Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7901
Peer-review started: April 10, 2021
First decision: May 11, 2021
Revised: May 24, 2021
Accepted: July 22, 2021
Article in press: July 22, 2021
Published online: September 16, 2021
Processing time: 152 Days and 14.2 Hours
Colorectal mucinous adenocarcinoma is a distinct subtype of colorectal adenocarcinoma that is not sensitive to chemotherapy and radiotherapy, and its prognosis is worse than that of nonmucinous adenocarcinoma. Early diagnosis and aggressive surgical treatment may be the key to improving the prognosis of patients. Ascending colon mucinous adenocarcinoma with the primary manifestation of a local abscess caused by non-intestinal perforation has never been reported. Moreover, since the lumen of the ascending colon is large, and early stage ascending colon cancer lacks typical clinical manifestations, the diagnosis may be delayed easily. We herein report three cases of delayed diagnosis of colorectal mucinous adenocarcinoma.
We present three patients (two females and one male) with mucinous ascending colon mucinous adenocarcinoma with the primary manifestation of a local abscess (the right area of the lumbar spine, right groin, and lower right abdomen) caused by non-intestinal perforation. At the initial clinical visit, the common causes of those abscesses, including spinal tuberculosis and urinary tract infection, were excluded. The treatment of the abscess was through an incision and drainage. However, the source of the abscess was not made clear, which led to an abscess recurrence and a delayed diagnosis of colorectal mucinous adenocarcinoma. After the patients were referred to our hospital, a definitive diagnosis of ascending colon mucinous adenocarcinoma was made with the help of tumor markers and colonoscopic findings. Because of the delayed diagnosis of the disease, two patients (case 1 and case 2) missed the chance of surgery due to disease progression and died in a short follow-up period. Only case 3 underwent radical surgery for the tumor in the right colon and partial abdominal wall resection and achieved a better prognosis.
Abscesses in the right area of the lumbar spine, right groin, or right lower quadrant caused by non-intestinal perforation as the primary clinical manifes
Core Tip: This paper presents three cases of delayed diagnosis of mucinous adenocarcinoma in the ascending colon with local abscess (caused by non-intestinal perforation) as the primary manifestation and emphasizes the importance of colonoscopy in the early diagnosis and surgical treatment of this malignancy.
- Citation: Han SZ, Wang R, Wen KM. Delayed diagnosis of ascending colon mucinous adenocarcinoma with local abscess as primary manifestation: Report of three cases. World J Clin Cases 2021; 9(26): 7901-7908
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7901.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7901
Colorectal cancer (CRC) is a malignant tumor with a high incidence and mortality rate. According to “Global cancer statistics in 2018”, CRC ranks as the third most common cancer and the second most frequent cause of cancer-related death in both sexes combined[1]. The most common histologic subtype of CRC is adenocarcinoma, of which colorectal mucinous adenocarcinoma is a distinct subtype and is characterized by abundant mucinous components that comprise at least 50% of the tumor volume. Statistics indicate that 10%–20% of CRC patients are of the mucinous subtype[2]. Currently, patients with colorectal mucinous adenocarcinoma receive treatments based on the same standard guidelines as those for CRC, which means that the main treatments are surgery, chemotherapy, radiotherapy, and molecular targeted therapy. However, the related literature has shown that colorectal mucinous adenocarcinoma is not sensitive to radiotherapy and chemotherapy, and the prognosis of mucinous adenocarcinoma is worse than that of nonmucinous adenocarcinoma[3,4]. The mechanism may be related to distinct molecular biological characteristics of colorectal mucinous adenocarcinoma and the presence of a large amount of mucus. Therefore, early diagnosis and aggressive surgical treatment may be the key to improving the prognosis of patients with colorectal mucinous adenocarcinoma. Colorectal mucinous adenocarcinoma with a local abscess as the primary manifestation is extremely rare. We herein present three patients with ascending colon mucinous adenocarcinoma with a local abscess as the primary manifestation.
Case 1: A 61-year-old woman had right waist swelling and pain for 1 year.
Case 2: A 75-year-old man had right groin swelling and pain for 6 mo.
Case 3: A 73-year-old woman had a mass in the right lower quadrant for 2 mo.
Case 1: One year ago, this patient presented swelling and pain without any causative factors in the right area of the lumbar spine. She went to a primary hospital and underwent abdominal computed tomography (CT) and spinal radiology. CT scanning did not find new organisms in the colon, but its reliability cannot be traced back. A local abscess of the right waist was suspected. Common causes of abscess formation, including spinal tuberculosis, urinary tract infection, and periappendiceal abscess, were excluded, and the cause of the abscess formation was unknown. Treatment of the abscess was through an incision and drainage under local anesthesia, and local symptoms improved after draining the abscess and taking antibiotics. However, the abscess recurred after she discharged from the hospital. The patient was treated many times for an abscess at a local hospital. Due to the poverty and economic backward
Case 2: Six months ago, the patient went to a local hospital because of right groin swelling and pain. An abscess in the right inguinal region was suspected after conducting abdominal CT. Then, the treatment of the abscess was through an incision and drainage under local anesthesia, and an indwelling drainage tube remained after the procedure. The drainage tube was removed 2 wk later due to the reduction in the drainage. However, after removing the drainage tube, the local mass formed again, and a small amount of viscous liquid seeped from the drainage tube site 1 wk later. The dressing was changed many times in the hospital over a long period. After 5 mo, the patient was admitted to our hospital for further treatment.
Case 3: Two months ago, a mass appeared in the right lower quadrant without any causative factors. Abdominal CT and ultrasound revealed an abscess near the appendix. Therefore, treatment of the periappendiceal abscess was performed through an incision and drainage, which was performed at the initial clinical visit; the local symptoms of the patient improved, and the patient was discharged. However, the mass appeared again in the right lower quadrant after 3 d. The size of the mass was approximately that of soybean at first; however, the mass had been increasing in size over time. The lower right abdomen was accompanied by persistent pain.
All patients did not have a noteworthy previous medical history, including pulmonary tuberculosis and urolithiasis.
All patients did not have a noteworthy previous medical history, including gastrointestinal cancer. Only the male patient (case 2) occasionally smoked and consumed alcohol.
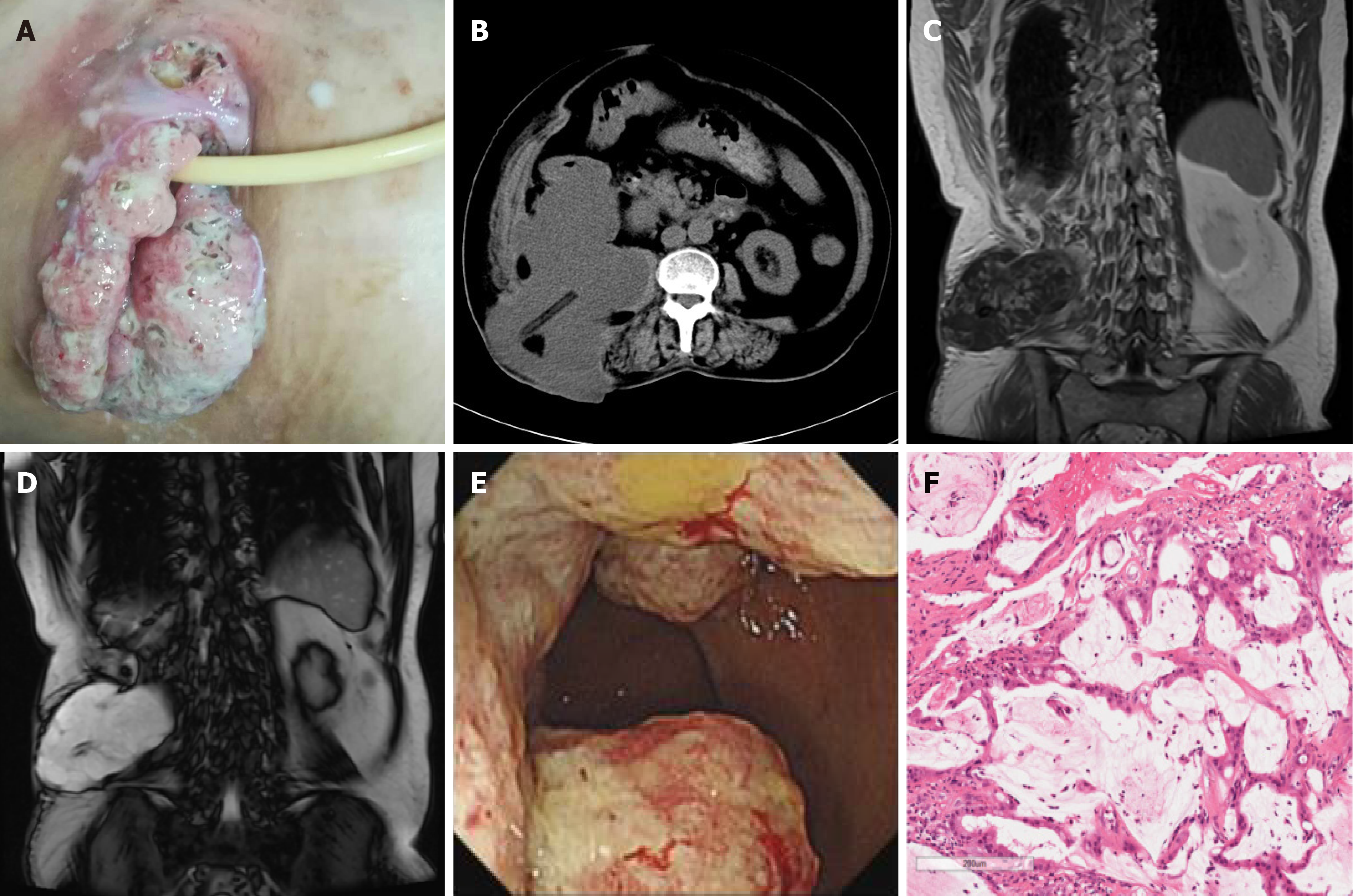
Case 1: Vital signs, which included body temperature, respiration, heart rate, and blood pressure, were within their normal ranges. The patient was anemic. There was an incision approximately 6 cm in length on the right side of the waist, and the incision split open, with pus overflowing. There was one drainage tube that was draining purulent liquid (Figure 1).
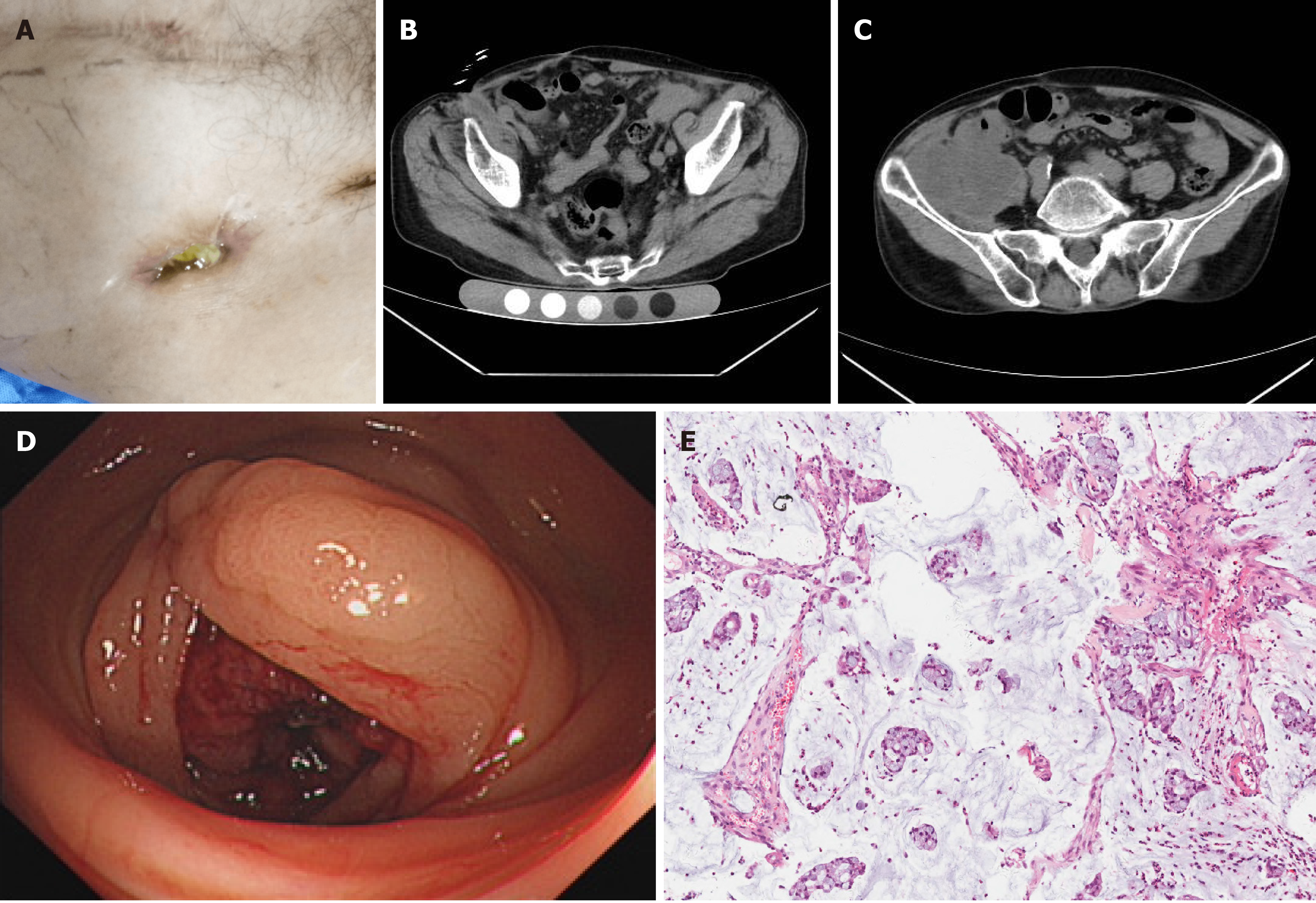
Case 2: Vital signs, including body temperature, respiration, heart rate, and blood pressure, were within their normal ranges. There was a scar approximately 5 cm in length in the lower abdomen and a sinus approximately 2 cm deep in the right inguinal region, with mucous flowing out (Figure 2A).
Case 3: Vital signs, including body temperature, respiration, heart rate, and blood pressure, were within their normal ranges. There was a mass approximately 10 cm × 5 cm in the right lower abdomen, and a part of the mass protruded on the surface of the skin; observations of this area were dark red skin, elevated skin temperature, fluctuations, tenderness, and an unclear boundary.
Case 1: Laboratory data showed the following: Red blood cell count, 2.56 × 1012 /L; hemoglobin, 60 g/L; white blood cell (WBC) count, 5.92 × 109 /L; erythrocyte sedimentation rate (ESR), 13.38 mm/h; C-reactive protein (CRP), 10.1 mg/L. Carcinoembryonic antigen (CEA) was elevated to 8.47 µg/L, and carbohydrate antigen 199 (CA199) was elevated to 98.80 U/mL. Wound pus culture suggested the growth of multiple Gram-negative bacilli. Other laboratory examination results were in their respective normal ranges.
Case 2: Laboratory data showed the following: WBC count, 4.20 × 109 /L; ESR, 2.0 mm/h; and CRP, 4.20 mg/L. CEA was elevated to 5.42 µg/L, and CA199 was 16.30 U/mL. Wound pus culture suggested the growth of Enterococcus faecium. Other laboratory examination results were in their respective normal ranges.
Case 3: Laboratory data showed the following: WBC count, 9.56 × 109 /L; ESR, 5.2 mm/h; and CRP, 9.80 mg/L. CEA was elevated to 21.87 µg/L, and CA199 was elevated to 134.80 U/mL. Wound pus culture suggested the growth of Enterococcus faecium and Aerobacter. Other laboratory examination results were in their respective normal ranges. Table 1 presents the results of partial laboratory examinations.
| Case 1 | Case 2 | Case 3 | Reference range | |
| WBC (109 /L) | 5.92 | 4.20 | 9.56 | 3.5-9.5 |
| RBC (1012 /L) | 2.56 | 4.22 | 5.20 | 3.8-5.1 |
| HGB (g/L) | 60.00 | 122.38 | 126.25 | Female: 115-150; Male: 125-175 |
| ESR (mm/h) | 13.38 | 2.00 | 10.22 | < 43 |
| CRP (mg/L) | 10.01 | 4.20 | 9.80 | 0.068-8.2 |
| CEA (µg/L) | 8.47 | 5.42 | 21.87 | no smoking: < 3.0smoking: < 5.0 |
| CA199 (U/mL) | 98.80 | 16.30 | 134.80 | < 35 |
Case 1: An abdominal CT scan revealed a 180 mm × 11 mm irregular mass below the right kidney, and the boundary between the psoas muscle and the ascending colon was unclear. The drainage tube was visible in the center. The soft tissue of the right iliac fossa was thickened, and the kidney was significantly displaced forward (Figure 1B). Magnetic resonance imaging (MRI) of the abdomen showed an irregular mass with a long T1 and long T2 signal along with the right posterior abdominal paravertebral, right posterior renal, and lower right lumbar regions that crossed the abdominal cavity, and the posterior margin reached the subcutaneous tissue (Figure 1C and D). Colonoscopy results showed a horseshoe-shaped mass in the ascending colon, occupying two-thirds of the lumen (Figure 1E). Pathological biopsy confirmed the diagnosis of colorectal mucinous adenocarcinoma (Figure 1F).
Case 2: An abdominal CT scan revealed a slightly irregular lower density tumor approximately 87 mm × 83 mm in the right iliac fossa, with edge enhancement. Local and cecal boundaries were unclear, and the mass wrapped around the humeral wing and extended to the subcutaneous area, showing a small density shadow of gas (Figure 2B and C). Colonoscopy and pathological biopsy results revealed a mass in the ascending colon in the lumen, which confirmed the diagnosis of ascending colon mucinous adenocarcinoma (Figure 2D and E).
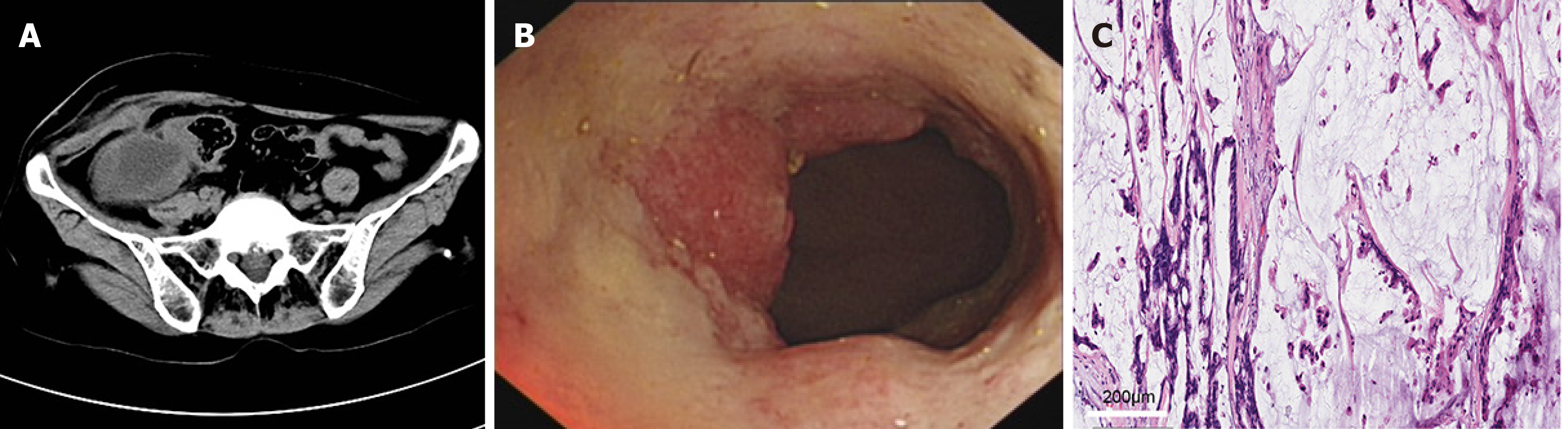
Case 3: An abdominal CT scan showed that the intestinal wall was irregularly thickened in the ileocecal area, the intestinal wall was unevenly enhanced, the intestinal lumen was expanded, the density of the right lower abdomen fat had increased, the irregular low-density area had extended to the subcutaneous region, the boundary of the intestine was unclear, and multiple enlarged lymph nodes were seen in the abdominal cavity and retroperitoneum (Figure 3A). Colonoscopy and pathological biopsy results revealed a mass in the lumen (Figure 3B), which confirmed the diagnosis of colon mucinous adenocarcinoma. The results of the pathological examination confirmed the diagnosis of right colon mucinous adenocarcinoma; the tumor had infiltrated into the serous layer of the intestinal wall, and cancer nodules were seen in the mesentery. No tumors were involved in the two cutting edges of the intestine and mesentery. Tumor metastasis was noted in a mesenteric lymph node (1/13) (Figure 3C). Immunohistochemistry results were: CDX2(+); CEA(+); CK20(+); Villin (++); Ki-67(30%); CA125(-); and CK17(-).
Case 1: Ascending colon mucinous adenocarcinoma.
Case 2: Ascending colon mucinous adenocarcinoma.
Case 3: Ascending colon mucinous adenocarcinoma, which was classified as stage IIIC (T4bN1aM0) according to the TNM staging system.
Case 1: Surgery was not performed because the lesions were extensive.
Case 2: Surgery was not performed because of the advanced age of the patient and the advanced stage of the tumor.
Case 3: Radical surgical resection of cancer of the right colon and partial abdominal wall resection were performed.
Case 1: The patient died after 2 mo of follow-up.
Case 2: The patient died after 11 mo of follow-up.
Case 3: To date, the patient has not had tumor recurrence after 1 year of follow-up.
Currently, for the special pathological histological subtype of mucinous colorectal adenocarcinoma, the most effective treatment is surgical resection. Therefore, early diagnosis and aggressive surgical treatment may be the key to improving the prognosis. In this article, we report the formations of a local abscess (the right area of the lumbar spine, right groin, and lower right abdomen) as the first manifestations of three right mucinous colonic adenocarcinoma patients. Moreover, the abscesses were not formed by the perforation of the intestine, because most of them were secondary to an infection of adjacent organs. After they arrived at our hospital, we excluded common causes, including spinal tuberculosis, urinary tract infection, and periappendiceal abscess. Then, we utilized a few tests with clinical suspicions of the patients having malignant tumors in the intestine. With the help of tumor marker detection and enteroscopy methods, the diagnosis was clear. However, the diagnosis of the disease in case 1 was delayed for 1 year, and the diagnosis of case 2 was delayed for half a year; this resulted in the patients being unable to undergo surgical treatment due to the advanced disease stage. Only case 3 successfully underwent radical resection of the right colon cancer.
The mechanism of abscess formation in these three patients with ascending colon mucinous adenocarcinoma is unclear. Cases of abscess formation after perforation of CRC have been reported[5], but we excluded the possibility of abscess formation due to the perforation of the intestine. The reasons are summarized as follows: (1) There was no intestinal content overflow or perforation in the previous operation; (2) There were no severe infection symptoms such as shivering and high fever; (3) No perforation was found by enteroscopy at our hospital; and (4) In case 3, there was no intestinal perforation in the specimen test after surgery. Therefore, the reasons for abscess formation may be as follows: (1) There are a large number of bacteria in the large intestine, and the mucosal barrier of the intestinal wall is damaged after the tumor occurs, which is prone to bacterial translocation[6]; (2) Due to the presence of a large number of mucus components in mucinous adenocarcinoma, the ability of phagocytic cells to phagocytize bacteria is weakened; and (3) In addition, the tumor grows rapidly, the blood supply is insufficient, and local tissue may develop necrosis. While intestinal pathogens translocate, infections and even abscess formation are likely to occur. The ascending colon is an inter-peritoneal organ, and while the tumor infiltrated at different locations, the lesions were different. This may be one of the mechanisms of abscess formation.
When we analyzed the factors of the delayed diagnoses, we found that all tumor lesions were located in the right colon. The diameter of the ascending colon is large, and the stools are thin. Compared with the diagnosis of left colon cancer, there are few symptoms of intestinal obstruction in the early stage of right colon cancer. The initial symptoms of right colon cancer are not typical. With the development of the disease, it is manifested as systemic symptoms, which include general malaise, weight loss, anemia, etc. Therefore, an early diagnosis is difficult for right colon cancer. In addition, it has been reported that the prognosis of right colon cancer is generally worse than that of left colon cancer, and this may also be related to the difficulty in the timely diagnosis of right colon cancer patients[7]. The three cases reported in this article also had a rare local abscess as the primary manifestation. Diagnosis is difficult in the absence of colonoscopy and pathological biopsy. If colonoscopy and pathological biopsy are performed early, it is expected that the lesion will be found early and the diagnosis will be clear.
In summary, abscess in the right area of the lumbar spine, right groin, or right lower quadrant caused by non-intestinal perforation as the primary clinical manifestation of ascending colon mucinous adenocarcinoma is extremely rare. In the diagnosis and treatment of patients with a local abscess, the source of the abscess must be clear. Mucinous adenocarcinoma of the ascending colon is one of the causes. A colonoscopy is essential for the diagnosis and identification of intestinal tumors, and regular colonoscopy may have prevented the delayed presentations of colonic tumors as an abdominal wall abscess by early detection of the lesions. For patients with colorectal mucinous adenocarcinoma with abscess formation, the source of the abscess should be identified as early as possible, and early diagnosis and aggressive surgical treatment are essential for improving the prognosis of patients.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bairwa BL, Valiveti CK S-Editor: Liu M L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55772] [Article Influence: 7967.4] [Reference Citation Analysis (132)] |
| 2. | Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 3. | Zong Z, Luo Y, Ying H, Wang A, Li H, Yi C. Trends of incidence and survival in patients with gastrointestinal mucinous adenocarcinoma. Oncol Lett. 2018;16:5791-5798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | McCawley N, Clancy C, O'Neill BD, Deasy J, McNamara DA, Burke JP. Mucinous Rectal Adenocarcinoma Is Associated with a Poor Response to Neoadjuvant Chemoradiotherapy: A Systematic Review and Meta-analysis. Dis Colon Rectum. 2016;59:1200-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Okita A, Kubo Y, Tanada M, Kurita A, Takashima S. Unusual abscesses associated with colon cancer: report of three cases. Acta Med Okayama. 2007;61:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 6. | Han S, Gao J, Zhou Q, Liu S, Wen C, Yang X. Role of intestinal flora in colorectal cancer from the metabolite perspective: a systematic review. Cancer Manag Res. 2018;10:199-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- vs left-sided colon cancers? Ann Surg Oncol. 2008;15:2388-2394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 387] [Article Influence: 22.8] [Reference Citation Analysis (0)] |