Published online Sep 26, 2021. doi: 10.12998/wjcc.v9.i27.8120
Peer-review started: April 3, 2021
First decision: July 15, 2021
Revised: July 25, 2021
Accepted: August 5, 2021
Article in press: August 5, 2021
Published online: September 26, 2021
Processing time: 165 Days and 18 Hours
Gastrointestinal stromal tumor (GIST) with cutaneous metastasis is very rare. As a result, cutaneous GISTs have not been well characterized. Focal segmental glomerulosclerosis (FSGS) is also a rare symptom among paraneoplastic nephritic syndromes (PNS).
In this case report, we describe a patient with cutaneous metastatic GIST accompanied by nephrotic syndrome occurring as a malignancy-associated PNS, for whom symptomatic treatment was ineffective, but clinical remission was achieved after surgery. Moreover, the patient has a missense mutation in NPHP4, which can explain the occurrences of GIST and FSGS in this patient and indicates that the association is not random.
This is the first reported case of a GIST with cutaneous metastasis accompanied by nephrotic syndrome manifesting as a PNS.
Core Tip: This paper reports the case of patient with cutaneous metastatic gastro
- Citation: Zhou J, Yang Z, Yang CS, Lin H. Paraneoplastic focal segmental glomerulosclerosis associated with gastrointestinal stromal tumor with cutaneous metastasis: A case report. World J Clin Cases 2021; 9(27): 8120-8126
- URL: https://www.wjgnet.com/2307-8960/full/v9/i27/8120.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i27.8120
Gastrointestinal stromal tumor (GIST) is one of the most common mesenchymal neoplasms that occurs in different areas of the gastrointestinal tract. The most frequent site of GIST is the stomach (60%), followed by the small intestine (35%) and the colon and rectum (< 5%)[1]. Immunohistochemistry is helpful for the diagnosis because GISTs show immunoreactivity for CD117 (95%), CD34 (70%), and DOG1, a complementary marker to CD117[2]. GISTs are usually benign with a malignant transfor
In this case report, we describe a patient with cutaneous metastatic GIST accompanied by nephrotic syndrome occurring as a malignancy-associated PNS for whom symptomatic treatment was ineffective but clinical remission was achieved after surgery. To the best of our knowledge, this is the first time that we present a unique case of cutaneous metastatic GIST accompanied by focal segmental glomerulosclerosis (FSGS) occurring as a malignancy-associated PNS.
A 64-year-old Chinese man was admitted to our hospital with edema that had persisted over 4 mo.
The patient reported edema of the face and lower limbs, particularly in the afternoon, and he had not received any special treatment for this symptom.
No other relevant history of past illness was reported.
No other relevant family history was reported.
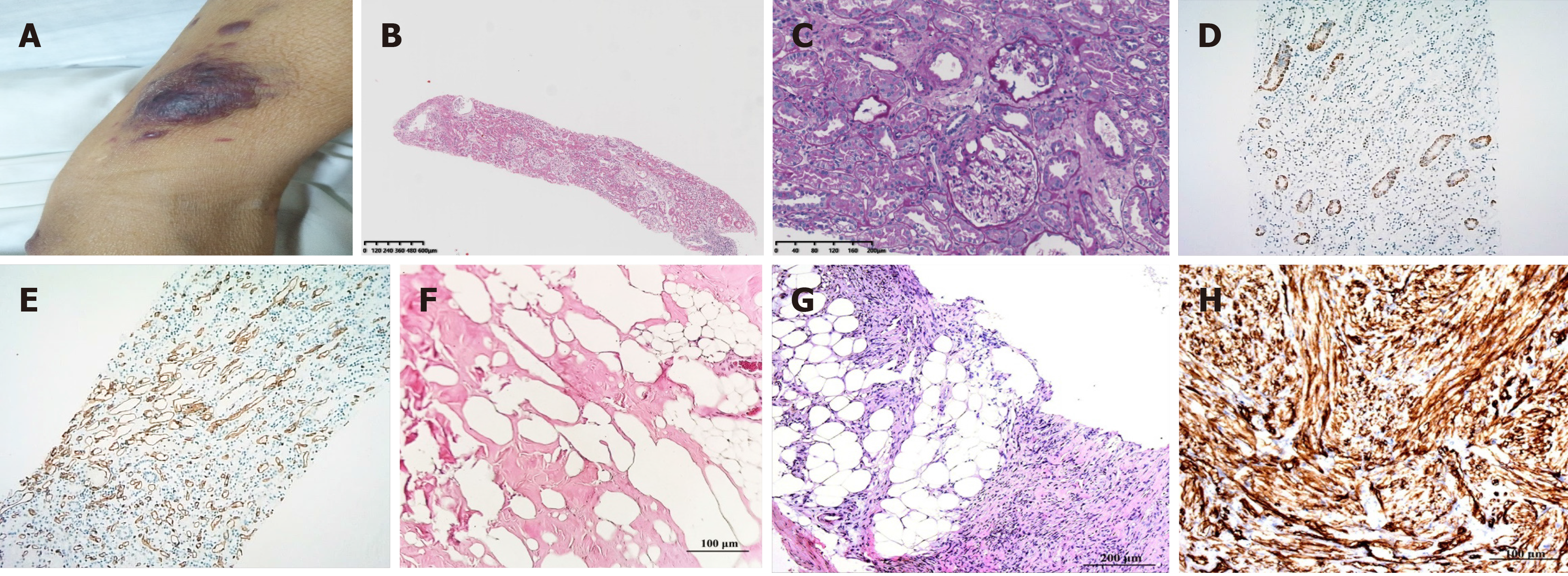
On examination, the patient presented with multiple nodules and lumps with a smooth surface that could be seen throughout the body (Figure 1A). Enlargement of the cervical, axillary, and inguinal lymph nodes was clearly observed.
Laboratory examinations revealed severe proteinuria (3.77 g/24 h; normal range, 0–0.15 g/24 h), hypoalbuminemia (1.8 g/dL), hyperlipidemia (cholesterol 15.7 mmoL, triglyceride 3.34 mmol/L), and increased serum creatinine levels (152 μmoL; normal range, 44–133 μmoL). These clinical parameters suggested that the patient’s symptoms were a result of nephrotic syndrome.
A kidney biopsy was performed and the analysis of nine glomeruli revealed one glomerulus indicative of glomerulosclerosis and one indicative of segmental glomerulosclerosis with peripheral podocytosis, vacuolation, and granular degeneration in the renal tubular epithelial cells (Figure 1B and C). These pathological findings were indicative of FSGS, not otherwise specified (NOS). Immunohistochemistry of the kidney revealed that the glomerular capillary loop, peritubular capillary, and arterioles were positive for CD34 (Figure 1D), and the proximal tubule cells were positive for CD117 (Figure 1E) but negative for DOG1. Fat degeneration and necrosis were observed in the lymph node, and no tumor metastasis was revealed (Figure 1F). Hematoxylin and eosin staining of the skin showed spindle cell tumor-like hyperplasia, which was slightly heteromorphic with focal necrosis and rarely mitotic (Figure 1G). Immunohistochemistry staining revealed that the tumor cells were positive for CD34, Bcl-2, CD99, Ki67, and vimentin but negative for smooth muscle actin (SMA) and S-100. Representative immunohistochemical staining for CD34 is shown in Figure 1H. The diagnosis was a desmoid tumor (DT) based on the above immunohistochemistry results.
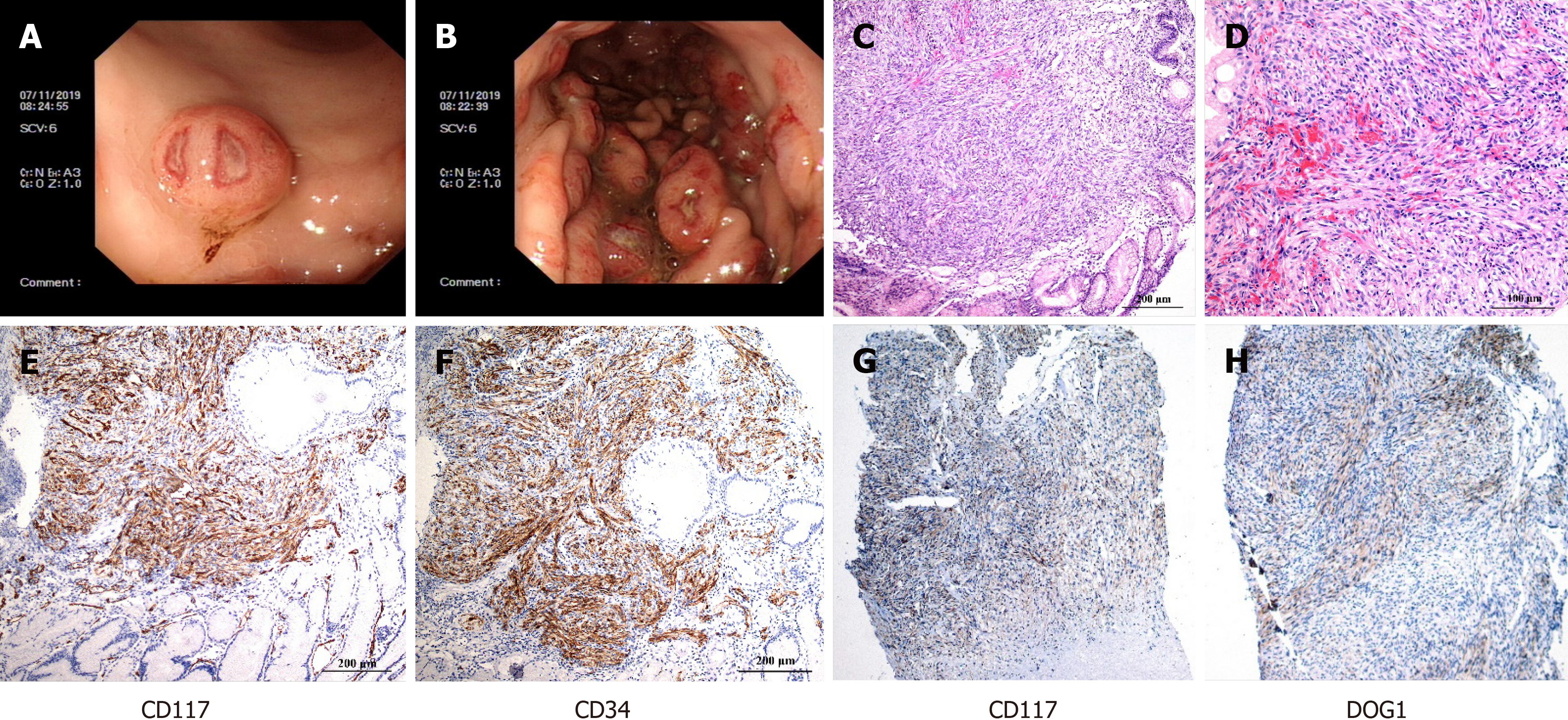
Then, gastroscopy was performed and the results revealed a 0.6 cm mucosal mass that was hard to the touch, and poor mobility could be seen in the greater curvature of the stomach (Figure 2A). Several 0.4-2 cm masses were observed in the anterior wall of the middle and upper of the gastric fundus with fractured surfaces and fresh blood (Figure 2B). Moreover, pathological examination revealed the destruction of the gastric solid membrane structure and spindle cell tumor-like hyperplasia with mild dysplasia (Figure 2C), and epithelioid cells were occasionally seen with 6-8 mitotic figures/50 high power fields (Figure 2D). CD117 (Figure 2E), CD34 (Figure 2F), and SMA were positive by immunohistochemistry, and DOG1 was probably positive, and S-100 and CK were negative. These findings supported the diagnosis of GIST. Additional immunohistochemistry revealed that the skin was positive for CD117 (Figure 2G) and DOG1 (Figure 2H), the two most sensitive and specific markers for the diagnosis of GIST[6]. In addition, a heterozygous missense mutation was found in the NPHP4 gene of the patient (NPHP4: NM_015102: exon17: c.2198G>A: p.G733D), which explained the occurrence of GIST and FSGS and indicated that this was not a random association[6].
The final diagnosis of the patient presented in this case report was a metastatic cutaneous GIST.
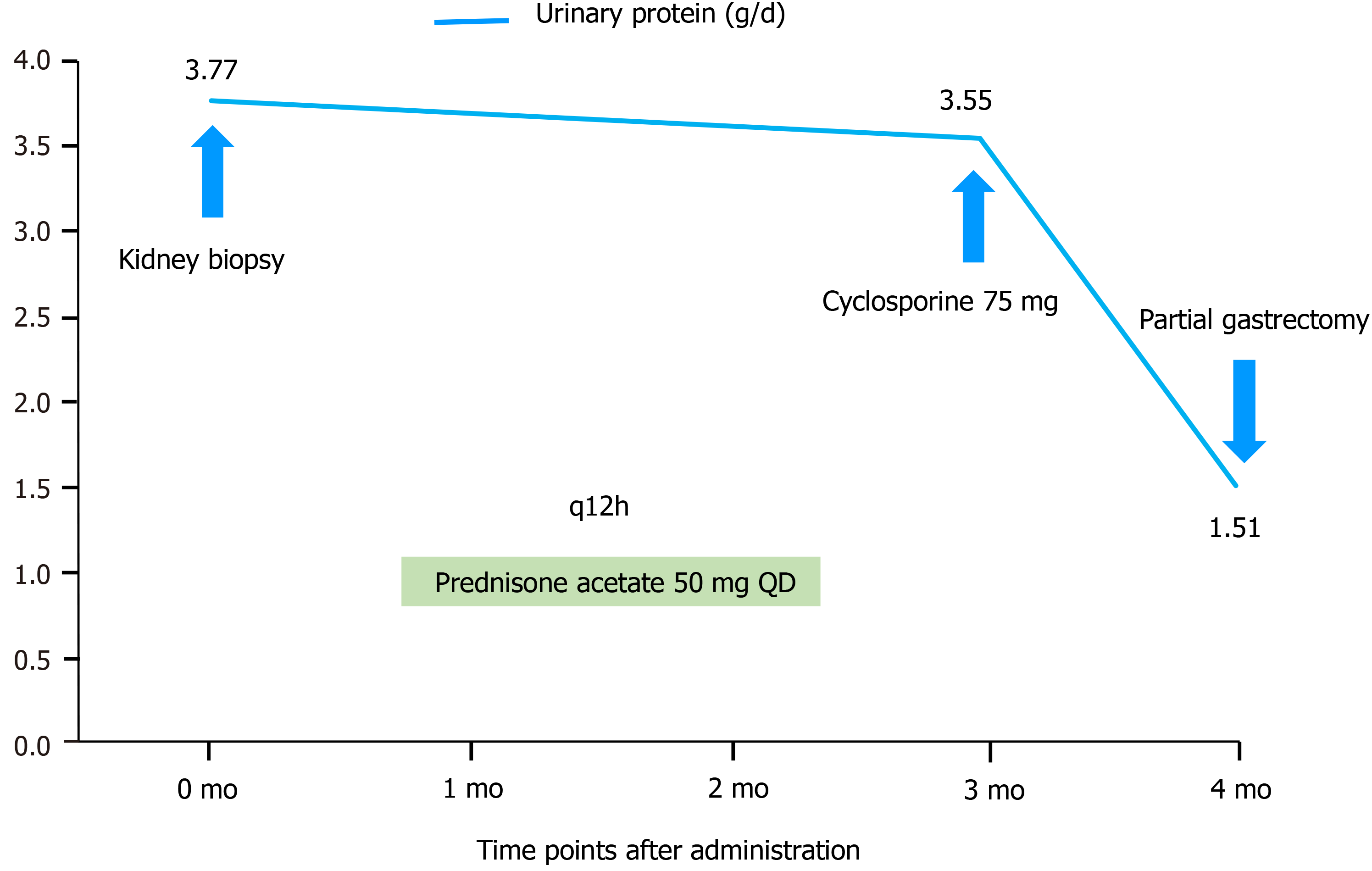
Cyclosporine was administered in an outpatient setting for 3 d, and the discomfort of the patient was alleviated. Moreover, the patient received prednisone acetate 50 mg QD for 3 mo. After 4 mo, a partial gastrectomy was performed.
Despite receiving prednisone for 3 mo, the patient’s severe proteinuria and hypoalbuminemia did not improve. After 4 mo of follow-up, a partial gastrectomy was performed. Two weeks after the tumor was removed by surgery, the patient’s serum albumin and urinary protein levels improved remarkably (Figure 3).
GISTs arise from the interstitial cells of Cajal, which serve as a pacemaker for the gastrointestinal tract by creating slow wave potentials that direct smooth muscle to contract. GISTs primarily metastasize to the liver and peritoneum, while cutaneous metastases are the least common. However, the mechanism of GIST metastasis to the skin remains unknown. It is hypothesized that the presence of skin metastases may indicate multiple internal metastases[3]. This patient’s disease was initially misdiagnosed as DT prior to the recognition of cutaneous metastatic GIST by assessing the expression of CD117, CD34, and DOG1 in the skin. Patients with a history of GISTs should be advised to undergo a full skin examination to detect visible clues that indicate metastatic tumor burden.
In our case, the patient had a missense mutation of NPHP4. NPHP4 is located on chromosome 1p36 and encodes nephrocystin-4/nephroretinin[7]. Nephrocystin-4 colocalizes and interacts with nephrocystins 1 and 3 in primary cilia and associated appendages, adherens junctions, and focal adhesions[8]. Individuals with mutations in NPHP4 most frequently present with nephronophthisis[9]. Some researchers have determined that NPHP4 is a negative regulator of the Hippo pathway[10]. In acute renal injury, the Hippo signaling pathway may be involved in the apoptosis of tubule epithelial cells, epithelial-mesenchymal transition, and acute renal injury progression to chronic kidney disease, among other processes[11]. In addition, the Hippo signaling pathway is involved in the development and progression of several chronic kidney diseases, including FSGS, diabetic nephropathy, and polycystic kidney disease[12]. The conserved Hippo signaling pathway regulates organ size in Drosophila melanogaster and mammals and plays an essential role in tumor suppression and cell proliferation[13]. NPHP4 is indeed a driving force of the proliferation of tumor cells. Therefore, the mutation of NPHP4 in this patient could explain the occurrence of GIST and FSGS and this was therefore not a random association.
Our patient presented with cutaneous metastatic GIST which needed to be differentiated from Gardner’s syndrome, an autosomal hereditary disease characterized by multiple adenomatous polyps in the colorectal region and the presence of some extracolonic lesions. Gardner’s syndrome is characterized by multiple adenomatous polyps in the colorectal region with osteoma, soft-tissue tumor, and tooth abnormality. In addition, 30%-75% of patients with Gardner’s syndrome also have dental abnor
Theoretically, the diagnosis of paraneoplastic glomerulopathy should rely on three strong criteria. First, clinical and histologic remission occurs after complete surgical removal of the tumor or chemotherapy-induced complete remission of the disease. In our patient, symptomatic treatment was ineffective but clinical remission was achieved after surgery. Therefore, the patient’s nephrotic syndrome could be diagnosed as a PNS. Second, a renal relapse accompanies the recurrence of neoplasia. In other words, proteinuria should directly correlate with tumor activity. Third, a pathophysiologic link is established between the two diseases, including the detection of tumor antigens and antitumor antibodies within subepithelial immune deposits[15]. In our patient, the immunohistochemistry of the kidney revealed positivity for CD117 and CD34, but negativity for DOG1. Telocytes (TCs) were indicated as a distinctive cell type after being previously described as “interstitial Cajal-like cells”[16]. Qi et al[17] reported TCs in the interstitium of the human kidney cortex. Renal TCs were found to express CD34 and CD117 with variable intensity[17]. Therefore, the positive CD34 and CD117 results in the kidney were not significant. Nephrotic syndrome can occur as malignancy-associated PNS, and it has been estimated that cancer occurs in 11%–22% of patients with nephrotic syndrome[5]. The most common pathological type of tumor-associated nephropathy is membranous nephropathy (44%–49%). FSGS is extremely rare among PNS patients and has been observed in association with renal cell carcinoma, invasive thymoma, and lung cancer[18]. There has been only one reported case of GIST of the stomach that was associated with nephrotic syndrome occurring as a malignancy-associated PNS. Nephrotic syndrome improves after surgery in 78% of patients[5]. Considering that cancer is a potential cause of nephrotic syndrome, surgical resection should be performed in the presence of PNS. In our case, the patient’s proteinuria and hypoalbuminemia did not respond to symptomatic treatment. After tumor removal, clinical remission of nephrotic syndrome was immediately achieved. Thus, FSGS can be considered a GIST-associated PNS. However, the variant of FSGS that has been reported in PNS is the collapsing variant, due to the overexpression of vascular endothelial growth factor, which leads to collapsing FSGS. Our patient, on the other hand, had FSGS NOS.
Some studies have indicated that α-actinin-4 mutations play an important role in the development of PNS[19]. Unfortunately, this patient did not have any ACTN4 mutations. However, these hypotheses seem insufficient to explain the occurrence of GIST accompanied by FSGS occurring as a malignancy-associated PNS. Tyrosine kinase inhibitors (TKIs) are effective for treating GISTs, but researchers have mainly focused on TKI-associated renal injury leading to FSGS. In the future, we will use cytotoxic chemotherapy for this patient with intensive follow-up. GISTs are currently regarded as potentially malignant tumors. Discrimination of benign GISTs from malignant GISTs is performed by postoperative histological analysis (tumor diameter, mitotic index, whether the tumor has metastasized, and Ki67 expression level)[20]. The diagnosis of cutaneous metastatic GIST indicates that this case represents a high-risk patient with a poor prognosis. Joensuu’s group recommended shorter imaging intervals of approximately 3-4 mo for high-risk patients during a period of approximately 2 years following discontinuation of imatinib[21]. Our patient’s risk for recurrence would be reduced by this follow-up schedule.
In conclusion, FSGS caused by cutaneous metastatic GIST is extremely rare, and to the best of our knowledge, this is the first report of such a case. The NPHP4 mutation in this case can explain that the occurrence of GISTs and FSGS was not a random association.
We thank Professor Qiu P for editing the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shalaby MN S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Vassos N, Agaimy A, Hohenberger W, Croner RS. Coexistence of gastrointestinal stromal tumours (GIST) and malignant neoplasms of different origin: prognostic implications. Int J Surg. 2014;12:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Phan M, Jones S, Jenkins J, Pant S, Khawandanah M. Pancreatic GIST in a Patient with Limited Stage Small Cell Lung Cancer: A Case Report and Review of Published Cases. Case Rep Oncol Med. 2016;2016:9604982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Kim YJ, Lee WJ, Won CH, Choi JH, Lee MW. Metastatic Cutaneous Duodenal Gastrointestinal Stromal Tumor: A Possible Clue to Multiple Metastases. Ann Dermatol. 2018;30:345-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Aickara DJ, McBride J, Morrison B, Romanelli P. Multidrug resistant gastrointestinal stromal tumor with multiple metastases to the skin and subcutaneous soft tissue: A case report and review of literature. J Cutan Pathol. 2020;47:398-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Takane K, Midorikawa Y, Yamazaki S, Kajiwara T, Yoshida N, Kusumi Y, Takayama T. Gastrointestinal stromal tumor with nephrotic syndrome as a paraneoplastic syndrome: a case report. J Med Case Rep. 2014;8:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Novelli M, Rossi S, Rodriguez-Justo M, Taniere P, Seddon B, Toffolatti L, Sartor C, Hogendoorn PC, Sciot R, Van Glabbeke M, Verweij J, Blay JY, Hohenberger P, Flanagan A, Dei Tos AP. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology. 2010;57:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Salomon R, Saunier S, Niaudet P. Nephronophthisis. Pediatr Nephrol. 2009;24:2333-2344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Delous M, Hellman NE, Gaudé HM, Silbermann F, Le Bivic A, Salomon R, Antignac C, Saunier S. Nephrocystin-1 and nephrocystin-4 are required for epithelial morphogenesis and associate with PALS1/PATJ and Par6. Hum Mol Genet. 2009;18:4711-4723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Simms RJ, Hynes AM, Eley L, Sayer JA. Nephronophthisis: a genetically diverse ciliopathy. Int J Nephrol. 2011;2011:527137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Habbig S, Bartram MP, Müller RU, Schwarz R, Andriopoulos N, Chen S, Sägmüller JG, Hoehne M, Burst V, Liebau MC, Reinhardt HC, Benzing T, Schermer B. NPHP4, a cilia-associated protein, negatively regulates the Hippo pathway. J Cell Biol. 2011;193:633-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Zhao S, Yin J, Zhou L, Yan F, He Q, Huang L, Peng S, Jia J, Cheng J, Chen H, Tao W, Ji X, Xu Y, Yuan Z. Hippo/MST1 signaling mediates microglial activation following acute cerebral ischemia-reperfusion injury. Brain Behav Immun. 2016;55:236-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Mistry K, Ireland JH, Ng RC, Henderson JM, Pollak MR. Novel mutations in NPHP4 in a consanguineous family with histological findings of focal segmental glomerulosclerosis. Am J Kidney Dis. 2007;50:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Zanconato F, Piccolo S. Eradicating tumor drug resistance at its YAP-biomechanical roots. EMBO J. 2016;35:459-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Seehra J, Patel S, Bryant C. Gardner's Syndrome revisited: a clinical case and overview of the literature. J Orthod. 2016;43:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Ronco PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int. 1999;56:355-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 158] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Popescu LM, Faussone-Pellegrini MS. TELOCYTES - a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 455] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 17. | Qi G, Lin M, Xu M, Manole CG, Wang X, Zhu T. Telocytes in the human kidney cortex. J Cell Mol Med. 2012;16:3116-3122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Jeyabalan A, Geara AS, Frey NV, Palmer MD, Hogan JJ. Paraneoplastic Focal Segmental Glomerulosclerosis Associated With Acute Lymphocytic Leukemia. Kidney Int Rep. 2019;4:1494-1498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Meng L, Cao S, Lin N, Zhao J, Cai X, Liang Y, Huang K, Lin M, Chen X, Li D, Wang J, Yang L, Wei A, Li G, Lu Q, Guo Y, Wei Q, Tan J, Huang M, Huang Y, Liu Y. Identification of a Novel ACTN4 Gene Mutation Which Is Resistant to Primary Nephrotic Syndrome Therapy. Biomed Res Int. 2019;2019:5949485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (9)] |
| 21. | Joensuu H, Martin-Broto J, Nishida T, Reichardt P, Schöffski P, Maki RG. Follow-up strategies for patients with gastrointestinal stromal tumour treated with or without adjuvant imatinib after surgery. Eur J Cancer. 2015;51:1611-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |