Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7930
Peer-review started: April 19, 2021
First decision: May 24, 2021
Revised: June 1, 2021
Accepted: July 22, 2021
Article in press: July 22, 2021
Published online: September 16, 2021
Processing time: 143 Days and 17.7 Hours
Malignant fibrous histiocytoma (MFH) is one of the most common soft tissue sarcomas among adults. It is characterized by large size, high grade, and biological aggressiveness. There are many reports of MFH after local stimulation, such as bone fracture, implants, and chronic osteomyelitis. In this paper, we report a patient who developed MFH 6 years after amputation, suggesting that wound healing and mechanical force play a role in the local stimulation of this disease.
A 66-year-old man complained of persistent pain in his residual mid-thigh. He had undergone amputation surgery due to a traffic accident 6 years prior. Physical examination showed tenderness but no abnormalities in appearance. X-ray radiographs and magnetic resonance imaging supported the diagnosis of a tumor, and a biopsy confirmed that the lesion was MFH. The patient received neoadjuvant chemotherapy and left hip disarticulation. During the 6-mo follow-up, there were no symptoms of recurrence.
Postsurgery MFH has been reported before, and many studies have attributed it to the biological effects of implants. Our case report shows that this disease can develop without an implant and thus highlights the importance of local stimulation. The wound-healing process and mechanical force can both promote this tumor, but whether they directly cause MFH needs further investigation.
Core Tip: In this paper, we report a patient who developed malignant fibrous histiocytoma 6 years after amputation, suggesting the role of wound-healing and mechanical force as local stimulation in this disease.
- Citation: Zhao KY, Yan X, Yao PF, Mei J. Malignant fibrous histiocytoma of the bone in a traumatic amputation stump: A case report and review of the literature. World J Clin Cases 2021; 9(26): 7930-7936
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7930.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7930
Malignant fibrous histiocytoma (MFH), also referred to as undifferentiated pleomorphic sarcoma, is one of the most common soft tissue sarcomas among adults and has a peak incidence between 60 and 70 years of age[1]. Most MFHs occur in extremities (49% in lower and 19% in upper extremities), followed by the trunk and retroperitoneum[2].
The relationship between MFH and local stimulation has been discussed for a long time. There are many studies attributing postsurgery MFH to the biological effect of the implants[3-6]. Keel et al[7] diagnosed four patients with MFH in their case series, all of whom had previously undergone surgery with implants. Reports of MFH occurring from 10 mo to 12 years after femoral fracture suggest that this tumor can be promoted by trauma[8,9]. In addition to implants and injury, MFH occurring at the site of chronic osteomyelitis is seen in the course of treatment[10-13]. The role of amputation was also mentioned by Inoshita et al[14] in their case report. In this paper, we present an MFH arising from the stump 6 years after amputation and discuss how local stimulation could induce MFH.
A 66-year-old man presented at the orthopedics clinic with a history of persistent pain in his amputation stump.
The patient’s pain started 3 mo ago and was not relieved spontaneously.
Six years ago, the patient underwent a traumatic amputation at his mid-thigh after a traffic accident.
None of the patient’s family members developed tumors before.
On physical examination, there was tenderness of the patient’s residual limb, and the appearance of his amputation stump was normal.
Routine laboratory tests, including routine blood examination, erythrocyte sedimen
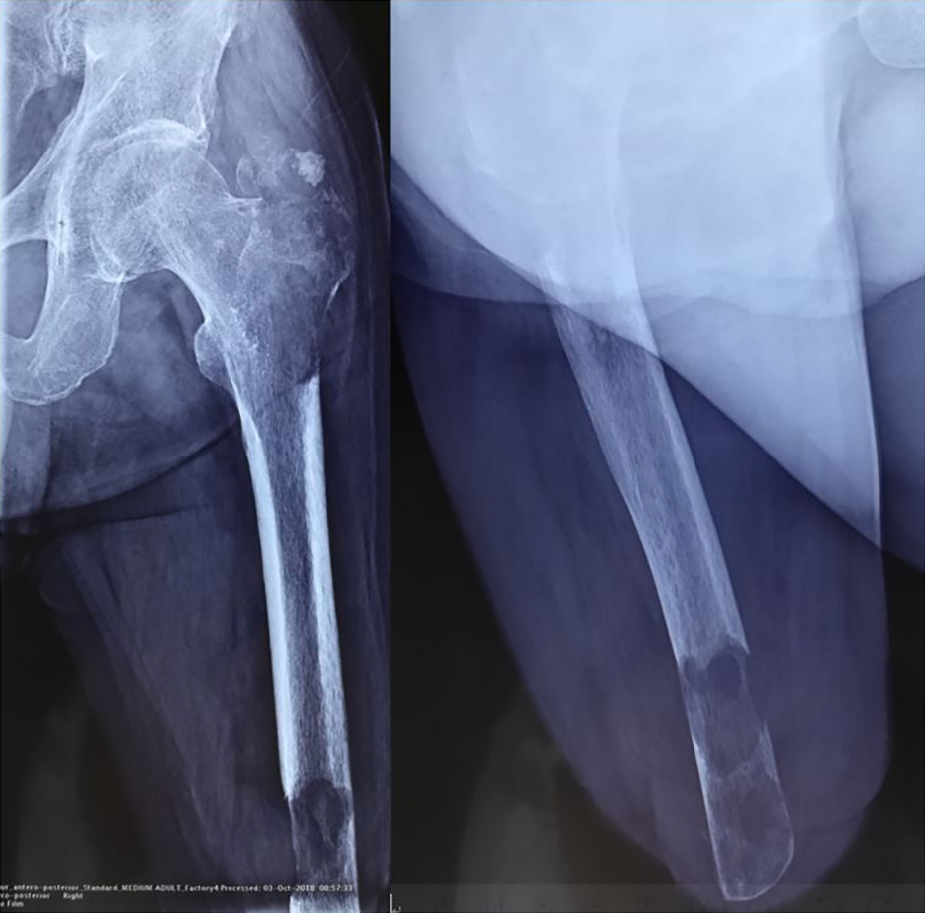
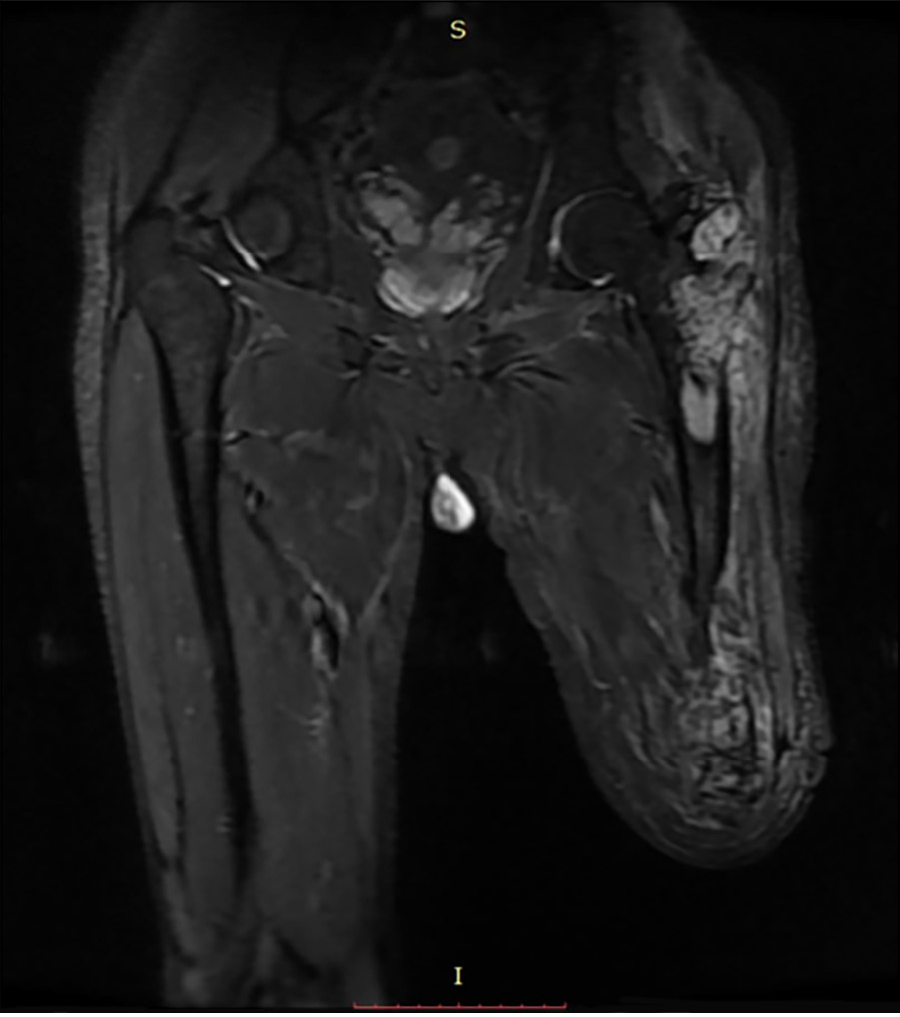
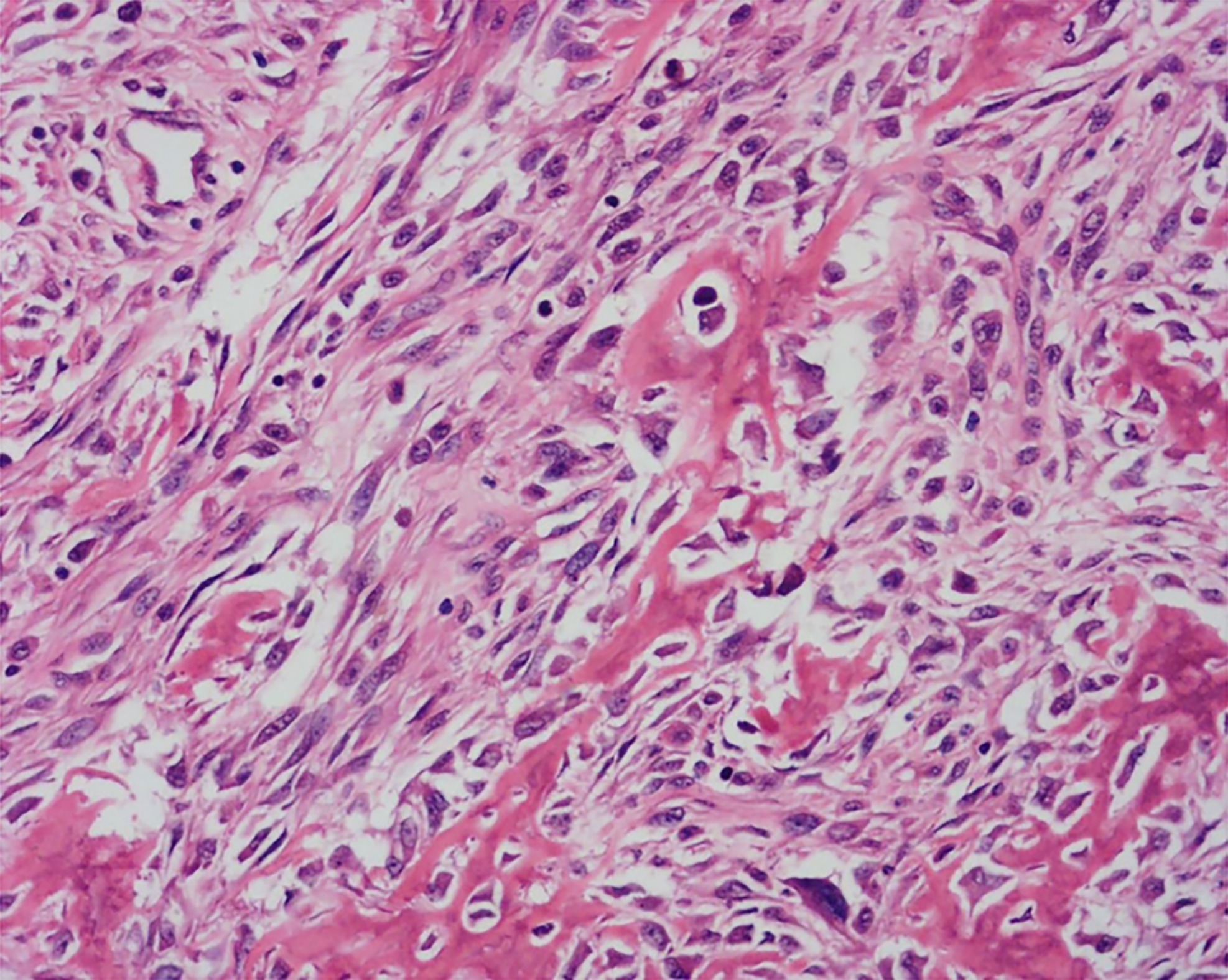
X-ray radiography showed a soft tissue mass and bone lesions in the femoral greater trochanter and residual femoral shaft without clear boundaries or periosteal reactions (Figure 1). Magnetic resonance imaging (MRI) also showed a soft tissue mass, supporting the diagnosis of a tumor (Figure 2). To obtain a definitive diagnosis, the patient underwent biopsy, which confirmed the diagnosis of MFH (Figure 3). Whole-body emission computed tomography did not reveal metastasis.
Malignant fibrous histiocytoma.
The patient underwent neoadjuvant chemotherapy and left hip disarticulation.
At the 6-mo follow-up, the patient remained asymptomatic with no recurrence of MFH.
MFH is a pleomorphic sarcoma that was originally described by Ozzello et al[15] in 1963 and O’Brien and Stout[16] in 1964. It is characterized by large size, high grade, and biological aggressiveness. Tumors involving extremities frequently present as painless masses that grow over a period of months[17].
The factors possibly related to MFH have been discussed for a long time. Some investigators reported postsurgery MFH, and most of them tended to attribute the tumor to implants rather than surgery[3-6] because the components of the implants might be carcinogenic. Keel et al[7] considered the biological effects of metal components (e.g., cobalt, chromium, stainless steel, nickel, iron, and manganese) and organic components (e.g., polymethylmethacrylate, polyethylene, silicone, and aliphatic polyurethane). Another commonly used component, titanium, though regarded as a relatively safe material, has been reported to induce genomic instability in vitro[18]. However, in this case, the patient did not receive any implants, necessi
Another theory is that it is a coincidence. MFH usually occurs in males in the femur, and patients with MFH arising in preexisting bone abnormalities were older than those with MFH in normal bone[19], and all of these features were demographic features of this patient. This patient might have developed MFH spontaneously after amputation.
However, reports of MFH after amputation are rare but exist[14], indicating the possible role of amputation in tumorigenesis. After amputation surgery, the stump undergoes a wound-healing process and is then subject to mechanical force from the prosthesis. First, during wound healing, cell proliferation is promoted by certain growth factors (e.g., epidermal growth factor, hepatocyte growth factor, vascular endothelial growth factor, insulin-like growth factor, fibroblast growth factor, and neuregulin) and signaling pathways (e.g., mTOR, Hippo, Wnt, Bmp, and Notch signaling)[20]. These growth factors and signaling pathways are closely correlated with the development of neoplasms. Additionally, hypoxia frequently occurs in mechanically challenged tissue; this induces the expression of hypoxia inducible factor-1α, upregulating several genes involved in promoting epithelial-mesenchymal transition and stem-like characteristics in tumor cells[21]. The compression force can lead to extracellular matrix stiffness, activating TGF-β[22], WNT[23], and Hippo signaling[24]. These biological processes can promote the proliferation of tumor cells.
In contrast to a number of studies on these types of carcinogenic mechanisms, only a few studies are available on molecular mechanisms involved in the tumorigenesis of MFH[25]. Idbaih et al[26] identified a high level of genomic complexity with the recurrent amplification of the 5p chromosome region, the biological significance of which is unknown. Perot et al[27] found that MFH was associated with the inactivation of the RB1 gene or frequent loss of p53 function. Matsuo et al[28] detected telomerase activity and the expression of human telomerase reverse transcriptase in tumor samples. Current studies have shown no association with signaling pathways related to wound healing and mechanical force.
The amputated femur of the patient showed two separate lesions in the greater trochanter and the residual limb. The two lesions were not connected by any intramedullary or extraosseous tissue. This is common in musculoskeletal tumors[29]. In the literature, multiple lesions in one bone are usually described as skip metastasis or synchronous multifocal tumors[29,30].
Skip metastasis can occur in osteosarcoma, Ewing sarcoma, and rarely in chondrosarcoma[29]. It presents as intramedullary lesions separated by normal marrow without any distant metastasis, such as lung metastasis. Skip metastasis usually emerges in the same bone, while those occurring in different bones are named transarticular skip metastasis. Synchronous multifocal lesions refer to more than one lesion at presentation without visceral metastasis[31,32]. Synchronous multifocal lesions have been reported in osteosarcoma, MFH, and chondrosarcoma[30]. Whether skip metastasis (or synchronous multifocal lesions) is metastasis in the traditional sense or multicentric tumorigenesis lacks strong evidence for differential diagnosis. The patient described in this paper exhibited two lesions in his greater trochanter and residual limb. The two lesions presented similar radiographic characteristics on MRI and looked similar pathologically. Therefore, we think that the lesions were more likely to be synchronous.
In this case, a 66-year-old man developed MFH 6 years after amputation. The disease course may suggest that the mechanical force from the prosthesis can promote MFH. However, based on the patient’s demographic characteristics, the tumor could also have occurred spontaneously without any correlation to the previous amputation surgery. More investigation is needed to determine whether the prosthesis or other types of local stimulation affect MFH after surgery.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sharma S S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Guo X
| 1. | Chen KH, Chou TM, Shieh S. Management of extremity malignant fibrous histiocytoma: A 10-year experience. Formosan J Surg. 2015;48:1-9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978;41:2250-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Richter H, Vinh TN, Mizel MS, Temple HT. Malignant fibrous histiocytoma associated with remote internal fixation of an ankle fracture. Foot Ankle Int. 2006;27:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Li W, Li D, Zhu X, Lu S, He C, Yang Q. Low-grade myxofibrosarcoma following a metal implantation in femur: a case report. Diagn Pathol. 2014;9:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Cole BJ, Schultz E, Smilari TF, Hajdu SI, Krauss ES. Malignant fibrous histiocytoma at the site of a total hip replacement: review of the literature and case report. Skeletal Radiol. 1997;26:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Olmedo DG, Michanié E, Oivi L, Santini-Araujo E, Cabrini RL. Malignant fibrous histiocytoma associated with coxofemoral arthrodesis. Tumori. 2007;93:504-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Keel SB, Jaffe KA, Petur Nielsen G, Rosenberg AE. Orthopaedic implant-related sarcoma: a study of twelve cases. Mod Pathol. 2001;14:969-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Bader H, Spohner F, Gerlitzky W, Meyer D. [Malignant fibrous histiocytoma after supracondylar femoral fracture (author's transl)]. Dtsch Med Wochenschr. 1981;106:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Hautamaa PV, Gaither DW, Thompson RC Jr. Malignant fibrous histiocytoma arising in the region of a femoral fracture. A case report. J Bone Joint Surg Am. 1992;74:777-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Zlowodzki M, Allen B, Schreibman KL, Vance RB, Kregor PJ. CASE REPORTS: malignant fibrous histiocytoma of bone arising in chronic osteomyelitis. Clin Orthop Relat Res. 2005;439:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Chow LT, Wong SK. Primary osseous inflammatory malignant fibrous histiocytoma masquerading as chronic osteomyelitis. Orthopedics. 2014;37:e940-e945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Czerwiński E, Skolarczyk A, Frasik W. Malignant fibrous histiocytoma in the course of chronic osteomyelitis. Arch Orthop Trauma Surg. 1991;111:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Heliö H, Kivioja A, Karaharju EO, Elomaa I, Knuutila S. Malignant fibrous histiocytoma arising in a previous site of fracture and osteomyelitis. Eur J Surg Oncol. 1993;19:479-484. [PubMed] |
| 14. | Inoshita T, Youngberg GA. Malignant fibrous histiocytoma arising in previous surgical sites. Report of two cases. Cancer. 1984;53:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Ozzello L, Stout Ap, Murray MR. Cultural characteristics of malignant histiocytomas and fibrous xanthomas. Cancer. 1963;16:331-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | O'Brien JE, Stout AP. Malignant fibrous xanthomas. Cancer. 1964;17:1445-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Murphey MD, Gross TM, Rosenthal HG. From the archives of the AFIP. Musculoskeletal malignant fibrous histiocytoma: radiologic-pathologic correlation. Radiographics. 1994;14:807-26; quiz 827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 78] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Coen N, Kadhim MA, Wright EG, Case CP, Mothersill CE. Particulate debris from a titanium metal prosthesis induces genomic instability in primary human fibroblast cells. Br J Cancer. 2003;88:548-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Capanna R, Bertoni F, Bacchini P, Bacci G, Guerra A, Campanacci M. Malignant fibrous histiocytoma of bone. The experience at the Rizzoli Institute: report of 90 cases. Cancer. 1984;54:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Ricci L, Srivastava M. Wound-induced cell proliferation during animal regeneration. Wiley Interdiscip Rev Dev Biol. 2018;7:e321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Northey JJ, Przybyla L, Weaver VM. Tissue Force Programs Cell Fate and Tumor Aggression. Cancer Discov. 2017;7:1224-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 22. | Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell. 2012;23:781-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 23. | Fernández-Sánchez ME, Barbier S, Whitehead J, Béalle G, Michel A, Latorre-Ossa H, Rey C, Fouassier L, Claperon A, Brullé L, Girard E, Servant N, Rio-Frio T, Marie H, Lesieur S, Housset C, Gennisson JL, Tanter M, Ménager C, Fre S, Robine S, Farge E. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature. 2015;523:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 24. | Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4707] [Cited by in RCA: 4190] [Article Influence: 299.3] [Reference Citation Analysis (0)] |
| 25. | Widemann BC, Italiano A. Biology and Management of Undifferentiated Pleomorphic Sarcoma, Myxofibrosarcoma, and Malignant Peripheral Nerve Sheath Tumors: State of the Art and Perspectives. J Clin Oncol. 2018;36:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 26. | Idbaih A, Coindre JM, Derré J, Mariani O, Terrier P, Ranchère D, Mairal A, Aurias A. Myxoid malignant fibrous histiocytoma and pleomorphic liposarcoma share very similar genomic imbalances. Lab Invest. 2005;85:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Pérot G, Chibon F, Montero A, Lagarde P, de Thé H, Terrier P, Guillou L, Ranchère D, Coindre JM, Aurias A. Constant p53 pathway inactivation in a large series of soft tissue sarcomas with complex genetics. Am J Pathol. 2010;177:2080-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Matsuo T, Shay JW, Wright WE, Hiyama E, Shimose S, Kubo T, Sugita T, Yasunaga Y, Ochi M. Telomere-maintenance mechanisms in soft-tissue malignant fibrous histiocytomas. J Bone Joint Surg Am. 2009;91:928-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Saifuddin A, Sharif B, Oliveira I, Kalus S, Barnett J, Pressney I. The incidence of skip metastases on whole bone MRI in high-grade bone sarcomas. Skeletal Radiol. 2020;49:945-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Walter H, Schneider-Stock R, Mellin W, Günther T, Nebelung W, Roessner A. Synchronous multifocal bone sarcomas--a case report and molecular pathologic investigation. Gen Diagn Pathol. 1995;141:67-74. [PubMed] |
| 31. | Maheshwari AV, Jelinek JS, Seibel NL, Meloni-Ehrig AM, Kumar D, Henshaw RM. Bilateral synchronous tibial periosteal osteosarcoma with familial incidence. Skeletal Radiol. 2012;41:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Araki Y, Hayashi K, Yamamoto N, Takeuchi A, Miwa S, Igarashi K, Higuchi T, Abe K, Taniguchi Y, Yonezawa H, Morinaga S, Asano Y, Nojima T, Tsuchiya H. Reconstruction using a frozen autograft for a skull and humeral lesion of synchronous multicentric osteosarcoma after undergoing successful neoadjuvant chemotherapy: a case report and review of the literature. BMC Surg. 2021;21:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |