Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7923
Peer-review started: April 22, 2021
First decision: May 24, 2021
Revised: May 27, 2021
Accepted: July 19, 2021
Article in press: July 19, 2021
Published online: September 16, 2021
Processing time: 140 Days and 22.6 Hours
Inborn error of bile acid synthesis type 4 is a peroxisomal disease with impaired bile acid synthesis caused by a-methylacyl-CoA racemase (AMACR) gene mutation. The disease is usually found in children with mild to severe liver disease, cholestasis and poor fat-soluble vitamin absorption. At present, there is no report of inborn errors of bile acid synthesis type 4 in adults with liver disease and poor fat-soluble vitamin absorption.
A 71-year-old man was hospitalized in our department for recurrent liver dysfunction. The clinical manifestations were chronic liver disease and yellow skin and sclera. Serum transaminase, bilirubin and bile acid were abnormally increased; and fat-soluble vitamins decreased. Liver cirrhosis and ascites were diagnosed by computed tomography. The patient had poor coagulation function and ascites and did not undergo liver puncture. Genetic testing showed AMACR gene missense mutation. The patient was diagnosed with inborn error of bile acid synthesis type 4. He was treated with ursodeoxycholic acid, liver protection and vitamin supplementation, and jaundice of the skin and sclera was reduced. The indicators of liver function and the quality of life were significantly improved.
When adults have recurrent liver function abnormalities, physicians should be alert to genetic diseases and provide timely treatment.
Core Tip: Inborn error of bile acid synthesis type 4 is a peroxisomal disease with impaired bile acid synthesis caused by a-methylacyl-CoA racemase gene mutation. This is the first report of an adult patient with liver disease and fat-soluble vitamin deficiency. The patient had significantly improved prognosis after treatment. In adult patients with recurrent liver function abnormalities, physicians should be alert to the possibility of genetic disorders, which can be diagnosed by genetic testing or, if possible, combined with mass spectrometry.
- Citation: Wang SH, Hui TC, Zhou ZW, Xu CA, Wu WH, Wu QQ, Zheng W, Yin QQ, Pan HY. Diagnosis and treatment of an inborn error of bile acid synthesis type 4: A case report. World J Clin Cases 2021; 9(26): 7923-7929
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7923.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7923
Inborn error of bile acid synthesis is a rare genetic disease characterized by neonatal cholestasis, neurological disorders or fat-soluble vitamin deficiency. At present, an increasing number of inborn errors of bile acid synthesis have been reported, which constitutes an expanding category of rare inherited diseases. Most of these diseases have autosomal recessive inheritance, which has led to 1%–2% of neonatal cholestasis[1]. The congenital defects in bile acid synthesis and biotransformation are divided into three categories according to defects in the modification of the sterol nucleus, defects in the modification of the side chains, and defects in bile acid amidation. A-methylacyl-CoA racemase (AMACR) belongs to side-chain modification[2].
These diseases are characterized by failure to produce normal bile acids and abnormal accumulation of bile acids and bile acid intermediates. Patients with inborn errors of bile acid synthesis usually show normal or low serum bile acid concentration, normal γ-glutamyl transpeptidase concentration and no pruritus. If the disease is not diagnosed in time, it may lead to progressive chronic liver disease or liver failure[3].
In this case, the patient’s disease type was inborn error of bile acid synthesis type 4, with missense mutation in the AMACR gene; commonly known as AMACR deficiency. The clinical features of this disease are neonatal cholestasis with severe liposoluble vitamin deficiency or adult paroxysmal peripheral neuropathy[4]. How
We here present a 71-year-old patient at the time of diagnosis, who is the oldest patient with untreated AMACR deficiency described to date.
A 71-year-old man presented in the liver disease department of our hospital with abdominal distension without obvious inducement and no other discomfort.
The patient had recurrent bloating symptoms started a month previously, which worsened the week before admission.
The patient underwent physical examinations and liver function test at a local hospital two years ago. The patient received liver protection treatment in the local hospital, but his liver function did not improve and he had yellow urine occasionally, but had no obvious physical discomfort.
The patient’s personal and family history was unremarkable.
At admission, his body temperature was 36.8 °C, heart rate was 68 bmp, breathing rate was 18 breaths per minute, blood pressure was 134/74 mmHg, and blood oxygen saturation was 99%. The patient presented with chronic liver disease, moderately yellowish skin and sclera, but no liver palms and spider angioma. The patient's abdomen was soft, without obvious tenderness and rebound pain. Moreover, the patient's liver area was negative for percussion pain, the liver and spleen were not touched under the ribs, and Murphy's sign was negative. No abnormalities were found on nervous system examination.
Liver function showed albumin 23.9 g/L (normal 40-55 g/L) alanine aminotransferase 135 U/L (normal < 50 U/L), aspartate aminotransferase 127 U/L (normal < 40 U/L), γ-glutamine acyltranspeptidase 131 U/L (normal < 60 U/L), total bilirubin 49.3 μmol/L (normal < 24 μmol/L), and direct bilirubin 17.3 μmol/L (normal < 6.8 μmol/L). The blood test showed hemoglobin 116 g/L (normal 160-175 g/L), and platelet count 75 × 109/L (normal 125-350 × 109/L). The coagulation function test showed that the prothrombin time was 16.4 s (normal 9.8-13.2 s), and the international normalized ratio was 1.46 s (normal 0.85-1.20 s) (Table 1). The erythrocyte sedimentation rate, neutrophil count, and hypersensitivity C-reactive protein were normal. Stool and urine routine tests were also normal.
| Parameters | Day 1 | Day 7 | Day 15 | 1 mo | 2 mo | 3 mo | Reference range |
| ALB (g/L) | 28.2 | 23.9 | 27.6 | 31.3 | 27.5 | 27.8 | 40.0-55.0 |
| ALT (U/L) | 212 | 135 | 35 | 183 | 93 | 43 | 9-50 |
| AST (U/L) | 191 | 127 | 43 | 181 | 121 | 118 | 15-40 |
| GGT (U/L) | 152 | 131 | 75 | 55 | 58 | 62 | 10-60 |
| ALP (U/L) | 115 | 105 | 93 | 108 | 125 | 147 | 42-125 |
| TBIL (μmol/L) | 60.5 | 49.3 | 49.1 | 74.1 | 44.9 | 43.5 | 3.4-24.0 |
| DBIL (μmol/L) | 21.7 | 17.3 | 21.9 | 31.0 | 16.5 | 16.9 | 0.0-6.8 |
| PT (s) | 16.4 | 15.3 | 16.6 | 15.9 | 9.8-13.2 | ||
| INR | 1.46 | 1.36 | 1.48 | 1.42 | 0.85-1.20 | ||
| PLT (× 109/L) | 75 | 72 | 65 | 84 | 125-350 |
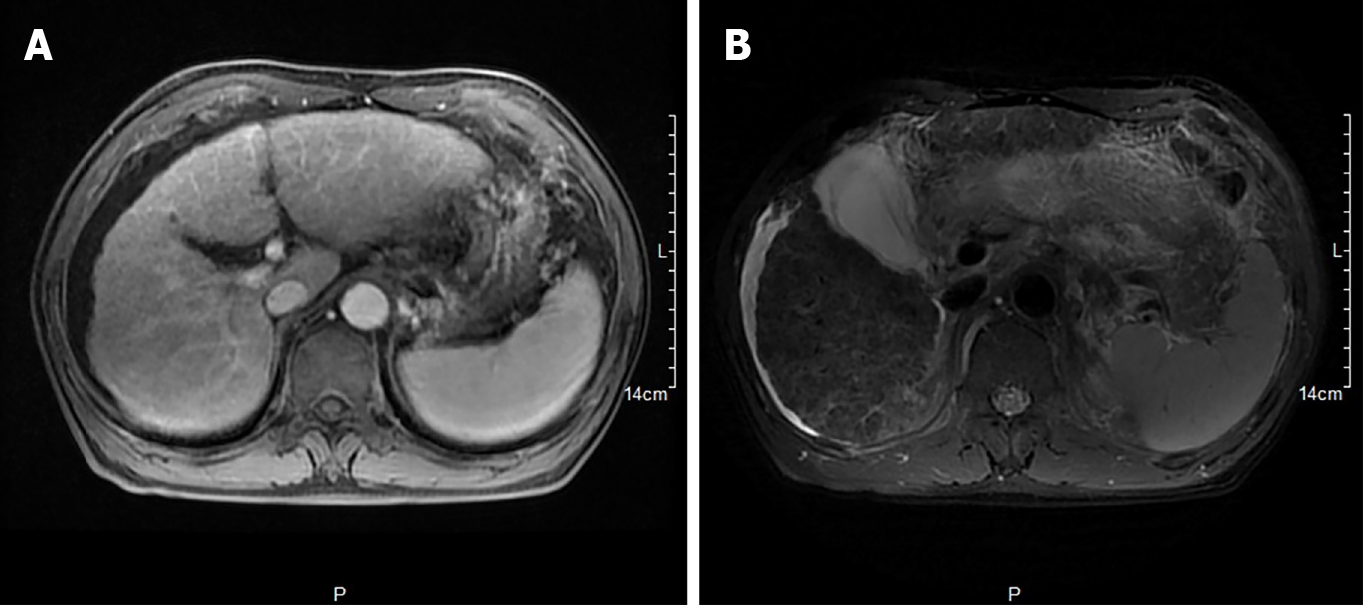
Hepatobiliary enhanced magnetic resonance imaging showed that the liver contour was unsmooth, the liver proportion was imbalanced, the left lobe of the liver was enlarged, and the right lobe of the liver was reduced. The liver parenchymal signal was not uniform, and multiple diffuse nodules could be seen. T1-weighted imaging showed isointensity, T2-weighted imaging showed low signal, OutPhase signal was not lower than InPhase, and there were no obvious abnormalities in the liver parenchyma after enhancement. The internal diameter of the main portal vein was within the normal range, and the intrahepatic bile duct was not significantly dilated. The volume of the spleen increased, and there was no obvious abnormality in the signal. The examination revealed liver cirrhosis, splenomegaly, and ascites (Figure 1).
Abnormal liver functions are associated with viral infection, alcohol consumption, drug use, autoimmunity, and genetic diseases. In the present case, coxsackie virus antibody, Epstein-Barr virus antibody, hepatitis B surface antigen, and cytomegalovirus-DNA were examined and results were all negative, thus viral factors could be ruled out. In addition, as the patient had no history of drinking and inappropriate drug use, alcoholic and drug-related factors were also ruled out. The patient's antinuclear antibody and anti-neutrophil cytoplasmic antibodies test were negative, which excluded autoimmune factors. Therefore, we speculated that the patient may have a genetic disease that caused abnormal liver function, so genetic test was carried out for genetic mutations.
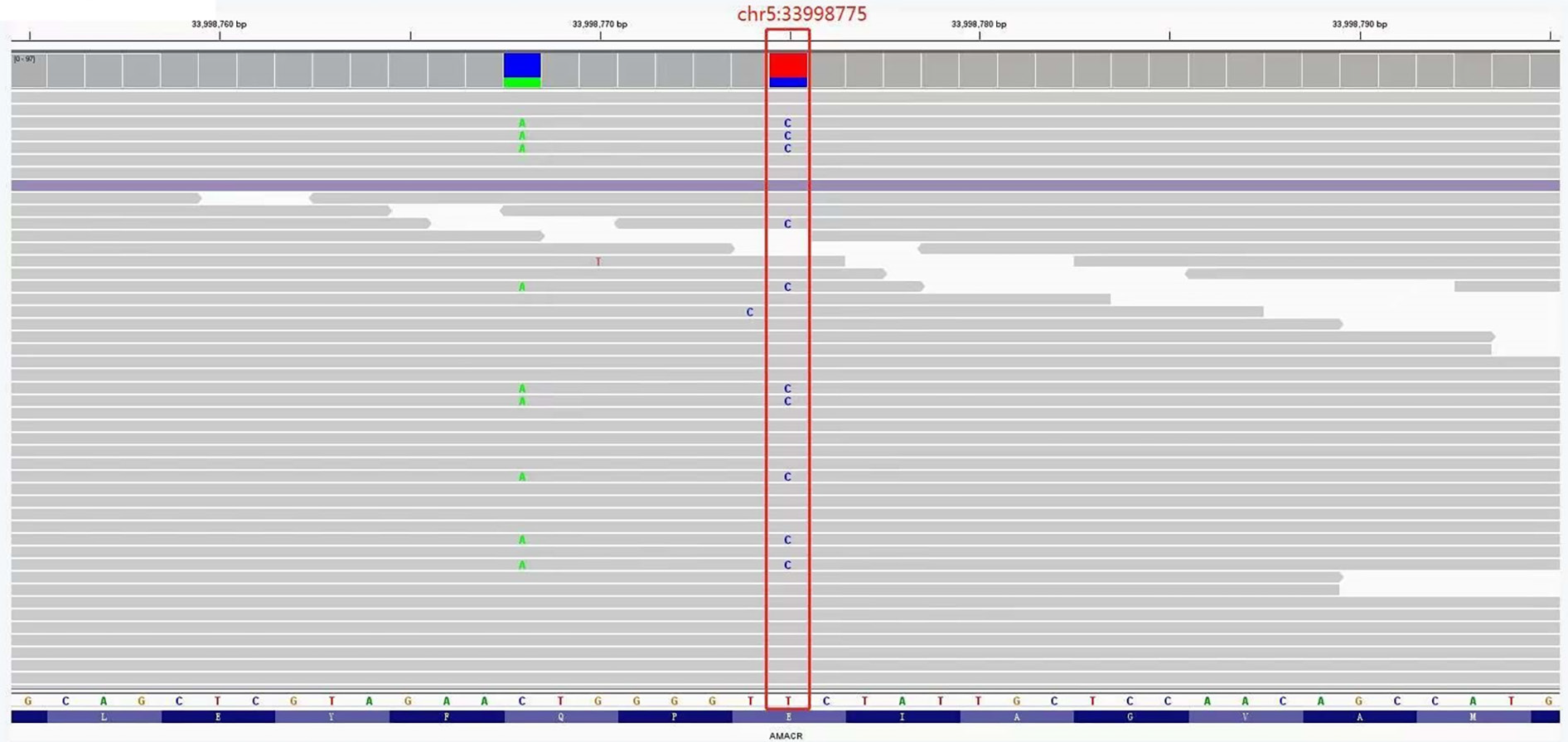
The detection range included 20000 gene combinations such as AARS, ANO5 and CAPN3, for genetic diseases. Exons and their adjacent ± 10 bp introns were tested and analyzed by high-throughput sequencing. Regarding genes related to the digestive system, a heterozygous missense mutation (c.710A>G) in the 33998775 position of exon 5 of the AMACR gene was found (Figure 2), which occurred in the protein CoA-transferase family Ⅲ domain. According to the National Center for Biotechnology Information and Online Mendelian Inheritance in Man, the frequency of this site in the normal East Asian population is 0.0001. SIFT and Polyphen-2 software was used to predict its protein function, and the results were all positive.
The final diagnosis was abnormal liver function caused by inborn error of bile acid synthesis type 4.
He was given liver-protecting treatments such as intravenous infusion of compound glycyrrhizin 80 mL qd, adenosylmethionine 1000 mg qd, and glutathione 1.2 g qd. As the patient had hypoalbuminemia, 10 g qd human albumin was injected intravenously. Spironolactone tablets were also administered orally at 20 mg qd. After the diagnosis, the patient started oral ursodeoxycholic acid 0.25 g tid. Subsequently, the fat-soluble vitamin was tested, and the result showed that vitamin A was 83.10 ng/mL (normally 300-800 ng/mL), so vitamin A capsules of 25000 units bid were supplemented. After 2 wk of continuous use of liver protection and diuretics, the patient's liver function improved significantly, the abdominal distension was markedly relieved, and the skin and sclera were slightly yellowish, the patient was then discharged from hospital.
The patient was advised to take ursodeoxycholic acid 0.25 g tid, bicyclol 25 mg tid, spironolactone tablets 20 mg qd, peptides 80 mg tid and vitamin A capsules of 25000 units bid.
The patient had good compliance with medication and regular check-ups every month. During the follow-up, the patient had no obvious symptoms such as abdominal distension, and the liver function indicators basically returned to normal. It was recommended that the patient should continue treatment and medication and regular inspections.
The clinical phenotype caused by AMACR deficiency is known as inborn error of bile acid synthesis type 4. AMACR deficiency is an autosomal recessive defect that affects or even hinders bile acid and fatty acid synthesis by inhibiting oxidative AMACR deficiency of cholesterol side chain[5,6]. AMACR catalyzes conversion of (25R) trihydroxy-cholestanoic acid (THCA) to its 25S isomer; a step required for subsequent oxidation of peroxisomal β-oxidate to primary bile acids[2].
In 2000, Ferdinandusse et al[6] reported three adult patients with progressive sensory neuropathy, but presenting without fat-soluble vitamin malabsorption and liver disease. AMACR gene detection in these three patients revealed gene mutations, and fibroblast culture confirmed the damage to the synthetic pathway. In 2003, Setchell et al[7] reported a case of AMACR deficiency in a child with fat-soluble-vitamin deficiency, coagulopathy and mild cholestatic liver disease in the neonatal period. Analysis of the patient’s blood and urine showed significant elevation of 25R-THCA. Genetic testing confirmed a mutation in the AMACR gene, and fibroblast studies also confirmed AMACR deficiency[8].
Among the currently reported patients with AMACR deficiency, there are not only adults with delayed peripheral neuropathy, but also infants with cholestasis with absorption of fat-soluble vitamins. However, the case we report here is an adult with cholestasis but without any neurological manifestations. The patient had been treated in a local hospital, but the cause of disease was unknown repeated liver function examinations found that serum transaminase and total bilirubin were elevated, and conjugated bilirubin was the main component, and γ-glutamyl transpeptidase and total bile acid were also elevated. Because the patient had contraindications for liver puncture, histological examination could not be performed. It was impossible to clarify the specific cause based on the clinical characteristics of the patient alone. After genetic testing, a heterozygous missense mutation (c.710A>G) in the AMACR gene was found, which provided an evidence for the diagnosis. It was reported that the clinical manifestations of this disease are cholestatic liver disease (usually present in infancy) and progressive nervous system disease (present in late childhood or adulthood). Infantile cholestatic disease is characterized by concomitant hyperbilirubinemia with elevated transaminases, but normal g-glutamyl transpeptidase, and biopsy demonstrates giant cell hepatitis. Neurological manifestations usually include signs of upper motor neuron damage (spastic paralysis)[4,8]. Clinically, if there are neonatal or adult cholestasis and chronic liver disease similar to the above disease characteristics, a high degree of clinical suspicion is required when making a diagnosis. The diagnosis of inborn error of bile acid synthesis requires comprehensive clinical symptoms, laboratory examinations, auxiliary examinations and pathological biopsy. The current diagnosis mainly relies on genetic testing and urine mass spectrometry. For inborn error of bile acid synthesis, it is safe and effective to supplement primary bile acids such as ursodeoxycholic acid as soon as possible[9]. Oral supplementation of fat-soluble vitamins such as K1, E, A, and D is needed. Oral administration of primary bile acids has two purposes: One is to provide the human body with primary bile acids, and the other is to down-regulate the synthesis of abnormal bile acids through negative feedback, thereby reducing the production of abnormaltoxic intermediate products of defective liver cells[2]. Therefore, the use of primary bile acid replacement therapy for this disease can improve liver function and avoid further liver damage[10,11]. Liver transplantation is the only option for patients whose condition cannot be controlled by medication. The patient's liver function improved progressively after oral administration of ursodeoxycholic acid. But the long-term prognosis needs to be followed up. This case shows that patients with inborn error of bile acid synthesis type 4 can survive the neonatal period, childhood, and adulthood without bile acid therapy. It is still not clear why some patients die in the early stages of cholestasis disease while this patient can live for decades without treatment. At the same time, pediatricians and hepatologists need to improve their understanding of the diagnosis and treatment of congenital bile acid deficiency for early diagnosis and treatment.
This report systematically analyzed the clinical symptoms, signs, and laboratory features of an adult patient with inborn error of bile acid synthesis type 4. The patient was diagnosed by mutations of the AMACR gene. Cholic acid treatment has achieved good results. This study for the first time indicated the inherent relationship between age and clinical manifestations, and the clinical features of AMACR gene mutations in this disease.
We greatly appreciate the patient, his family and the medical staff involved in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali FE, Costache RS S-Editor: Fan JR L-Editor: A P-Editor: Guo X
| 1. | Bove KE, Heubi JE, Balistreri WF, Setchell KD. Bile acid synthetic defects and liver disease: a comprehensive review. Pediatr Dev Pathol. 2004;7:315-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Monte MJ, Marin JJ, Antelo A, Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol. 2009;15:804-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 364] [Cited by in RCA: 404] [Article Influence: 25.3] [Reference Citation Analysis (9)] |
| 3. | Ferdinandusse S, Denis S, IJlst L, Dacremont G, Waterham HR, Wanders RJ. Subcellular localization and physiological role of alpha-methylacyl-CoA racemase. J Lipid Res. 2000;41:1890-1896. [PubMed] |
| 4. | Vaz FM, Ferdinandusse S. Bile acid analysis in human disorders of bile acid biosynthesis. Mol Aspects Med. 2017;56:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Watkins PA, McGuinness MC, Raymond GV, Hicks BA, Sisk JM, Moser AB, Moser HW. Distinction between peroxisomal bifunctional enzyme and acyl-CoA oxidase deficiencies. Ann Neurol. 1995;38:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Ferdinandusse S, Denis S, Clayton PT, Graham A, Rees JE, Allen JT, McLean BN, Brown AY, Vreken P, Waterham HR, Wanders RJ. Mutations in the gene encoding peroxisomal alpha-methylacyl-CoA racemase cause adult-onset sensory motor neuropathy. Nat Genet. 2000;24:188-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Setchell KD, Heubi JE, Bove KE, O'Connell NC, Brewsaugh T, Steinberg SJ, Moser A, Squires RH Jr. Liver disease caused by failure to racemize trihydroxycholestanoic acid: gene mutation and effect of bile acid therapy. Gastroenterology. 2003;124:217-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Van Veldhoven PP, Meyhi E, Squires RH, Fransen M, Fournier B, Brys V, Bennett MJ, Mannaerts GP. Fibroblast studies documenting a case of peroxisomal 2-methylacyl-CoA racemase deficiency: possible link between racemase deficiency and malabsorption and vitamin K deficiency. Eur J Clin Invest. 2001;31:714-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Clayton PT. Disorders of bile acid synthesis. J Inherit Metab Dis. 2011;34:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Gonzales E, Gerhardt MF, Fabre M, Setchell KD, Davit-Spraul A, Vincent I, Heubi JE, Bernard O, Jacquemin E. Oral cholic acid for hereditary defects of primary bile acid synthesis: a safe and effective long-term therapy. Gastroenterology. 2009;137:1310-1320.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Sundaram SS, Bove KE, Lovell MA, Sokol RJ. Mechanisms of disease: Inborn errors of bile acid synthesis. Nat Clin Pract Gastroenterol Hepatol. 2008;5:456-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |