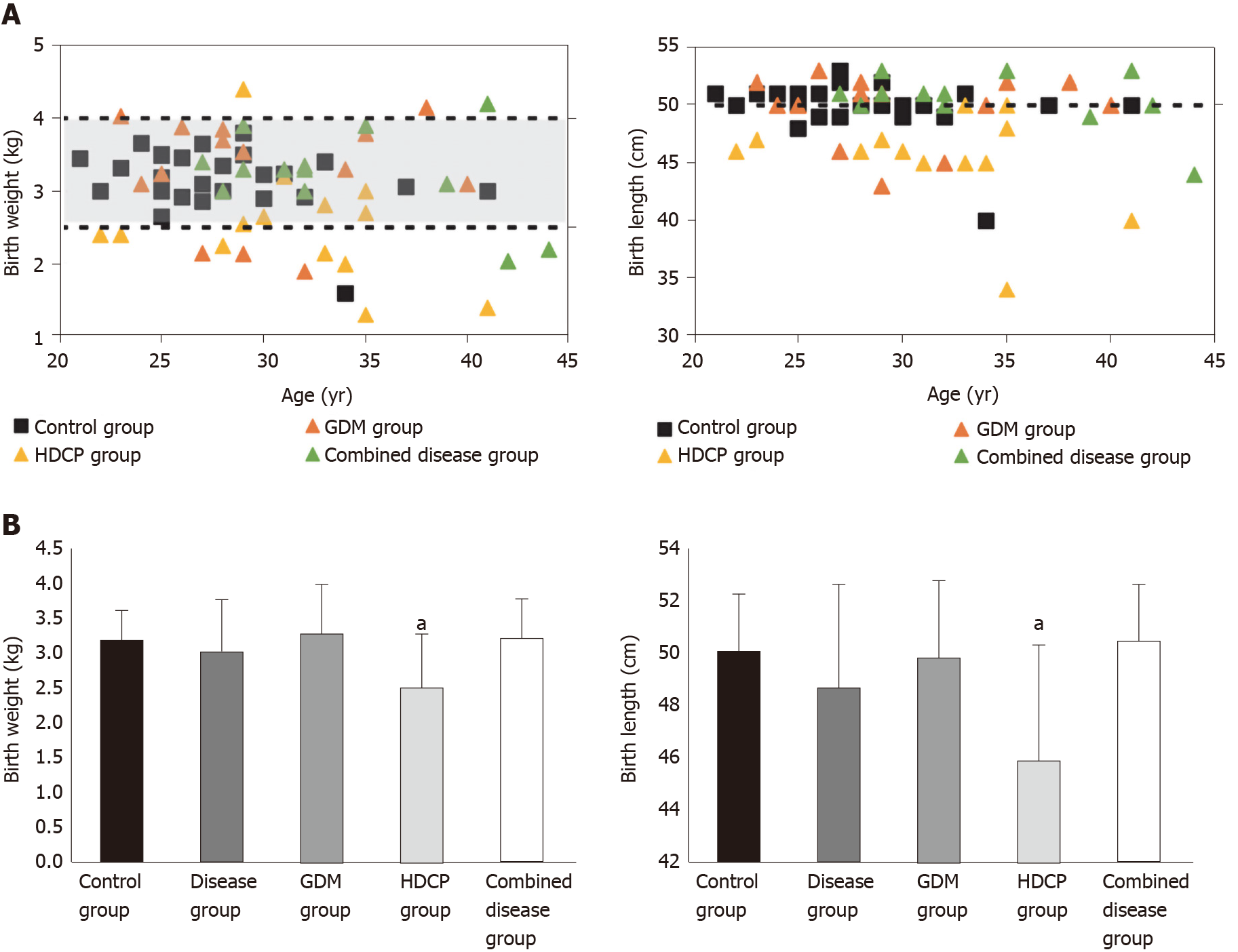
A total of 72 pregnant women participated in this study; all were over 18 years of age (range: 21–44 years). The average age of pregnant women was 28 years in the control group, 30 years in the GDM group, 31 years in the HDCP group and 33 years in the disease combination group. The average body mass index (BMI) was 25.8 (kg/m2) in the control group, 28 (kg/m2) in the GDM group, 27.6 (kg/m2) in the HDCP group and 29.7 (kg/m2) in the disease combination group. All 72 pregnant women were compared to the control group, and the age and BMI of pregnant women in the HDCP group and in the disease combination group were significantly higher (P < 0.05), while only BMI was significantly higher in the GDM group. In addition, the Apgar score of newborns in the three disease groups (GDM only, HDCP only, and the combination group) was significantly lower at 1 min and 5 min than that in newborns in the control group (P < 0.05). Figure 1A shows neonatal birth weight and birth body length in the control group and the disease groups. The dotted lines and shading represent neonatal birth weights and length within the normal range and the standard values (2.5–4.0 kg and 50 cm). Neonatal birth weight and birth body lengths were generally within the normal range, and these parameters in the disease group were 37% greater than the normal range. Compared with the control group, birth weight and body length in the GDM group and disease combination group were not significantly different (P > 0.05), but these parameters were significantly reduced in the HDCP group (P < 0.05, Figure 1B).
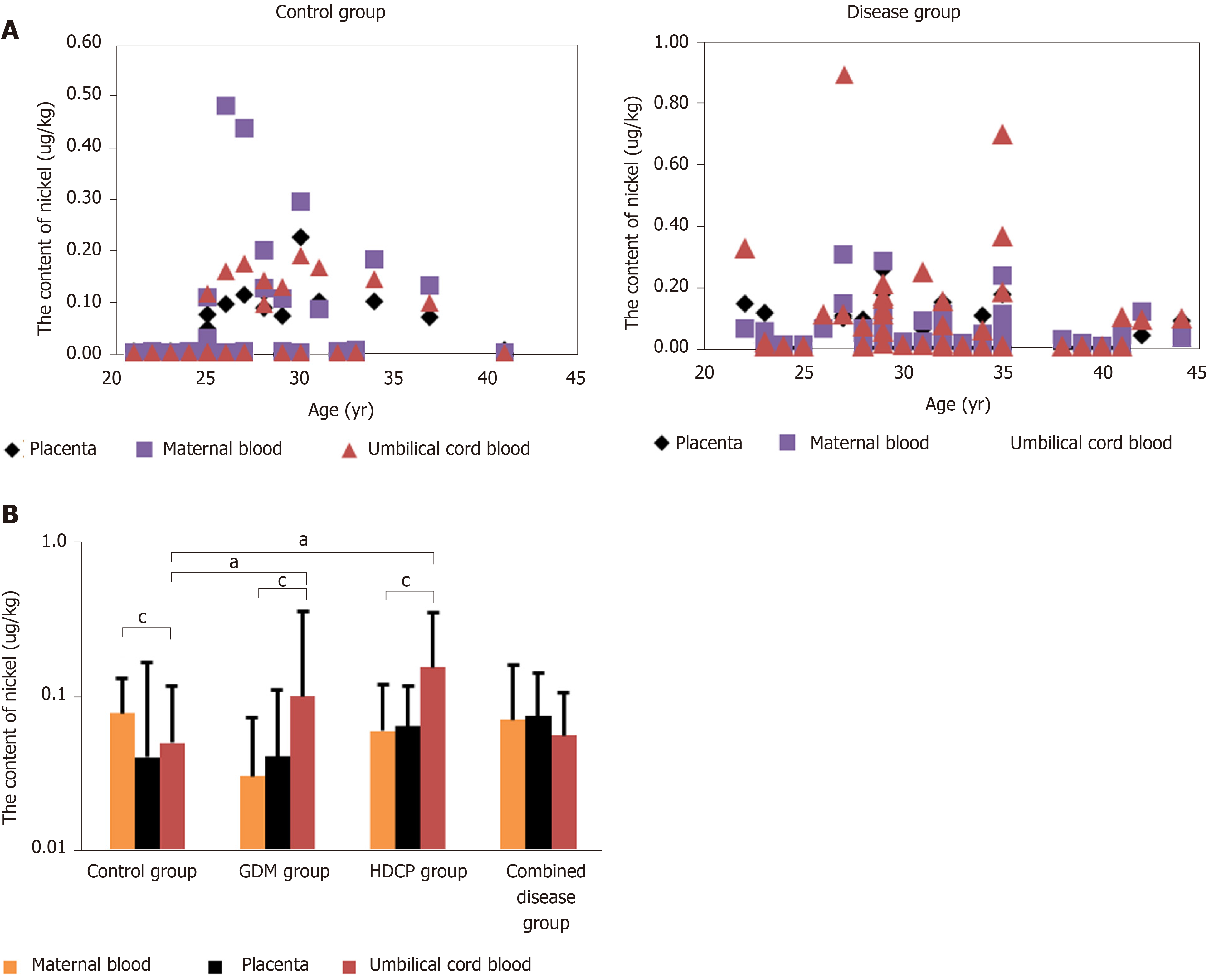
Ni was detected in all paired samples in the control group and disease group (Figure 2A). Compared with the content of Ni in maternal blood, the content of Ni in umbilical cord blood in the control group was significantly reduced, whereas the content of Ni in umbilical cord blood in the GDM group and HDCP group was significantly enhanced (P < 0.05, Figure 2B). The content of Ni in maternal blood and umbilical cord blood was not significantly different in the disease combination group (Figure 2B). In addition, compared with the content of Ni in umbilical cord blood in the control group, the content of Ni in umbilical cord blood in the GDM group and HDCP group was significantly increased (P < 0.05, Figure 2B); and the content of Ni in umbilical cord blood was not significantly different between the control group and the disease combination group (Figure 2B).
The placenta can act as a barrier to heavy metals in the maternal-fetal system. A higher ratio of heavy metals in maternal blood to heavy metals in umbilical blood greater than 1 indicates better placental barrier function[14]. In this study, we found that the proportion of women with a ratio greater than 1 was 85% in the control group, 60.47% in the entire disease group, 71.43% in the GDM group, 50.00% in the HDCP group, and 60.00% in the disease combination group. The effect of the placental barrier against Ni was weakened in the disease groups.
Weight management during pregnancy is an important part of pregnancy health care, and it has attracted the attention of researchers for many years. The high risk factors for gestational diseases mainly include individual factors, genetic factors and environmental factors[15]. Studies have shown that BMI during pregnancy is related to HDCP, GDM and pregnancy outcomes[16-18]. The BMI of pregnant women with HDCP is positively correlated with blood pressure[19]. Pregnant women with HDCP may present with fluid retention, which makes them heavier than healthy pregnant women[20]. The results of this study are consistent with current reports in the literature, in that they show that there is an interaction between the basic characteristics of pregnant women and pregnancy complications. However, specific metabolic mechanisms require further study. It has been long established that attention must be paid to maternal health and physical condition during pregnancy in order to improve the health of the mother and the infant.
The birth weight of the newborn is an important index in judging whether the fetus has grown normally in the uterus[21]. The health status of pregnant women is one of the factors affecting the birth weight of the newborn. Studies on HDCP have shown that the pathological mechanism involved in abnormal fetal intrauterine growth is complex and mainly attributed to placental vascular dysfunction as a result of reduced placental blood flow[22]. Fetal intrauterine growth depends on the effective transportation of nutrients by the placenta[23,24]. A decrease in placental blood flow leads to chronic fetal hypoxia and nutritional deficiency, resulting in intrauterine growth restriction (IUGR), premature delivery and even the possibility of death[4]. We found that there were significant differences between the HDCP group and the control group in terms of birth weight, length and Apgar score, which was consistent with the results reported in the literature. However, we assessed a limited number of indicators and samples; thus, we could not directly determine which factors (environmental exposure, individual differences, genetic factors) influenced fetal growth status in the HDCP group and the underlying mechanisms.
This study shows that pregnant women in Kunming, Yunnan Province experience exposure to environmental Ni. The detection of Ni in cord blood showed that Ni can be transferred to the fetus through the placental barrier. The placenta plays an important role as a barrier between maternal environmental exposure and transfer to the fetus, which influences their development[25]. By detecting Ni content in maternal blood, placenta blood and cord blood in the control group and disease group, we found that the placental barrier in the control group had a certain protective role, but the detection of Ni in cord blood in the control group showed that some Ni could still pass through the placenta and transfer to the fetus via the umbilical cord. Although the placenta has a high affinity for Ni, which prevents its transfer, the placental barrier does not protect the fetus from Ni[26].
The experimental data showed that the content of Ni in placenta blood and cord blood of pregnant women with gestational diseases (GDM group, HDCP group, disease combination group) was higher than that in the control group. Pregnant women with pregnancy complications may accumulate more Ni in the placenta through environmental exposure. Nickel has embryotoxicity and can induce lipid peroxidation in the placenta. This metabolic change can lead to a decrease in placental vitality and potential embryotoxicity. This affects embryo development[27], resulting in fetal IUGR. The transport of Ni in the placenta will change the morphology and permeability characteristics of the placenta during the development phase[28], resulting in weakening of the placental barrier function against Ni. As the intermediate medium for Ni transfer from mother to fetus, the placenta allows more Ni to enter the fetal side through the placenta. Although it has been reported that Ni can pass through the placenta[14], little is known about the toxic metabolic mechanism of Ni in the placenta.
The placenta is an important selective barrier to toxic substances during pregnancy[29]. However, some heavy metals (such as Ni) can interfere with the placental transport system and then cross the placenta[30,31]. Although the environmental exposure level is far lower than the international standard[29,32,33], because the fetal physiological and biochemical levels are different to those of adults, the fetus is highly sensitive to harmful substances, even trace exposure levels[34]. From our data analysis, we established that in pregnant women with pregnancy complications related to environmental exposure, the Ni placental barrier function showed different degrees of damage. Nickel placental barrier function varied from strong to weak in the following order: Control group, GDM group, disease combination group and the HDCP group. The placental barrier function against Ni in the control group was significantly better than that in all disease groups. The placental barrier function against Ni in the maternal-fetal system of the GDM group, HDCP group, and disease combination group was damaged to varying degrees, and it did not play a good role as a placental barrier. However, studies are needed to establish the mechanism by which Ni is transported and metabolized between the mother, placenta and fetus, to determine the toxic metabolic mechanism of Ni in the maternal-fetal system and to determine how prenatal exposure to Ni affects fetal growth in utero. These studies should involve more paired samples and more detailed follow-up of the health status of newborns, as well as the use of advanced molecular biology methods to conduct in-depth studies on the samples.
The first advantage of this study is that the included population was in the third trimester, which can be used to evaluate exposure to Ni during pregnancy. The second advantage is the complete detection of Ni content in paired samples of maternal blood, placenta and cord blood, which can describe the dynamic changes in Ni in the maternal-fetal system. The third advantage of this study is the assessment of the placental distribution of Ni in the control, GDM and HDCP groups, and to compare the placental distribution of Ni in the maternal-fetal system in both healthy women and those with gestational diseases (GDM and HDCP) in the general population. The diagnosis of gestational diseases was based on the standard hospital formal diagnosis, and other interference factors (such as the age of pregnant women 20-45-years-old and a non-occupationally exposed population) were strictly controlled. It also provides important clinical value for disease prevention in the future.
This study also has some limitations. Firstly, the sample size was small. Secondly, the research involved a case-control design, and the findings were not confirmed in an animal model and at the cell level. Thirdly, this study only screened the pregnant women living in the study area for a long time, and did not investigate and classify their diet and living habits, and did not take into account the potential influencing factors and the detection of Ni in other stages of pregnancy. In addition, this study was conducted in a provincial hospital. Although there were differences in some of the aspects studied, it does not represent the whole Kunming population. Therefore, we plan to increase the sample size and expand the scope of the study population in a follow-up study, with multi-dimensional assessment and analysis of the distribution and transfer characteristics of Ni in the maternal-fetal system in healthy women and in those with gestational diseases in the general population, as well as the toxicity of Ni.
This study has both advantages and disadvantages. It was found that pregnant women in Kunming, Yunnan Province experienced environmental exposure to Ni, which can be transferred to the fetus through the placental barrier. In the maternal-fetal system of women with pregnancy complications, the barrier effect of the placenta against Ni is weakened, thus affecting healthy growth of the fetus in the uterus. This study indicated that more attention should be focused on reducing Ni environmental exposure during pregnancy and improving the quality of the living environment in order to ensure normal development of the fetus.
Gestational diabetes mellitus and gestational hypertension disease are common pregnancy complications. In addition to the traditional pathogenic factors, new types of environmental exposure have attracted more and more attention. With the continuous development of emerging technologies, nickel (Ni)-containing products are widely used in production and in life. Ni may accumulate in the human body and has biological toxicity and carcinogenicity. Ni has a more extensive impact on the health of pregnant women and fetuses during gestation.
This study has important reference significance for reducing Ni exposure during pregnancy, improving the quality of the living environment and ensuring the normal development of the fetus.
This study aimed to evaluate Ni exposure in pregnant women in Kunming, Yunnan Province, China.
Basic information on the 72 pregnant women was collected by questionnaire survey. Maternal blood, placenta blood and cord blood were collected immediately after delivery. The Ni content in paired samples was determined using inductively coupled plasma mass spectrometry.
It was found that pregnant women in Kunming, Yunnan Province experienced environmental exposure to Ni, which can be transferred to the fetus through the placental barrier.
In the maternal-fetal system of women with pregnancy complications, the barrier effect of the placenta against Ni is weakened, thus affecting healthy growth of the fetus in the uterus.
Further research into the mechanisms, from the perspective of advanced molecular biology, will reveal the key role of nickel in gestational disease, placental barrier and birth outcome.
Manuscript source: Unsolicited manuscript
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Paolino MPJ S-Editor: Wang JL L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Obid R, Redlich M, Tomeh C. The Treatment of Laryngeal Cancer. Oral Maxillofac Surg Clin North Am. 2019;31:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 2. | Soomro MH, Baiz N, Huel G, Yazbeck C, Botton J, Heude B, Bornehag CG, Annesi-Maesano I; EDEN mother-child cohort study group. Exposure to heavy metals during pregnancy related to gestational diabetes mellitus in diabetes-free mothers. Sci Total Environ. 2019;656:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Abell SK, Boyle JA, de Courten B, Soldatos G, Wallace EM, Zoungas S, Teede HJ. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Zhang S, Wang L, Leng J, Liu H, Li W, Zhang T, Li N, Tian H, Baccarelli AA, Hou L, Hu G. Hypertensive disorders of pregnancy in women with gestational diabetes mellitus on overweight status of their children. J Hum Hypertens. 2017;31:731-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Visentin S, Londero AP, Camerin M, Grisan E, Cosmi E. A possible new approach in the prediction of late gestational hypertension: The role of the fetal aortic intima-media thickness. Medicine (Baltimore). 2017;96:e5515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Denkhaus E, Salnikow K. Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hematol. 2002;42:35-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 590] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 7. | Haber LT, Bates HK, Allen BC, Vincent MJ, Oller AR. Derivation of an oral toxicity reference value for nickel. Regul Toxicol Pharmacol. 2017;87 Suppl 1:S1-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Vladimir I, Bojanic V, Biljana J. Epidemiological and pathogenetic aspects of nickel poisoning. Acta Medica Medianae. 2007;46:37-44. |
| 9. | Wang X, Gao D, Zhang G, Zhang X, Li Q, Gao Q, Chen R, Xu S, Huang L, Zhang Y, Lin L, Zhong C, Chen X, Sun G, Song Y, Yang X, Hao L, Yang H, Yang L, Yang N. Exposure to multiple metals in early pregnancy and gestational diabetes mellitus: A prospective cohort study. Environ Int. 2020;135:105370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 10. | Mohmand J, Eqani SA, Fasola M, Alamdar A, Mustafa I, Ali N, Liu L, Peng S, Shen H. Human exposure to toxic metals via contaminated dust: Bio-accumulation trends and their potential risk estimation. Chemosphere. 2015;132:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 168] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 11. | Grant K, Goldizen FC, Sly PD, Brune MN, Neira M, van den Berg M, Norman RE. Health consequences of exposure to e-waste: a systematic review. Lancet Glob Health. 2013;1:e350-e361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 268] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 12. | Hou YP, Gu JY, Shao YF, Song YF, Jing YH, Wu WS, Pu S. The characteristics of placental transfer and tissue concentrations of nickel in late gestational rats and fetuses. Placenta. 2011;32:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Szakmáry E, Morvai V, Náray M, Ungváry G. Haemodynamic effect of nickel chloride in pregnant rats. Acta Physiol Hung. 1995;83:3-12. [PubMed] |
| 14. | Li A, Zhuang T, Shi J, Liang Y, Song M. Heavy metals in maternal and cord blood in Beijing and their efficiency of placental transfer. J Environ Sci (China). 2019;80:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Lind JM, Hennessy A, McLean M. Cardiovascular disease in women: the significance of hypertension and gestational diabetes during pregnancy. Curr Opin Cardiol. 2014;29:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Thornton YS. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obstet Gynecol. 2013;122:696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Shiqiao H, Bei X, Yini Z, Lei J. Risk factors of gestational diabetes mellitus during assisted reproductive technology procedures. Gynecol Endocrinol. 2020;36:318-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Dos Santos PA, Madi JM, da Silva ER, Vergani DOP, de Araújo BF, Garcia RMR. Gestational Diabetes in the Population Served by Brazilian Public Health Care. Prevalence and Risk Factors. Rev Bras Ginecol Obstet. 2020;42:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Ephraim RK, Osakunor DN, Denkyira SW, Eshun H, Amoah S, Anto EO. Serum calcium and magnesium levels in women presenting with pre-eclampsia and pregnancy-induced hypertension: a case-control study in the Cape Coast metropolis, Ghana. BMC Pregnancy Childbirth. 2014;14:390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Valensise H, Vasapollo B, Novelli GP, Larciprete G, Andreoli A, Altomare F, Di Pierro G, Galante A, Arduini D, De Lorenzo A. Total body water estimation and maternal cardiac systolic function assessment in normal and gestational hypertensive pregnant women. Med Sci Monit. 2004;10:CR530-CR534. [PubMed] |
| 21. | Kurtoğlu S, Hatipoğlu N, Mazıcıoğlu MM, Akın MA, Çoban D, Gökoğlu S, Baştuğ O. Body weight, length and head circumference at birth in a cohort of Turkish newborns. J Clin Res Pediatr Endocrinol. 2012;4:132-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Mateus J, Newman RB, Zhang C, Pugh SJ, Grewal J, Kim S, Grobman WA, Owen J, Sciscione AC, Wapner RJ, Skupski D, Chien E, Wing DA, Ranzini AC, Nageotte MP, Gerlanc N, Albert PS, Grantz KL. Fetal growth patterns in pregnancy-associated hypertensive disorders: NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2019;221:635.e1-635.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 1202] [Article Influence: 133.6] [Reference Citation Analysis (0)] |
| 24. | Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62:1046-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 355] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 25. | Meyrueix L, Adair L, Norris SA, Ideraabdullah F. Assessment of placental metal levels in a South African cohort. Environ Monit Assess. 2019;191:500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Jasim S, Tjälve H. Effects of sodium pyridinethione on the uptake and distribution of nickel, cadmium and zinc in pregnant and non-pregnant mice. Toxicology. 1986;38:327-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Zhang N, Chen M, Li J, Deng Y, Li SL, Guo YX, Li N, Lin Y, Yu P, Liu Z, Zhu J. Metal nickel exposure increase the risk of congenital heart defects occurrence in offspring: A case-control study in China. Medicine (Baltimore). 2019;98:e15352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Wang XW, Gu JY, Li Z, Song YF, Wu WS, Hou YP. Gestational age and dose influence on placental transfer of 63Ni in rats. Placenta. 2010;31:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Al-Saleh I, Shinwari N, Mashhour A, Mohamed Gel D, Rabah A. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int J Hyg Environ Health. 2011;214:79-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Zhang YL, Zhao YC, Wang JX, Zhu HD, Liu QF, Fan YG, Wang NF, Zhao JH, Liu HS, Ou-Yang L, Liu AP, Fan TQ. Effect of environmental exposure to cadmium on pregnancy outcome and fetal growth: a study on healthy pregnant women in China. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2004;39:2507-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Osman K, Akesson A, Berglund M, Bremme K, Schütz A, Ask K, Vahter M. Toxic and essential elements in placentas of Swedish women. Clin Biochem. 2000;33:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 201] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Schoeters G, Den Hond E, Zuurbier M, Naginiene R, van den Hazel P, Stilianakis N, Ronchetti R, Koppe JG. Cadmium and children: exposure and health effects. Acta Paediatr Suppl. 2006;95:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Holmes P, James KA, Levy LS. Is low-level environmental mercury exposure of concern to human health? Sci Total Environ. 2009;408:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 242] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 34. | Wells PG, Lee CJ, McCallum GP, Perstin J, Harper PA. Receptor- and reactive intermediate-mediated mechanisms of teratogenesis. Handb Exp Pharmacol. 2010;131-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |