Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7845
Peer-review started: March 29, 2021
First decision: April 28, 2021
Revised: May 8, 2021
Accepted: August 13, 2021
Article in press: August 13, 2021
Published online: September 16, 2021
Processing time: 165 Days and 2.6 Hours
Chronic active Epstein-Barr virus infection (EBV) is a systemic EBV-positive lymphoproliferative disease, which may lead to fatal illness. There is currently no standard treatment regimen for chronic active EBV (CAEBV), and hematopoietic stem cell transplantation is the only effective treatment. We here report a CAEBV patient treated with PEG-aspargase, who achieved negative EBV-DNA.
A 33-year-old female Chinese patient who had fever for approximately 3 mo was admitted to our hospital in December 2017. EBV-DNA was positive with a high copy number. She was diagnosed with chronic active EB virus infection. PEG-aspargase was administered at a dose of 1500 U/m2 at a 14-d interval, resulting in eradication of EBV for more than 6 mo. The effect of PEG-aspargase in this patient was excellent.
A chemotherapy regimen containing PEG-aspargase for CAEBV may be further considered.
Core Tip: Chronic active Epstein-Barr virus (EBV) infection may lead to fatal diseases, including EBV-positive lymphoproliferative disorders, lymphomas, and hemophagocytic lymphohistiocytosis. We present a chronic active EBV (CAEBV) patient who was treated with PEG-aspargase and achieved decreased load of EBV-DNA. PEG-aspargase requires further investigation as a chemotherapy drug for CAEBV to reduce the load of EBV-DNA.
- Citation: Song DL, Wang JS, Chen LL, Wang Z. Chronic active Epstein-Barr virus infection treated with PEG-aspargase: A case report. World J Clin Cases 2021; 9(26): 7845-7849
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7845.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7845
Chronic active Epstein-Barr virus infection (EBV) is a systemic EBV-positive lymphoproliferative disease, which may lead to fatal illness. There is currently no standard treatment regimen for chronic active EBV (CAEBV), and the only effective treatment is hematopoietic stem cell transplantation (HSCT)[1]. We here report a patient with CAEBV who achieved eradication of EBV for more than 6 mo following the completion of PEG-aspargase treatment. PEG-aspargase may provide a new treatment regimen to reduce EBV load for CAEBV.
A 33-year-old female Chinese patient was admitted to our hospital with intermittent fever and weakness for 3 mo.
About 2 mo previously, this patient was admitted to a local hospital due to fever and decreased appetite. The laboratory examination showed leukopenia and liver function damage. EBV-DNA was 6.09 × 105 IU/mL in September 2017. Fever was not effectively improved with cephalosporin and ganciclovir. Interferon was given; EBV-DNA decreased slightly, but fever persisted. The fever had lasted 3 mo when she was admitted to our hospital in December 2017.
The patient had no other previous medical history.
The patient had no personal or family history of similar illnesses.
Physical examination revealed enlargement of multiple superficial lymph nodes and splenomegaly. Abdominal ultrasonography showed that the spleen was 15.7 cm in diameter.
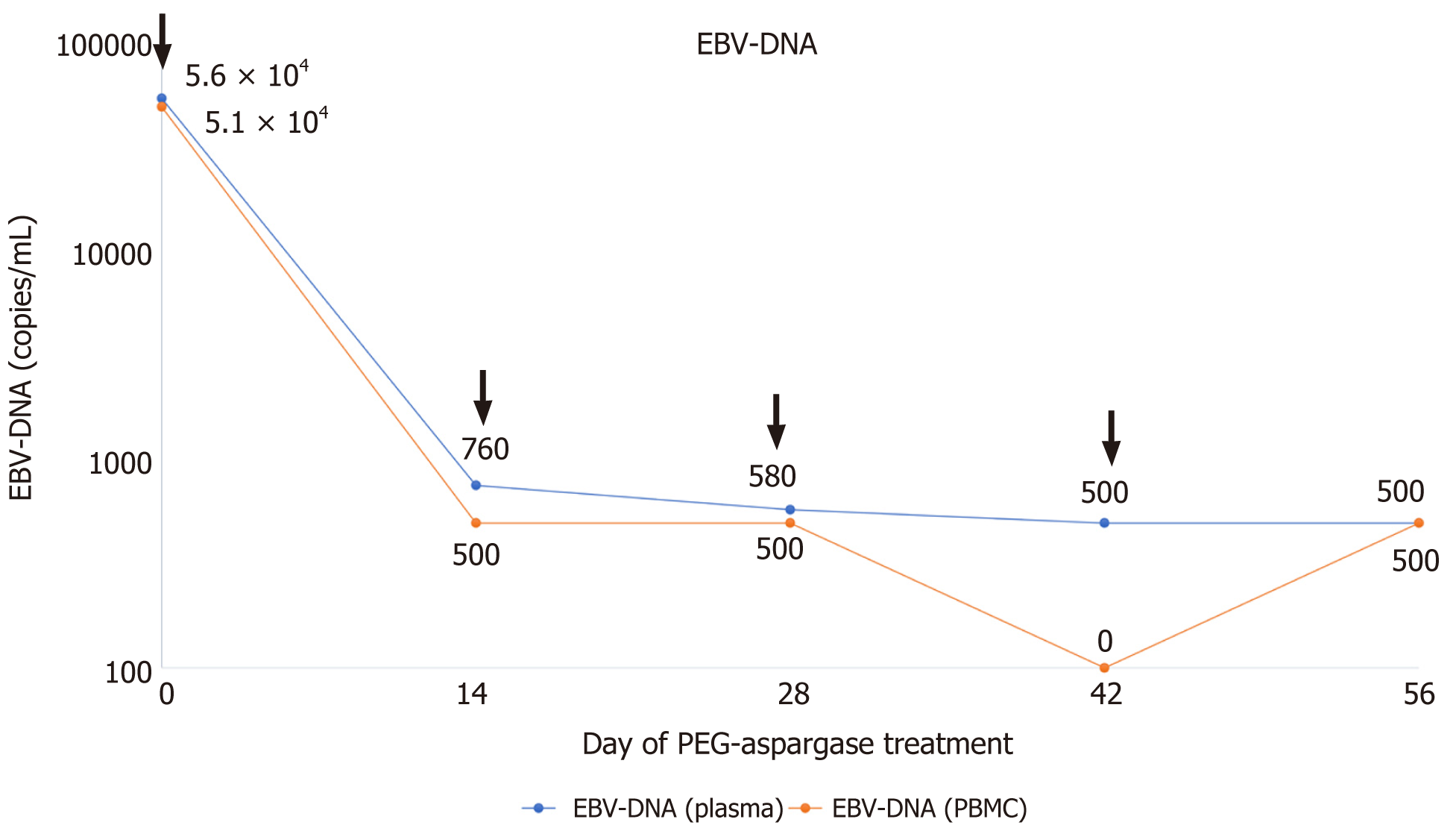
Complete blood count revealed bicytopenia with a white blood cell count of 1.39 × 109/L, hemoglobin 14.2 gm/dL, and platelet count of 89 × 109/L. ALT and AST were elevated to 95 U/L and 173.2 U/L, respectively. EBV-DNA (whole blood) was 5.1 × 104 copies/mL and EBV-DNA (plasma) was 5.60 × 104 copies/mL in December 2017. Natural killer cell was mainly involved in lymphocyte subsets of EBV infection, despite the accumulation of all lymphocyte subsets. Flow cytometry of the bone marrow revealed about 2.26% abnormal phenotype natural killer (NK) cells, expressing CD56bri, CD2, CD7, CD94bri, CD161, and CD159a. Biopsy was taken from the swollen left inguinal lymph node and bone marrow. However, no tumor was detected, and EBV-encoded small RNA (EBER) was not found by in situ hybridization of two biopsies. The tests of hepatitis virus, human immunodeficiency virus, antinuclear antibody, and rheumatoid antibody were all negative.
According to the diagnostic criteria for CAEBV, she was finally diagnosed with CAEBV.
The patient started on PEG-aspargase (1500 U/m2) treatment every 14 d from December 2017. The informed consent was obtained from the patient and this therapy was approved by the institutional ethics committee.
The patient's body temperature gradually dropped, and fever improved on the 7th day during the first treatment course with PEG-aspargase. On the 14th day, the patient's liver enzymes had returned to normal and the spleen shrank to normal size. EBV-DNA (whole blood) was 760 copies/mL and EBV-DNA (plasma) was < 500 copies/mL in January 2018. EBV-DNA had maintained negative for more than 6 mo (Figure 1) since February 2018. Re-examination of the bone marrow showed that abnormal NK cells disappeared. Hypofibrinogenemia was recorded during PEG-aspargase treatment, but bleeding or thromboembolism did not occur. No other side effects of PEG-aspargase, such as allergy, pancreatitis, and hepatotoxicity, were noted. The patient rejected allogeneic HSCT for personal reasons. Unfortunately, the patient eventually died due to relapse of CAEBV and occurrence of hemophagocytic lymphohistiocytosis (HLH) after more than 6 mo of PEG-aspargase treatment. Due to severe liver dysfunction at the time of relapse, the patient could no longer receive PEG-aspargase therapy.
CAEBV is a chronic disease with persistent infectious mononucleosis-like symptoms, such as fever, lymphadenopathy, and hepatosplenomegaly. CAEBV may develop into fatal diseases, including multi-organ failure, EBV-associated T/NK cell lymphoproliferative disorder, T or NK cell lymphomas, and HLH[1].
The diagnostic criteria for CAEBV are as follows: (1) Sustained or recurrent infectious mononucleosis-like symptoms persistent for > 3 mo; (2) Increased amounts of EBV detected by Southern blot hybridization, EBER-positive cells in affected tissues or the peripheral blood, or ≥ 102.5 copies/µg of EBV-DNA in peripheral blood mononuclear cells (PBMCs); and (3) No evidence of previous immunological abnor
There is currently no standard treatment for CAEBV. Sawada et al[5] suggested a sequential treatment strategy consisting of prednisolone, cyclosporine A, and etoposide, a so-called cooling therapy as the first step, and combination chemo
PEG-aspargase, a pegylated form of L-asparaginase, has a prolonged circulation time and diminished immunogenicity compared with native L-asparaginase[6]. Therefore, PEG-aspargase requires less frequent administration and has better treatment efficacy compared to L-asparaginase. Multiple studies on NK/T-cell lymphoma have found that chemotherapy regimens containing PEG-aspargase significantly reduce the load of EBV-DNA while treating lymphoma, and the reduced load of EBV-DNA after treatment suggests longer survival[7,8]. Our study on refractory relapsed EBV-HLH found that the L-DEP regimen (PEG-aspargase and DEP combination therapy) can reduce EBV-DNA load while effectively controlling HLH-induced fever and organ dysfunction[9]. Therefore, it can be speculated that PEG-aspargase may have an “eliminating” effect on EBV infection. In the present patient, EBV was eradicated after treatment with PEG-aspargase, and EBV-DNA remained negative for more than 6 mo.
The possible mechanisms underlying this may include the following aspects. First, PEG-aspargase accelerates apoptosis in EBV-positive T and NK cells, which has been confirmed in in vitro experiments. Ando et al[10] have reported specific antitumor activity of L-asparaginase against NK-cell tumors in vitro. Jinta et al[11] confirmed that L-asparaginase decreased the number of living cells in all examined EBV-positive cell lines in a dose-dependent manner, an effect not seen in the PBMCs. The “apoptosis” induced by L-asparaginase of EBV-infected T/NK cells may decompose asparagine, an essential amino acid for protein synthesis, such as the effect of treating NK/T cell lymphomas. Second, the expression of P-glycoprotein may be a cause of chemoresistance of EBV-positivity-related malignant disease. Yamaguchi et al[12] found that tumor cells from nine of 10 patients with Extranodal natural killer/T-cell lymphoma, an EBV-positive NK-LPD, were positive for P-glycoprotein. They also examined P-glycoprotein expression in ENKL by immunohistochemistry. Yoshimori et al[13] reported that EBV-infected T cells in EBV-T-LPDs expressed functional P-glycoprotein. The effect of PEG-aspargase is not influenced by P-glycoprotein. Therefore, compared to other chemotherapeutic drugs, PEG-aspargase, which is not a substrate of P-glycoprotein, can more effectively reduce EBV-positive T/NK cells.
Whether CAEBV patients are suitable for PEG-aspargase therapy remains unclear. Ando et al[10] found that the level of asparagine synthetase expression in NK-cell tumors was related to sensitivity and clinical response to L-asparaginase in ENKL. However, Jinta et al[11] suggested that the level of asparagine synthetase did not sufficiently determine the response to L-asparaginase on EBV-T/NK-LPDs. Therefore, which indicators reflect the response of patients to PEG-aspargase should be further explored. In addition, the mechanism of asparaginase therapy is asparagine depletion. However, the optimum target of asparaginase activity to achieve optimal asparagine depletion of PEG-aspargase still needs to be fully elucidated[14]. Therefore, further clinical study is required to confirm whether regimens containing PEG-aspargase are effective in treating CAEBV, who can respond to this treatment and how to achieve maximum therapeutic benefit.
PEG-aspargase may be effective in reducing the load of EBV-DNA in CAEBV patients. A chemotherapy regimen containing PEG-aspargase for treatment of CAEBV may warrant further study.
Manuscript source: Unsolicited manuscript
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Renzo ND S-Editor: Liu M L-Editor: A P-Editor: Zhang YL
| 1. | Arai A. Advances in the Study of Chronic Active Epstein-Barr Virus Infection: Clinical Features Under the 2016 WHO Classification and Mechanisms of Development. Front Pediatr. 2019;7:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, Naoe T, Esaki S, Kikuta A, Sawada A, Kawa K, Ohshima K, Nakamura S. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119:673-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 312] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 3. | Cohen JI, Jaffe ES, Dale JK, Pittaluga S, Heslop HE, Rooney CM, Gottschalk S, Bollard CM, Rao VK, Marques A, Burbelo PD, Turk SP, Fulton R, Wayne AS, Little RF, Cairo MS, El-Mallawany NK, Fowler D, Sportes C, Bishop MR, Wilson W, Straus SE. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011;117:5835-5849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Kawamoto K, Miyoshi H, Suzuki T, Kozai Y, Kato K, Miyahara M, Yujiri T, Choi I, Fujimaki K, Muta T, Kume M, Moriguchi S, Tamura S, Kato T, Tagawa H, Makiyama J, Kanisawa Y, Sasaki Y, Kurita D, Yamada K, Shimono J, Sone H, Takizawa J, Seto M, Kimura H, Ohshima K. A distinct subtype of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disorder: adult patients with chronic active Epstein-Barr virus infection-like features. Haematologica. 2018;103:1018-1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Sawada A, Inoue M, Kawa K. How we treat chronic active Epstein-Barr virus infection. Int J Hematol. 2017;105:406-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | van der Sluis IM, Vrooman LM, Pieters R, Baruchel A, Escherich G, Goulden N, Mondelaers V, Sanchez de Toledo J, Rizzari C, Silverman LB, Whitlock JA. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica. 2016;101:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 7. | Wang JH, Wang L, Liu CC, Xia ZJ, Huang HQ, Lin TY, Jiang WQ, Lu Y. Efficacy of combined gemcitabine, oxaliplatin and pegaspargase (P-gemox regimen) in patients with newly diagnosed advanced-stage or relapsed/refractory extranodal NK/T-cell lymphoma. Oncotarget. 2016;7:29092-29101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Liu T, Zhu F, Xiao Y, Li Q, Liu X, Yang K, Wu G, Zhang L. Pegaspargase, gemcitabine, dexamethasone, and cisplatin (P-GDP) combined chemotherapy is effective for newly diagnosed extranodal NK/T-cell lymphoma: a retrospective study. Cancer Manag Res. 2018;10:5061-5069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Wang J, Wang Y, Wu L, Zhang J, Lai W, Wang Z. PEG-aspargase and DEP regimen combination therapy for refractory Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. J Hematol Oncol. 2016;9:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Ando M, Sugimoto K, Kitoh T, Sasaki M, Mukai K, Ando J, Egashira M, Schuster SM, Oshimi K. Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol. 2005;130:860-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Jinta M, Imadome K, Komatsu H, Yoshimori M, Kurata M, Fujiwara S, Miura O, Arai A. L-Asparaginase monotherapy for EBV-positive T/NK lymphoproliferative diseases: A pilot Study. J Med Dent Sci. 2015;62:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Yamaguchi M, Kita K, Miwa H, Nishii K, Oka K, Ohno T, Shirakawa S, Fukumoto M. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. 1995;76:2351-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Yoshimori M, Takada H, Imadome K, Kurata M, Yamamoto K, Koyama T, Shimizu N, Fujiwara S, Miura O, Arai A. P-glycoprotein is expressed and causes resistance to chemotherapy in EBV-positive T-cell lymphoproliferative diseases. Cancer Med. 2015;4:1494-1504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Heo YA, Syed YY, Keam SJ. Pegaspargase: A Review in Acute Lymphoblastic Leukaemia. Drugs. 2019;79:767-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |