Published online Sep 26, 2021. doi: 10.12998/wjcc.v9.i27.8232
Peer-review started: May 4, 2021
First decision: June 24, 2021
Revised: July 8, 2021
Accepted: August 11, 2021
Article in press: August 11, 2021
Published online: September 26, 2021
Processing time: 135 Days and 4.5 Hours
Surgery, which is a major risk factor for venous thrombosis, has rarely been considered a risk factor for arterial thrombosis. Recent studies have suggested that venous and arterial thromboses share common risk factors and have a bidirectional relationship. Accordingly, there is a growing interest in the risk of arterial thrombosis after surgery. We report a case of acute bilateral lower extremity arterial thromboses that developed after a prolonged surgery.
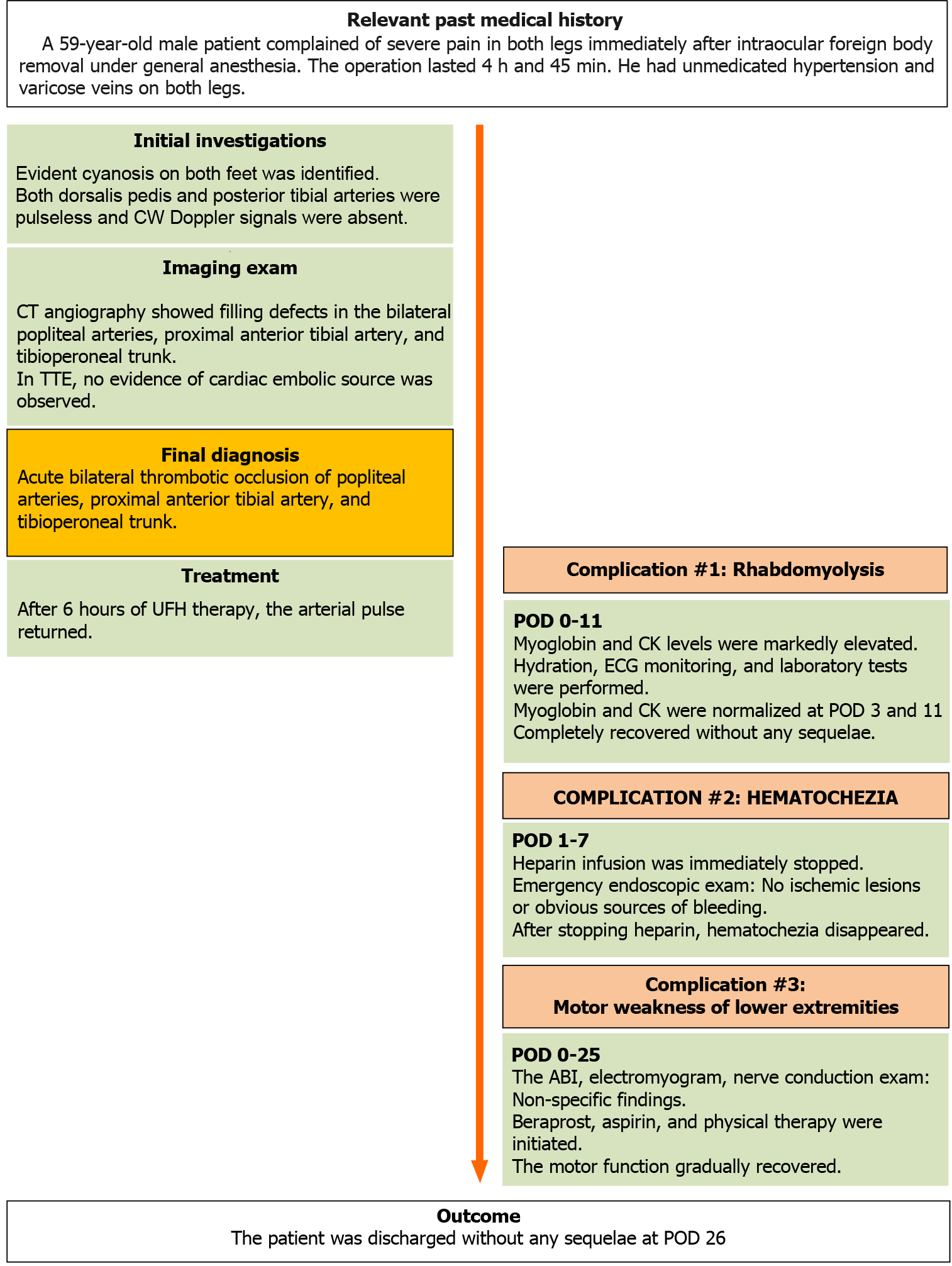
A 59-year-old man was hospitalized for intraocular foreign body removal surgery. He was a heavy-drinking smoker and had untreated hypertension and varicose veins in both legs. The operation was unexpectedly prolonged, lasting 4 h and 45 min. Immediately after emergence from general anesthesia, the patient compla
Acute lower extremity arterial thrombosis can occur after surgery. Anesthesiologists should pay particular attention to patients with risk factors for thrombo
Core Tip: The conventional literature emphasizes that surgery is a major risk factor for venous thrombosis rather than arterial thrombosis. However, recent studies have suggested that these two types of thromboses are closely related and share common risk factors. Accordingly, there has been a growing interest in the increased postope
- Citation: Jeon S, Hong JM, Lee HJ, Kim E, Lee H, Kim Y, Ri HS, Lee JJ. Acute lower extremity arterial thrombosis after intraocular foreign body removal under general anesthesia: A case report and review of literature. World J Clin Cases 2021; 9(27): 8232-8241
- URL: https://www.wjgnet.com/2307-8960/full/v9/i27/8232.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i27.8232
Thrombosis refers to the formation of a blood clot, which partially or fully blocks the blood flow, in a blood vessel[1]. The complications of thrombosis vary depending on the type and anatomical location of the blood vessel in which the clot is located. While venous thrombosis causes congestion in the upstream area, arterial thrombosis causes ischemia in the downstream area[2,3]. Traditionally, arterial thrombosis and venous thrombosis have been considered as distinct diseases, with different risk factors, underlying mechanisms, and treatments[4,5]. The well-established risk factors for venous thrombosis include trauma, surgery, and cancer, while the factors leading to arterial thrombosis include smoking, hypertension, and dyslipidemia[4].
Due to immobility and systemic hypercoagulability, surgery is a risk factor for venous thrombosis[4]. To reduce this preventable complication during surgery, pa
A 59-year-old male patient (163 cm, 70 kg) complained of severe pain in both legs immediately after intraocular foreign body removal under general anesthesia.
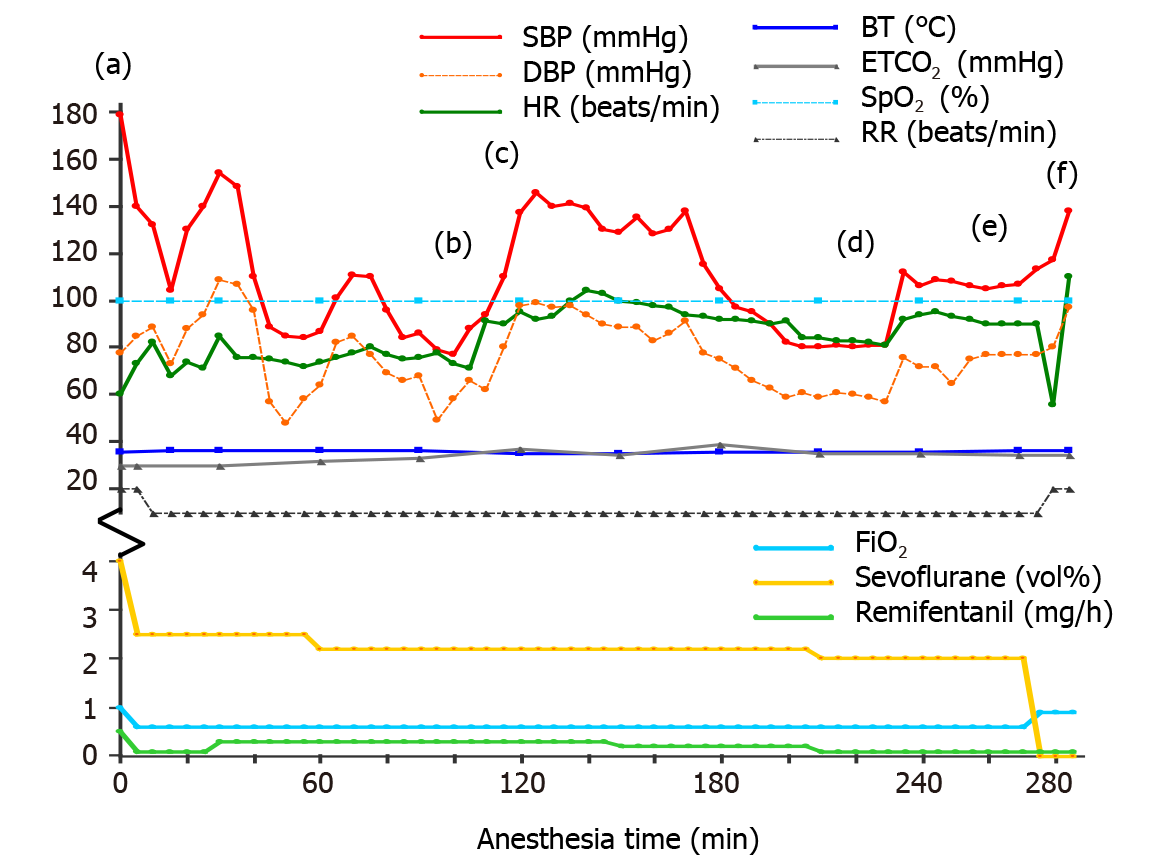
After an accident at a construction site, in which a 3 mm iron particle entered the patient’s left eye, the patient was hospitalized for foreign body removal surgery. Physical examination immediately after admission revealed no abnormal findings, except for the left eye injury. The patient did not complain of any discomfort in either leg. Preoperative electrocardiogram (ECG), chest radiography, and laboratory findings were unremarkable (Table 1). In the operating room, standard monitoring (ECG, pulse oximetry, noninvasive blood pressure, end-tidal CO2 (EtCO2), and esophageal stetho
| Preoperative | After surgery (POD 0) | After hematochezia (POD 1) | POD 2 | Reference range | |
| Complete blood count | |||||
| WBC (103/µL) | 6.80 | 13.11 | 10.00 | 8.4 | 4.0-11.0 |
| RBC (106/µL) | 4.44 | 4.6 | 3.89 | 3.25 | 4.5-6.0 |
| Hb (g/dL) | 14.7 | 15.1 | 12.6 | 10.4 | 14.0-17.0 |
| Hct (%) | 42.3 | 44.0 | 36.6 | 31.3 | 42.0-52.0 |
| Plt (103/µL) | 205 | 176 | 160 | 109 | 140-400 |
| PCT (%) | 0.2 | 0.18 | 0.16 | 0.11 | |
| MPV (fL) | 9.6 | 10.0 | 9.8 | 9.9 | 7-11 |
| PDW (fL) | 10.9 | 110. | 10.2 | 10.3 | 11-16 |
| Coagulation profile | |||||
| PT-INR | 1.07 | 1.03 | 1.03 | 0.88-1.12 | |
| aPTT (s) | 33.2 | 24.7 | 32.1 | 27-42 | |
| Liver and kidney function tests | |||||
| AST (U/L) | 20 | 136 | 114 | 10-40 | |
| ALT (U/L) | 18 | 62 | 59 | 6-40 | |
| ALP (U/L) | 107 | 75 | 64 | 40-129 | |
| T bil (mg/dL) | 0.75 | 0.51 | 0.88 | 0.1-1.2 | |
| Albumin (g/dL) | 4.9 | 3.7 | 3.7 | 3.3-5.2 | |
| T chol (mg/dL) | 213 | 169 | 156 | 175-210 | |
| BUN (mg/dL) | 12.5 | 32.9 | 20.1 | 6-26 | |
| Creatinine (mg/dL) | 0.76 | 0.98 | 0.77 | 0.4-1.2 | |
| GFR (mL/min/1.73 m2) | 105 | 78.3 | 103.4 | ||
| Uric acid (mg/dL) | 4.1 | 6.1 | 3.3 | 2.5-8.0 | |
| Electrolyte | |||||
| Sodium (mmol/L) | 142.2 | 144.2 | 139.4 | 139.7 | 138-148 |
| Potassium (mmol/L) | 4.08 | 3.70 | 4.25 | 4.01 | 3.5-5.3 |
| Calcium (mg/dL) | 9.2 | 7.7 | 7.8 | 8.5-10.3 | |
| Phosphorus (mg/dL) | 3.4 | 3.2 | 2.3 | 2.0-4.6 | |
| Anion gap | 11.7 | 20.4 | 14.3 | 10.4 | |
| Myoglobin and muscle enzyme | |||||
| Myoglobin (ng/mL) | 1192.8 | 295.0 | 96.7 | 15.2-91.2 | |
| Creatine kinase (U/L) | 7081.0 | 6198.6 | 4697 | 5-217 | |
| CK-MB (ng/mL) | 89.06 | 55.02 | 0.5-5.0 | ||
Although the patient was diagnosed with hypertension > 10 years earlier (baseline SBP/DBP, 160-180/100-78 mmHg), he had voluntarily not taken antihypertensive medication for years. He also had varicose veins in both legs.
The patient was a heavy-drinking smoker[8]; he would drink more than 50 g of alcohol and smoke 18 cigarettes per day. The patient had no family history of hypercoagulable disorders.
After the surgical drape was removed, cyanosis was evident in both feet of the patient. The pulse was not palpable in the bilateral dorsalis pedis and posterior tibial arteries. For further evaluation and treatment, the patient was referred for a consultation to the vascular surgery department of our hospital. A hand-held continuous-wave Doppler examination revealed that Doppler signals of the bilateral dorsalis pedis and posterior tibial arteries were absent (i.e., inaudible).
Immediately after the surgery, a series of laboratory tests were performed. Routine postoperative laboratory test findings are presented in Table 1. Except for a decrease in protein S activity [22% (reference range[9], 65-160)] and an increase in fibrinogen degradation products [146.3 µg/mL (0.0-5.0)] and the D-dimer level [35.2 µg/mL (0.0-0.5)], the results of the hypercoagulability work-up were not specific [protein C activity, 102.6% (73.0-142.0); fibrinogen, 277.2% (170.0-380.0); and antithrombin III activity, 91.7% (80.0-120.0)]; factor V Leiden, lupus anticoagulant, anti-cardiolipin immunoglobulin (Ig) M, anti-cardiolipin IgG, anti-cardiolipin IgA, anti-phospholipid IgG, and prothrombin G20210A mutation findings were all negative. Blood cultures were also negative. Lipid profile was as follows: Low-density lipoprotein cholesterol level, 108 mg/dL (< 160); high-density lipoprotein cholesterol level, 69.0 mg/dL (35.0-72.0); and triglyceride level, 64 mg/dL (58-250 mg/dL). Cardiac markers were as follows: myoglobin level, 1192.8 ng/mL (15.2-91.2); creatine kinase (CK) level, 7081 U/L (5-217); CK-myocardial band level, 89.06 ng/mL (0.5-5.0); troponin I level, 0.02 ng/mL (0-0.05); and brain natriuretic peptide level, 28 pg/mL (0-100). Urinalysis results were as follows: color, yellow; clarity, clear; pH, 7.0 (5.0-6.5); urine occult blood, trace; urine RBC, 11-15/high power field (HPF; 0-2); urine WBC, 0-2 (0-2); urine glucose, negative. HbA1c and blood glucose levels were 5.9% and 99 mg/dL, respec
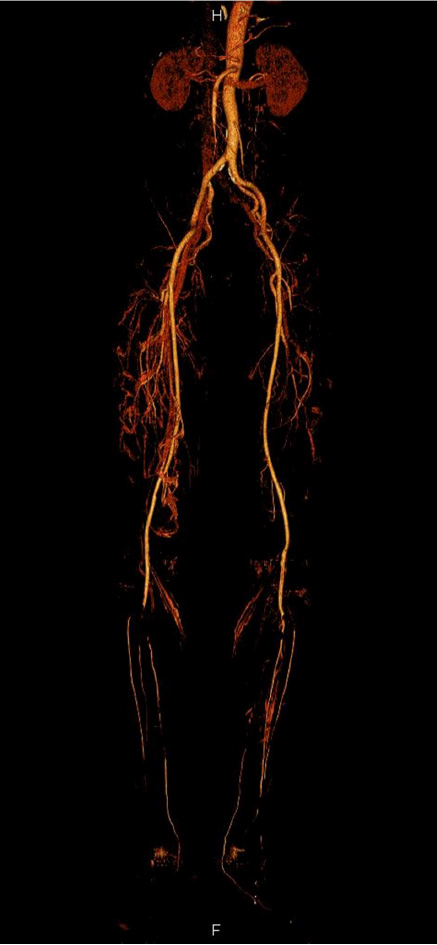
On computed tomography (CT) angiography, filling defects in the bilateral popliteal arteries, bilateral proximal anterior tibial artery, and bilateral tibioperoneal trunk were visible, which confirmed the Doppler findings (Figure 2). Concomitant venous throm
Transesophageal echocardiography revealed no structural or functional abnormalities, and there was no evidence of a cardiac embolic source. Postoperative ECG showed a normal sinus rhythm.
The patient was diagnosed with acute thrombotic occlusion of the bilateral popliteal arteries, proximal anterior tibial arteries, and tibioperoneal trunk.
After surgery, the patient was administered oxygen at the rate of 5 L/min using a nasal cannula, and the patient’s vital signs were stable, except for tachycardia caused by pain (HR, 119-125 beats/min; SpO2, 97%-100%; SBP, 110-120 mmHg; DBP, 55-80 mmHg; and RR, 15-20 breaths/min). For pain control, intravenous fentanyl 100 mcg and pethidine 25 mcg were administered immediately and 30 min after surgery, respectively. Immediately after the diagnosis was confirmed, intravenous unfractionated heparin (UFH) was administered for anticoagulation, with a bolus loading dose of 5000 units, followed by a maintenance dose of 800 units/h. After 2 h, heparin infusion was stopped, and surgical thrombectomy was planned. However, upon arrival in the operating room, that is 4 h after heparin cessation, the arterial pulse had returned in both lower limbs. Therefore, the surgery was canceled, and heparin therapy was reinitiated. Lipo-prostaglandin E1, a potent vasodilator and platelet aggregation inhibitor, was administered as an adjuvant treatment[10]. After 10 h of heparin reinitiation, the patient had hematochezia with a total volume of approximately 500 mL. Heparin infusion was immediately stopped, and the patient was closely monitored. The patient’s vital signs remained stable (HR: 74-92 beats/min; SpO2: 97%-99%; SBP: 120-140 mmHg; DBP: 80-82 mmHg; and RR: 20-21 breaths/min). Due to the repeated heparin infusion and discontinuation, activated partial throm
Although myoglobinuria was absent, the patient’s history, symptoms, and mar
Immediately after surgery, the patient complained of motor weakness in both lower extremities, and the muscle strength parameters according to the expanded Medical Research Council of Great Britain grading scale[11] were as follows, right/Left: hip flexion (2/5-), hip extension (2/5-), hip abduction (2/5-), hip adduction (2/5-), knee flexion (2/5-), knee extension (2/5-), ankle dorsiflexion (3/5-), ankle plantar flexion (3/5-), great toe extension (3/5-), and great toe flexion (3/5-). To evaluate the cause of motor weakness, the ankle brachial index (ABI) was measured; the right and left ABIs were within the normal range (1.26 and 1.21, respectively). Electromyogram and nerve conduction examinations showed non-specific findings. On POD 12, lipo-prosta
The conventional literature emphasizes the difference between arterial and venous thromboses[4]. The pathophysiology of venous thrombosis has been described as Virchow’s triad, that is, stasis, hypercoagulability, and alterations in the endothelium[1,3,4]. In contrast, the pathophysiology of acute arterial thrombosis includes rupture of an atherosclerotic plaque associated with high shear rates and disruption of the endothelium[1,3,4]. Moreover, it is still recommended to treat arterial thrombosis with drugs that target platelets and venous thrombosis with drugs that target proteins of the coagulation cascade[1,4].
However, recent epidemiological studies have suggested that venous thrombosis and arterial thrombosis are closely related[4,5]. The most probable biological expla
In the present case, the patient had multiple risk factors (advanced age, smoking, hypertension, varicose veins, and protein S deficiency) and developed acute arterial thromboses in the lower limbs during an unexpectedly prolonged operation.
The aging process involves degeneration of vessel walls, activation of the coagu
Cigarette smoking generates a prothrombotic environment by increasing the arterial intima-media thickness, promoting endothelial dysfunction, and increasing platelet activation and prothrombic biomarkers[17,18]. Smoking is a particularly strong risk factor for arterial thrombosis[17,18]. However, evidence on the effect of smoking on venous thrombosis remains controversial[13]. According to a recent large-scale, popu
In patients with hypertension, despite the continuous exposure of the vessel wall to high pressure, complications of hypertension are paradoxically more strongly asso
For venous thrombosis, varicose vein and protein S deficiency are well-documented risk factors; however, with regard to arterial thrombosis, the effects of varicose veins and protein S deficiency remain unclear[24,25]. In a retrospective cohort study using national health insurance data, Chang et al[24] found that varicose veins were signi
Protein S, a cofactor of protein C, inactivates coagulation factors Va and VIIIa and inhibits thrombin generation[25]. In a retrospective family cohort study, Mahmoodi et al[26] reported that protein S deficiency increases arterial thromboembolic risk in patients below 55 years of age (adjusted hazard ratio, 4.6; 95%CI: 1.1-18.3). Further
Surgery is an independent risk factor for venous thrombosis[28]. Surgery itself induces blood stasis, release of tissue factors, and a generalized hypercoagulable envi
Prevention is the most effective strategy for limiting the adverse consequences of thromboembolism in surgical patients[29,32]. Thromboprophylaxis includes mechani
As ophthalmic surgery is considered as a low-risk procedure, routine thromboprophylaxis is often overlooked, and relevant guidelines for thromboprophylaxis during ophthalmic surgery are scarce[35,36]. In a previous survey-based study of anesthesiologists involved in the management of ophthalmic surgeries, 45% of respondents reported experiencing thromboembolism after ophthalmic surgery; however, only 40% stated that there were routine assessments for indications and contraindications of thromboprophylaxis in preanesthetic clinics[36]. In this case too, the preoperative thromboembolism risk assessment was overlooked. Moreover, while it was planned for < 2 h, the surgery was unexpectedly prolonged. As prevention is the best policy, this case highlights the importance of preoperative thromboembolic risk assessment, intraoperative communication between the surgeon and anesthesiologist (particularly in the context of unexpectedly prolonged surgery), and the need for consensus guide
In summary, acute bilateral lower extremity arterial thromboses can occur unexpec
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chauhan S, Chilimuri S S-Editor: Gao CC L-Editor: A P-Editor: Yuan YY
| 1. | Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 940] [Cited by in RCA: 838] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 2. | Tsai FY, Kostanian V, Rivera M, Lee KW, Chen CC, Nguyen TH. Cerebral venous congestion as indication for thrombolytic treatment. Cardiovasc Intervent Radiol. 2007;30:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Previtali E, Bucciarelli P, Passamonti SM, Martinelli I. Risk factors for venous and arterial thrombosis. Blood Transfus. 2011;9:120-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 168] [Reference Citation Analysis (0)] |
| 4. | Lowe GD. Common risk factors for both arterial and venous thrombosis. Br J Haematol. 2008;140:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Jerjes-Sanchez C. Venous and arterial thrombosis: a continuous spectrum of the same disease? Eur Heart J. 2005;26:3-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Autar R. NICE guidelines on reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients undergoing surgery. J Orthop Nurs. 2007;11:169-176. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Agnelli G. Prevention of venous thromboembolism in surgical patients. Circulation. 2004;110:IV4-I12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Mirbaba M. Heavy-Drinking Smokers: Pathophysiology and Pharmacologic Treatment Options. Am J Psychiatry Resid J. 2016;11:8-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Myo Clinic Laboratories. Protein S Activity, Plasma. [cited 25 April 2021]. In: Myo Clinic Laboratories. [Internet]. Available from: https://hematology.testcatalog.org/show/S_FX. |
| 10. | Li J, Wang B, Wang Y, Wu F, Li P, Li Y, Zhao L, Cui W, Ding Y, An Q, Si J. Therapeutic effect of liposomal prostaglandin E1 in acute lower limb ischemia as an adjuvant to hybrid procedures. Exp Ther Med. 2013;5:1760-1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Barohn RJ, Dimachkie MM, Jackson CE. A pattern recognition approach to patients with a suspected myopathy. Neurol Clin. 2014;32:569-593, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Violi F, Loffredo L. Association between venous and arterial thrombosis. Lancet. 2008;371:809; author reply 809-809; author reply 810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 719] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 14. | Lowe GD. Venous and arterial thrombosis: epidemiology and risk factors at various ages. Maturitas. 2004;47:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 822] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 16. | Savji N, Rockman CB, Skolnick AH, Guo Y, Adelman MA, Riles T, Berger JS. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol. 2013;61:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 17. | Campbell RA, Machlus KR, Wolberg AS. Smoking out the cause of thrombosis. Arterioscler Thromb Vasc Biol. 2010;30:7-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Tsiara S, Elisaf M, Mikhailidis DP. Influence of smoking on predictors of vascular disease. Angiology. 2003;54:507-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Enga KF, Braekkan SK, Hansen-Krone IJ, le Cessie S, Rosendaal FR, Hansen JB. Cigarette smoking and the risk of venous thromboembolism: the Tromsø Study. J Thromb Haemost. 2012;10:2068-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Lip GY. Hypertension and the prothrombotic state. J Hum Hypertens. 2000;14:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Yameogo AR, Mandi G, Millogo G, Samadoulougou A, Zabsonre P. Assessing causes of death in the Cardiology Department of Yalgado Ouédraogo University Hospital. Pan Afr Med J. 2014;19:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Dorobantu M, Onciul S, Tautu OF, Cenko E. Hypertension and Ischemic Heart Disease in Women. Curr Pharm Des. 2016;22:3885-3892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Gordon T, Kannel WB. Predisposition to atherosclerosis in the head, heart, and legs. The Framingham study. JAMA. 1972;221:661-666. [PubMed] [DOI] [Full Text] |
| 24. | Chang SL, Huang YL, Lee MC, Hu S, Hsiao YC, Chang SW, Chang CJ, Chen PC. Association of Varicose Veins With Incident Venous Thromboembolism and Peripheral Artery Disease. JAMA. 2018;319:807-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 25. | Fearon A, Pearcy P, Venkataraman S, Shah P. Protein S Deficiency and Arterial Thromboembolism: A Case Report and Review of the Literature. J Hematol. 2019;8:37-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Mahmoodi BK, Brouwer JL, Veeger NJ, van der Meer J. Hereditary deficiency of protein C or protein S confers increased risk of arterial thromboembolic events at a young age: results from a large family cohort study. Circulation. 2008;118:1659-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Cho YP, Kwon TW, Ahn JH, Kang GH, Han MS, Kim YH, Kwak JH, Lee SG. Protein C and/or S deficiency presenting as peripheral arterial insufficiency. Br J Radiol. 2005;78:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Kim JY, Khavanin N, Rambachan A, McCarthy RJ, Mlodinow AS, De Oliveria GS Jr, Stock MC, Gust MJ, Mahvi DM. Surgical duration and risk of venous thromboembolism. JAMA Surg. 2015;150:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 29. | Marino PL. The ICU book. 4th ed. Lippincott Williams & Wilkins, 2013: 59-69. |
| 30. | Tuman KJ. Perioperative myocardial infarction. Semin Thorac Cardiovasc Surg. 1991;3:47-52. [PubMed] |
| 31. | Ng JL, Chan MT, Gelb AW. Perioperative stroke in noncardiac, nonneurosurgical surgery. Anesthesiology. 2011;115:879-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | O'Donnell M, Weitz JI. Thromboprophylaxis in surgical patients. Can J Surg. 2003;46:129-135. [PubMed] |
| 33. | Moran PS, Teljeur C, Harrington P, Ryan M. A systematic review of intermittent pneumatic compression for critical limb ischaemia. Vasc Med. 2015;20:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Nenci GG, Minciotti A. Low molecular weight heparins for arterial thrombosis. Vasc Med. 2000;5:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 35. | Yang V, Romeo P. Review: Ophthalmic Surgery as a cause of Pulmonary Emboli. J Cardiol Clin Res. 2020;8:1156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Kumar CM, Macachor J, Seet E. Venous thromboembolism prophylaxis during vitreoretinal surgery – a snapshot survey of international ophthalmic anaesthetists. Br J Anaesth. 2015;115:320-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |