Published online Sep 26, 2021. doi: 10.12998/wjcc.v9.i27.8020
Peer-review started: May 16, 2021
First decision: June 15, 2021
Revised: June 28, 2021
Accepted: August 3, 2021
Article in press: August 3, 2021
Published online: September 26, 2021
Processing time: 122 Days and 18 Hours
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related death worldwide, and has relatively high recurrence rates. Few studies have been published on the clinical stages of recurrent HCC.
To assess the applicability of the Barcelona Clinic Liver Cancer (BCLC) staging for recurrent HCC and the need to establish clinical stage criteria for recurrent HCC.
The clinicopathological data of 81 patients with recurrent HCC who were ad
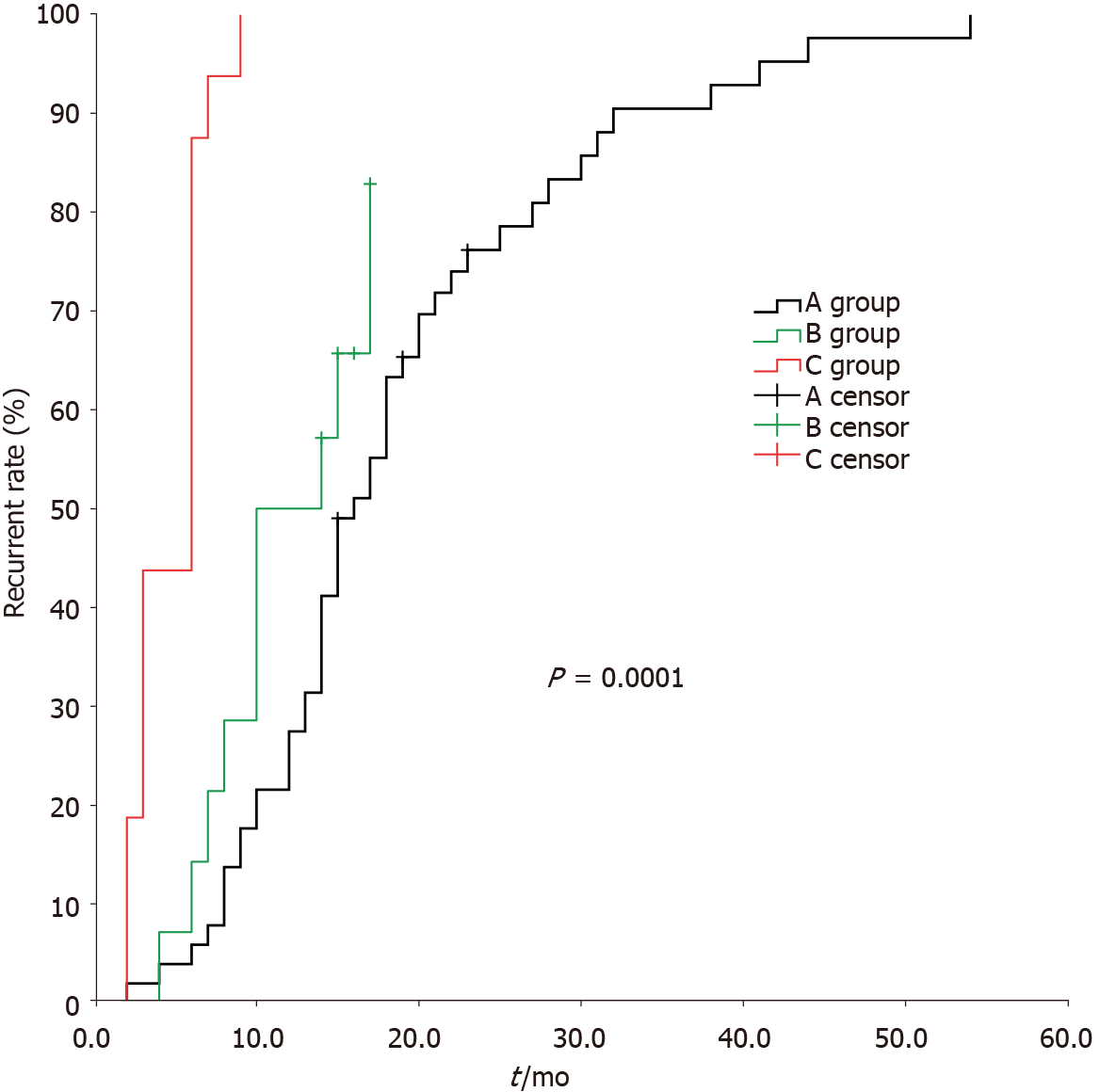
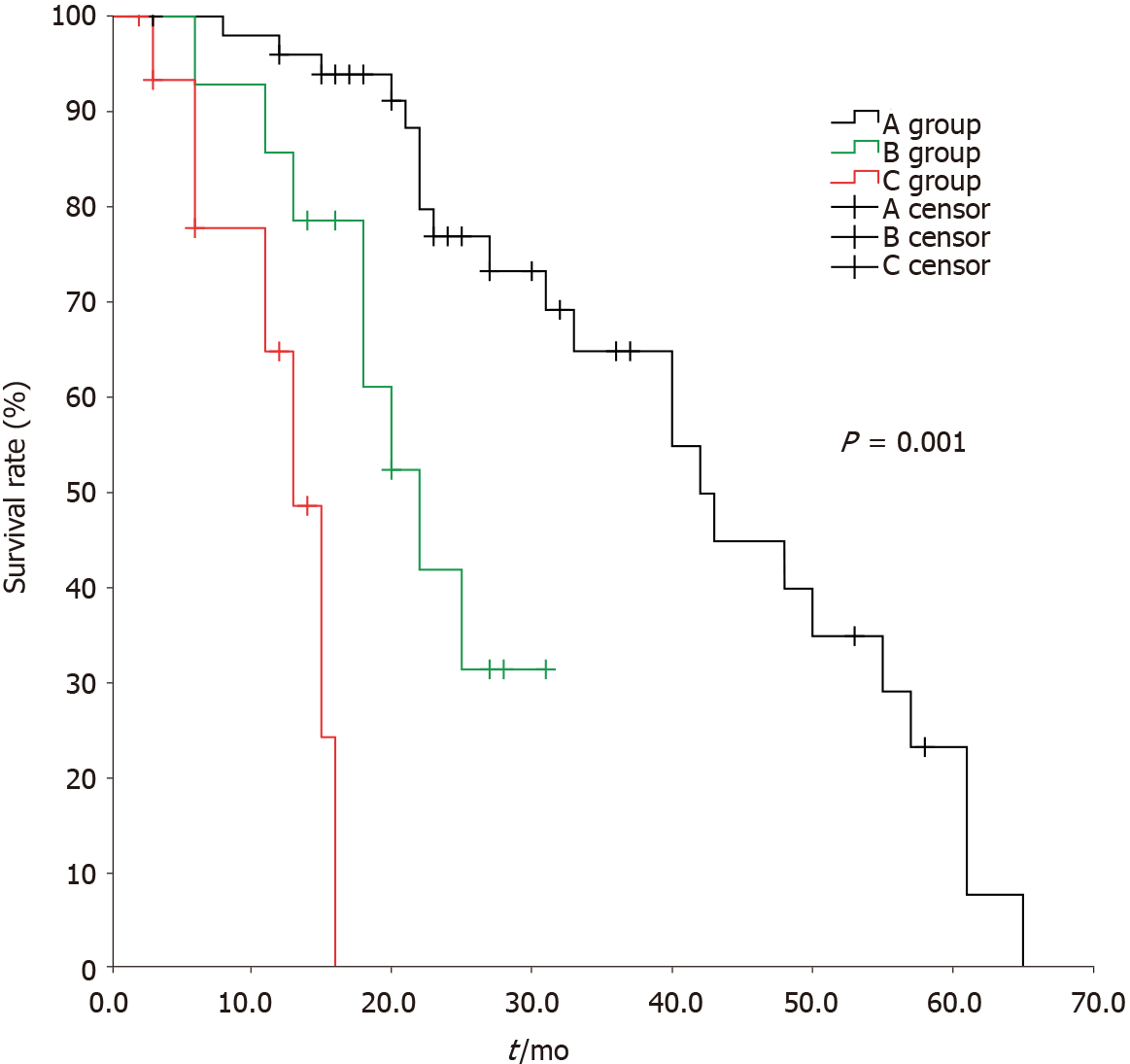
The median time to tumor recurrence in groups A, B, and C was 16 ± 1.5 mo, 10 ± 2.8 mo, and 6 ± 0.5 mo, respectively, with a statistically significant difference among them (χ2 = 70.144, P < 0.05); no statistically significant difference was noted between group A and group B (χ2 = 2.659, P > 0.05), although there were statistically significant differences between group A and group C and between group B and group C (χ2 = 62.110, and 19.972, P < 0.05). The median overall survival in groups A, B, and C were 42 ± 5.1 mo, 22 ± 3.1 mo, and 13 ± 1.8 mo, respectively, with a statistically significant difference among them (χ2 = 38.949, P < 0.05); there were statistically significant differences between group A and group B, group A and group C, and group B and group C (χ2 = 9.577, 37.172, and 7.183, respectively; P < 0.05).
There are different prognoses in recurrent HCC patients according to the BCLC staging. Therefore, BCLC staging is applicable to recurrent HCC and it is essential to formulate clinical stage criteria for recurrent HCC.
Core Tip: We analyzed the clinical and pathological data of 81 patients who developed recurrent hepatocellular carcinoma (HCC), with an aim to evaluate the applicability of the Barcelona Clinic Liver Cancer (BCLC) staging system for recurrent HCC. Our results indicate that BCLC staging is applicable to recurrent HCC and it is essential to formulate clinical stage criteria for recurrent HCC.
- Citation: Yao SY, Liang B, Chen YY, Tang YT, Dong XF, Liu TQ. Clinical stages of recurrent hepatocellular carcinoma: A retrospective cohort study. World J Clin Cases 2021; 9(27): 8020-8026
- URL: https://www.wjgnet.com/2307-8960/full/v9/i27/8020.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i27.8020
In 2012, there were 782500 patients with newly diagnosed hepatocellular carcinoma (HCC) (ranked 6th worldwide) and 745500 patients who died (ranked 2nd worldwide)[1]. The radical treatment methods for HCC include liver transplantation, surgical resection (SR), and radiofrequency ablation (RFA). Numerous studies have shown that although many patients receive curative treatment, tumor recurrence is quite common. For very early stage HCC patients, the 5-year disease-free survival (DFS) rates are 40.7% for SR and 29.3% for radiofrequency ablation. For early stage HCC patients, the 5-year DFS rates are 50.8% for SR and 14.1% for radiofrequency ablation[2]. However, even in patients who undergo liver transplantation, the tumor recurrence rate is up to 15%–20%[3]. Therefore, how to manage recurrent HCC is important in improving overall survival. To date, there have not been criteria of clinical stages for recurrent HCC. The Barcelona Clinic Liver Cancer (BCLC) staging system is regarded as the most reasonable staging criteria for primary HCC. However, whether it is suitable for recurrent HCC remains unclear. The aim of this study was to assess the applicability of the BCLC staging for recurrent HCC and the need to establish clinical stage criteria for recurrent HCC, and analyze the factors affecting the prognosis of recurrent HCC patients.
Three hundred and fifty-six recurrent HCC patients who received curative hepatic resection or RFA as an initial treatment at the People’s Hospital of Guangxi Zhuang Autonomous Region between January 2013 and December 2017 were considered candidates for this study. The inclusion criteria were as follows: (1) Pathological diagnosis of HCC; (2) No other malignant tumors or pregnancy-related disease, which may influence survival; (3) BCLC stage A, B, or C; (4) Child-Pugh level A or B; and (5) Complete clinicopathological data. Finally, 81 patients met these criteria and were enrolled. This study did not require approval from the institutional ethics committee or informed consent, and complied with the principles of the Declaration of Helsinki.
The patients were stratified into three groups based on BCLC criteria: A (per
All patients were regularly followed to identify recurrence by assessing the level of the tumor marker alpha-fetoprotein (AFP) or performing ultrasonography (US) or con
Clinical and pathological characteristics, including age, gender, AFP, HBsAg, HBV-DNA, tumor location, liver cirrhosis, tumor cell differentiation, treatment modalities, time to recurrence from last treatment, number of recurrences, and time of survival were collected from our electronic medical records or by telephone follow-up. All the patients were given antiviral treatment once they have positive HBV-DNA according to the guidelines of prevention and treatment for chronic hepatitis B (2010 version, China)[4].
Continuous variables were assessed for normality and are expressed as the mean ± SD, and comparisons among groups were evaluated by ANOVA. Categorical variables were compared by Chi-square test or Fisher’s exact test with small expected fre
We identified 81 patients, and all of them underwent a complete follow-up. The follow-up time ranged from 2 to 65 mo, with an average follow-up time of 23 ± 15 mo. There were 72 males and 9 females, with a mean age of 53 years (range, 25–82 years). There were 51 cases in group A, 14 cases in group B, and 16 cases in group C. No significant differences were detected among the three groups with respect to age, gender, AFP, HBsAg, HBV-DNA, tumor location, liver cirrhosis, tumor cell differentiation, treatment modalities, time to recurrence from last treatment, or number of recurrences (Table 1).
| Variable | Group A (n = 51) | Group B (n = 14) | Group C (n = 16) | χ2 value/F value | P value |
| Gender (male/female) | 44/7 | 13/1 | 15/1 | 0.622 | 0.784 |
| Age (yr) | 54 ± 13 (33-82) | 48 ± 11 (25-67) | 52 ± 10 (39-67) | 1.028 | 0.461 |
| AFP (μg/L) | 353.5 ± 104.5 (143.5-563.4) | 121.9 ± 47.5 (19.2-224.6) | 326.9 ± 90.6 (133.8-519.9) | 0.769 | 0.467 |
| HBsAg (negative/positive) | 4/47 | 2/12 | 1/15 | 0.968 | 0.728 |
| HBV-DNA (negative/positive) | 39/12 | 7/7 | 8/8 | 5.956 | 0.051 |
| Tumor location (left lobe/right lobe/both lobe ) | 9/37/5 | 0/10/4 | 2/9/5 | 7.459 | 0.089 |
| Liver cirrhosis (negative/positive) | 23/28 | 8/6 | 6/10 | 1.180 | 0.554 |
| Tumor cell differentiation (well/moderate/poor) | 9/32/10 | 4/8/2 | 2/10/4 | 1.655 | 0.832 |
| Treatment modality (RFA/RFA + PEI/TACE/LR) | 21/20/1/9 | 4/6/1/3 | 7/2/4/3 | 10.933 | 0.064 |
| Time to recurrence from last treatment (mo) | 26.6 ± 3.9 (18.6-34.5) | 22.6 ± 4.1 (13.7-31.6) | 17.3 ± 5.3 (5.9-28.5) | 0.856 | 0.429 |
| Number of recurrences (first/second) | 43/8 | 10/4 | 11/5 | 2.632 | 0.285 |
The median time to tumor recurrence for group A, group B, and group C was 16 ± 1.5 mo, 10 ± 2.8 mo, and 6 ± 0.5 mo, respectively, with a statistically significant difference among them (χ2 = 70.144, P < 0.05); no statistically significant difference was noted between group A and group B (χ2 = 2.659, P > 0.05), but there were statistically significant differences between group A and group C, and group B and group C (χ2 = 62.110 and 19.972, respectively, P < 0.05) (Figure 1).
The median time of overall survival for group A, group B, and group C was 42 ± 5.1 mo, 22 ± 3.1 mo, and 13 ± 1.8 mo, respectively, with a statistically significant difference among them (χ2 = 38.949, P < 0.05); there were statistically significant differences between group A and group B, group A and group C, and group B and group C (χ2 = 9.577, 37.172, and 7.183, respectively, P < 0.05) (Figure 2)
Since the BCLC staging system was put forward in 1999, it has been confirmed by a large number of clinical studies and is considered to be the most reasonable liver cancer staging criteria by combining tumor status, liver function, and treatment strategies.
Recurrence is one of the most important reasons why the prognosis of HCC is difficult to improve. At present, there have been guidelines for primary liver cancer, but for recurrent liver cancer, there is still much controversy[5,6]. Some researchers consider that the treatment for recurrent HCC can refer to that for primary HCC, including repeat hepatectomy, liver transplantation, local ablation, interventional therapy, radiotherapy, and systemic therapy[7,8]. Although SR is the best treatment option for patients with HCC, the 3-year recurrence rate is still as high as 50%-70%[9]. The treatment modalities for recurrent HCC include liver transplantation, SR, RFA, transcatheter arterial chemoembolization, and targeted therapy. Several studies show that 10%-30% of recurrent HCC patients underwent repeated SR, with a 5-year sur
Is the Barcelona Clinic Liver Cancer (BCLC) staging system applicable to recurrent HCC? In our study, the median time to tumor recurrence for group A, group B, and group C was 16 ± 1.5 mo, 10 ± 2.8 mo, and 6 ± 0.5 mo, respectively, with a statistically significant difference among them (P < 0.05); there was no statistically significant difference between group A and group B (χ2 = 2.659, P > 0.05), but there were statistically significant differences between group A and group C, and group B and group C (P < 0.05). Meanwhile, the median time of overall survival for group A, group B, and group C was 42 ± 5.1 mo, 22 ± 3.1 mo, and 13 ± 1.8 mo, respectively, with a statistically significant difference among them (P < 0.05); there were statistically significant differences between group A and group B, group A and group C, and group B and group C (P < 0.05). Our study showed the BCLC staging system is applicable to recurrent HCC, and there are different prognoses in recurrent HCC patients with different stages classified by BCLC, which is just similar to that for primary HCC. It is essential to formulate the standard of clinical stages for recurrent HCC, which would contribute to the development of more precise and individual treatment plans for recurrent HCC patients, and, improve the therapeutic efficacy for recurrent HCC. Our study showed as well that the regular examination and follow-up are important because they can increase the rate of early diagnosis and treatment for recurrent HCC. Further research is needed to provide a more exact staging basis for recurrent HCC.
The limitations of our study included its non-prospective nature and small cohort size, which would lead to recall bias. Therefore, there is clearly a need for larger sample, prospective, multicenter clinical trials to confirm our conclusion in the future, and it is essential to formulate a better clinical staging system for recurrent HCC.
There are different prognoses in recurrent HCC patients with different stages classified by BCLC, which is just similar to that for primary HCC. BCLC staging system is applicable to recurrent HCC, but not precisely enough. It is essential to formulate the standard of clinical stages for recurrent HCC, which would contribute to the develop
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related death worldwide, and has relatively high recurrence rates. At present, there has not been a unanimous opinion for the treatment of recurrent HCC, and clinical stages of recurrent HCC remain controversial.
This study showed that the Barcelona Clinic Liver Cancer (BCLC) staging system is applicable to recurrent HCC, and it is essential to formulate the standard of clinical stages for recurrent HCC, which would contribute to the development of more precise and individual treatment plans for recurrent HCC patients.
The aim of this study was to assess the applicability of the BCLC staging for recurrent HCC and the need to establish clinical stage criteria for recurrent HCC.
The clinicopathological data of 81 patients with recurrent HCC were collected. The patients were divided into three groups according to the BCLC staging system as follows: (1) Group A with BCLC stage A, 51 patients; (2) Group B with BCLC stage B, 14 patients; and (3) Group C with BCLC stage C, 16 patients. The median time to tumor recurrence time and the median overall survival were compared.
The median time to tumor recurrence in groups A, B, and C was 16 ± 1.5 mo, 10 ± 2.8 mo, and 6 ± 0.5 mo, respectively, with a statistically significant difference among them; no statistically significant difference was noted between group A and group B, al
There are different prognoses in recurrent HCC patients according to the BCLC. Therefore, BCLC staging is applicable to recurrent HCC and it is essential to formulate clinical stage criteria for recurrent HCC.
Recurrent HCC patients with different clinical stages have different prognoses, and it is essential to formulate more precise clinical stage criteria for recurrent HCC.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hann HW S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21370] [Article Influence: 2137.0] [Reference Citation Analysis (3)] |
| 2. | Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 3. | Welker MW, Bechstein WO, Zeuzem S, Trojan J. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int. 2013;26:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Chinese Society of Hepatology and Chinese Society of Infectious Diseases; Chinese Medical Association. [The guideline of prevention and treatment for chronic hepatitis B (2010 version)]. Zhonghua Gan Zang Bing Za Zhi. 2011;19:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 83] [Reference Citation Analysis (0)] |
| 5. | Saraswat VA, Pandey G, Shetty S. Treatment algorithms for managing hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S80-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer. 2018;7:235-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 7. | Erridge S, Sodergren MH. The Chengdu system for recurrent hepatocellular carcinoma: A step in the right direction. Hepatobiliary Surg Nutr. 2019;8:298-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Wen T, Jin C, Facciorusso A, Donadon M, Han HS, Mao Y, Dai C, Cheng S, Zhang B, Peng B, Du S, Jia C, Xu F, Shi J, Sun J, Zhu P, Nara S, Millis JM; MDT of West China Hospital*. Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: an international expert consensus. Hepatobiliary Surg Nutr. 2018;7:353-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Torzilli G, Donadon M, Cimino M. Are Tumor Exposure and Anatomical Resection Antithetical during Surgery for Hepatocellular Carcinoma? Liver Cancer. 2012;1:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Chan DL, Morris DL, Chua TC. Clinical efficacy and predictors of outcomes of repeat hepatectomy for recurrent hepatocellular carcinoma - a systematic review. Surg Oncol. 2013;22:e23-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 12. | Sun WC, Chen IS, Liang HL, Tsai CC, Chen YC, Wang BW, Lin HS, Chan HH, Hsu PI, Tsai WL, Cheng JS. Comparison of repeated surgical resection and radiofrequency ablation for small recurrent hepatocellular carcinoma after primary resection. Oncotarget. 2017;8:104571-104581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Jiang H, Wan SY, Hou H, Zhou LB, Yu ZF, Geng XP. A meta-analysis of the efficacy of rehepatectomy and radiofrequency ablation for recurrence liver cancer. Zhonghua Putong Waike Zazhi. 2015;30:146-149. [DOI] [Full Text] |