Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7833
Peer-review started: April 6, 2021
First decision: April 28, 2021
Revised: May 9, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: September 16, 2021
Processing time: 156 Days and 20.9 Hours
Radical cystectomy is considered the first choice for the treatment of muscle-invasive bladder cancer. However, for some patients who have lost the indications for surgery, external beam radiotherapy is a non-invasive and effective treatment.
A 76-year-old patient with bladder cancer who had serious comorbidities and could not tolerate surgery or chemotherapy came to the Wuwei Heavy Ion Center. He received carbon ion radiotherapy (CIRT) with a whole-bladder dose of 44 GyE and tumor boost of 20 GyE. When he finished CIRT, his bladder cancer-related hematuria completely disappeared, and computed tomography examination showed that the tumor had obviously decreased in size. At the 3-mo follow-up, the tumor disappeared, and there were no acute or late adverse events. CIRT was well tolerated in this patient.
CIRT may allow for avoiding resection and was well tolerated with curative outcomes.
Core Tip: This case report is of a bladder cancer patient who received carbon ion radiotherapy (CIRT). The patient tolerated CIRT well. After the completion of CIRT, the hematuria associated with bladder cancer disappeared completely, and computed tomography examination showed that the tumor was significantly reduced. During the 3-mo follow-up, the tumor disappeared and there were no acute or late adverse events.
- Citation: Zhang YS, Li XJ, Zhang YH, Hu TC, Chen WZ, Pan X, Chai HY, Wang X, Yang YL. Carbon ion radiotherapy for bladder cancer: A case report. World J Clin Cases 2021; 9(26): 7833-7839
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7833.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7833
Radical cystectomy is considered the treatment of choice for muscle-invasive bladder carcinoma[1-6]. Surgery may not be possible, however, for patients with locally advanced tumors or for those considered medically unfit[7,8]. External beam radiotherapy (EBRT) is a noninvasive and efficient treatment modality[9-11], although it is based on very limited literature consisting mainly of small retrospective series of patients not eligible for curative treatment[12-14].
Carbon ion beams produce increased energy deposition at the end of their range to form the Bragg peak while minimizing radiation damage to the surrounding tissue, leading to a more precise dosage and localization than photon beams. Furthermore, carbon ion radiotherapy (CIRT) is cell cycle independent with a high relative biologic effectiveness (RBE) and low oxygen enhancement ratio. This kind of radiation also leads to double-strand breaks in DNA molecules, resulting in lethal damage to tumor cells. These properties are thus advantageous for treating radiation-insensitive tumors[15]. Here, we present 1 case of a bladder carcinoma patient who underwent CIRT who achieved good results.
A 76-year-old male patient with bladder cancer is presented. The patient complained of intermittent gross hematuria for 1 year.
The patient had previously been in good health.
No similar medical history in the family.
After the patient was transferred to our center, we performed a full work up. We found the patient had serious gross hematuria (Figure 1A) through the entire course of micturition with pain on straining to urinate.
No abnormalities in blood routine, biochemistry, and electrolytes tests.
Due to the renal and cardiac insufficiency, the patient only underwent abdominopelvic computed tomography (CT) scanning and magnetic resonance imaging without contrast. Imaging in August 2020 showed that the bladder wall was in-homogeneous thickened with mass formation, the mass protrusion into the lumen, and a mass size of 27.9 mm × 29.1 mm. The radiography diagnosis was bladder cancer. Given his co-morbidity, he had a high risk of cystoscopy; at our center, we took urine exfoliation cytology, which found transitional cell carcinoma. He had a serious macroscopic hematuria, and laboratory examination showed urinary red blood cell count (URBC) 14653/μL.
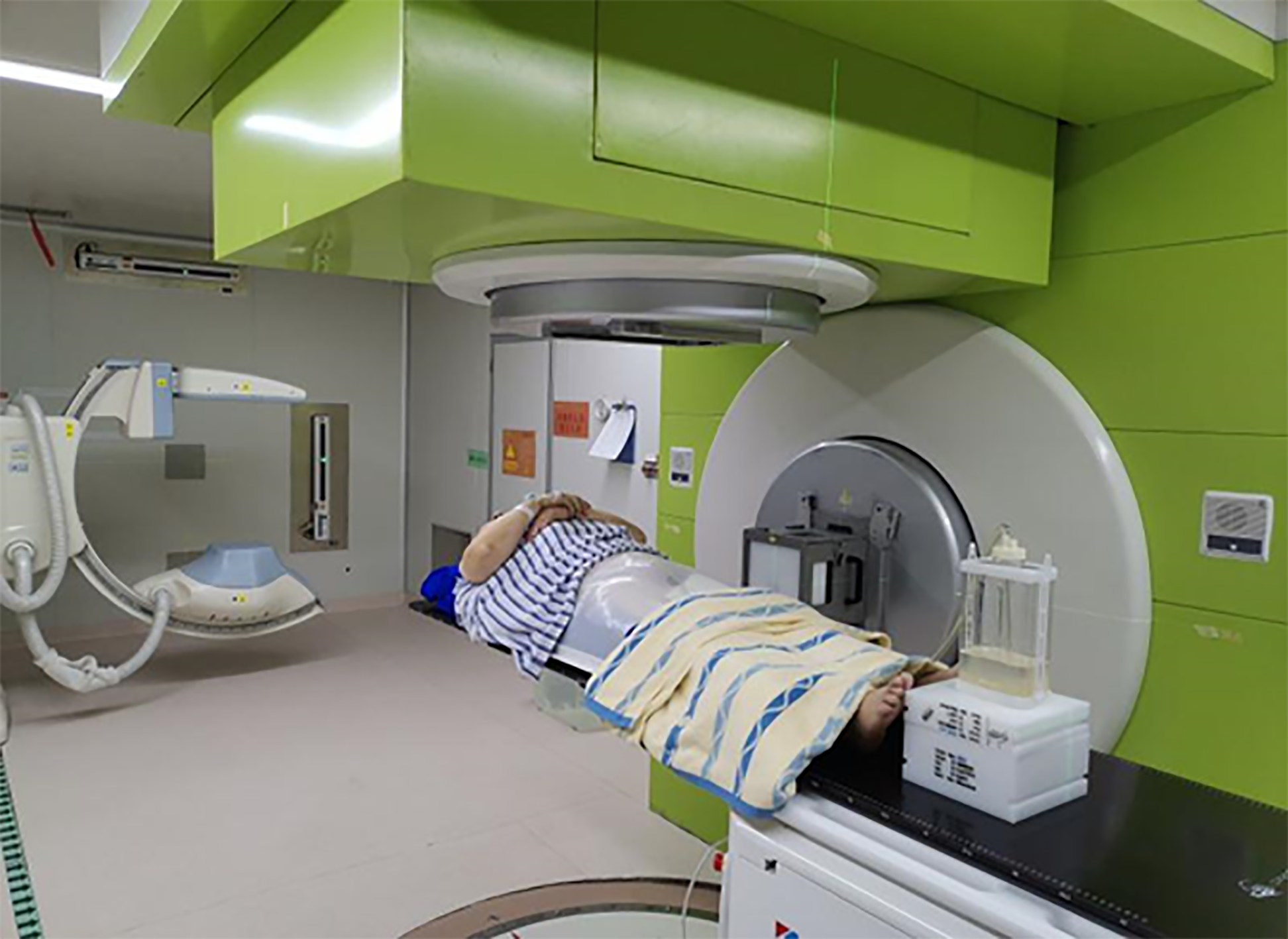
After detailed comprehensive evaluation, the patient’s definite diagnosis was bladder cancer, America Joint Committee on Cancer (8th edition), cT2N0M0, stage II, and Karnofsky score of 70 points. Due to the patient's underlying co-morbidity (diabetes, chronic renal and cardiac insufficiency), the patient had high risk of surgical treatment or cystoscopy. He and his family refused to accept surgery, cystoscopy, or chemotherapy. After MDT, we decided CIRT for the patient with the “galloping scheme” technique (as discussed in the next paragraph) (Figure 2).
Introduction of the "galloping scheme": Before CIRT, the patient underwent indwelling urinary catheter placement. The catheter needs to have a large inner diameter with double-lumen and have 21F-24F inner lumen diameter, which is connected to the bladder and closed thoracic drainage bottle, which has a large volume of more than 2000 mL. Physiological saline solution is injected into the water-sealed bottle (Figure 3). By using “the law of connected vessels”, bladder pressure is related to bladder volume, detrusor, sphincter contraction, abdominal pressure, etc. The fluid flow in and out of the water-sealed bottle was observed until a fluctuating level of relative equilibrium was reached. We adjusted the liquid level of the closed thoracic drainage bottle, and we acquired an ideal bladder volume when finally the water level of the closed thoracic drainage bottle and the bladder pressure is in equilibrium. The ideal bladder volume was obtained by adjusting the liquid level of the bottle according to the lesion location and irradiation range of the bladder cancer. The liquid level of the closed thoracic drainage bottle was measured and recorded. When the patient was asked to urinate forcibly, we found an increase in the level of the thoracic drainage bottle, and finally the water level came back to the original level. The bladder volume repeatability was observed. The specified pressure of closed thoracic drainage bottle was transmitted to the bladder, making the internal bladder pressure and volume almost constant during CIRT. In other words, during the treatment, we kept the precise level of the closed thoracic drainage bottle, and we reached a steady bladder volume and position.
In CT simulation positioning, the whole process of CIRT was simulated several times to determine the variation of urine volume and bladder contour with urine production during treatment, and the internal target volume (ITV) range of bladder was determined individually. Technically, we obtained the individualized data of constant bladder volume and pressure during CIRT (the fluid level in the water-sealed bottle has generally good reproducibility of 10-20 cm above anterior portion of the pubis). Before CIRT, the difference of bladder volume was observed at the simulated positioning session more than three times to determine the degree and range of bladder volume change during radiotherapy, so as to finally achieve the demand of precise treatment. The "galloping scheme" was used to control the constant bladder volume and position throughout the treatment. We had adopted this technique at CIRT for prostate cancer and cervical cancer patients and find it was very wonderful. During CT simulation and positioning, the whole CIRT session was simulated for several times, and the ITV range of the bladder was determined often as 2-4 mm.
Target area was delineated: Gross tumor volume (GTV) was delineated as bladder lesions visible on CT and magnetic resonance imaging. Internal GTV (IGTV) was formed by external expansion of 3 mm on the basis of GTV; GTV plus 3 mm formed primary GTV (PGTV). Clinical target volume included the whole bladder, and ITV was within 0.2-0.4 cm of uniform external expansion of the bladder. Primary tumor volume (PTV) was expanded by 3mm on the basis of ITV, and the 3 mm margins were modified if intestinal tract was close to the bladder; 4-field ‘box’ technique was used to treat both PTV 1 and PGTV.
IGTV was formed by external expansion of 3 mm on the basis of GTV and was formed by external expansion of 3 mm on the basis of IGTV. It was tailored appropriately according to the location of intestinal tract. The irradiation dose was expressed in Gy (RBE), defined as the carbon ion physical dose (Gy) multiplied by a RBE value of 3.0.
The patient was given total doses of 64 Gy (15 fractions, 5 d per week); 20 GyE in four fractions (5 GyE/Fx) was delivered in the first course to the PGTV covering the entire tumor, and then in the second course to PTV to the full dose [alternatively, the whole bladder receives an additional 44 Gy in 11 (4 GyE/Fx) fractions].
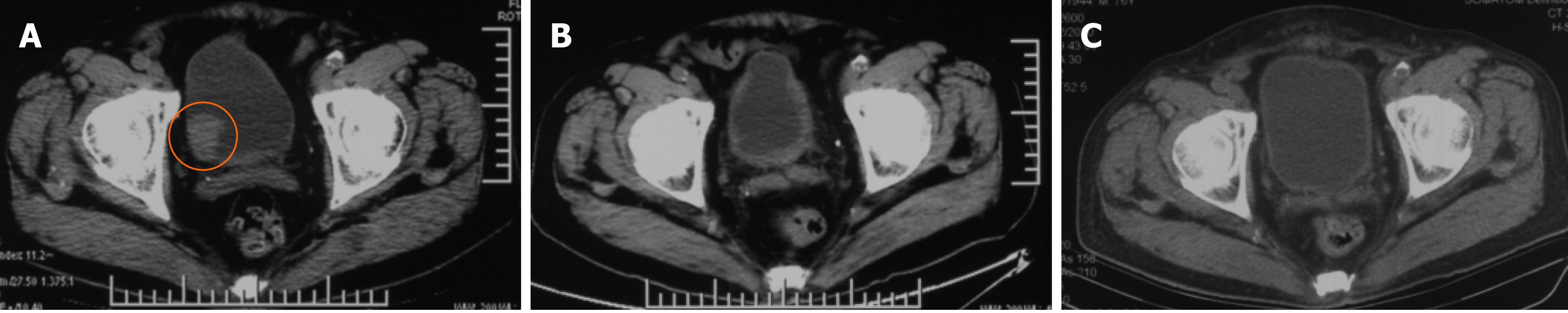
The treatment was started on August 28, 2020. Blood routine, biochemical items, and urine routine were regularly reviewed every 2-d during the treatment, and no toxicity and side effects of radiotherapy were found. After five times of CIRT (Figure 4), gross hematuria disappeared and dysuria was relieved. URBC value of routine URBC decreased significantly (14653 cases/μL to 31 cases/μL). After the initial six times of CIRT, the patient's gross hematuria was completely disappeared (Figure 1B). At the end of the CIRT, pelvic CT indicated that the tumor shrank to 7.6 mm × 17.7 mm, the maximum tumor diameter shrank to 39.1%, and the RECIST1.1 tumor efficacy was assessed as PR, and there were thickening changes of the bladder wall (Figure 5). At the 4-mo of follow-up, A large amount of urinary sediment was discharged during and until 50 d after treatment. About 30 d after treatment, his pain on straining to urinate disappeared.
There were no Grade II or more acute effect and late effects observed, and CT assessed the tumor completely disappeared and the bladder wall thickening change came back to normal. We assessed it was the complete response, and no any late effects were observed. Toxicities were assessed using the Common Terminology Criteria for Adverse Events (version 4.0).
To the best of our knowledge, there are no reports concerning CIRT in the management of bladder carcinomas. Compared to photons, carbon ions have a superior dose conformity, which is even better than that of modern radiotherapy techniques such as stereotactic body irradiation or intensity-modulated radiation therapy/volumetric-modulated arc radiotherapy[16]. CIRT also has greater biological advantages due to a higher linear energy transfer of heavy ions, resulting in better local tumor control and fewer adverse effects on normal tissue[17-20]. Hence, CIRT may be an effective treatment for patients with bladder carcinoma.
The most appropriate treatment algorithm for muscle invasive disease remains controversial. Although radical cystectomy has long been the standard treatment in the United States, organ-preserving regimens using irradiation with concurrent chemotherapy are emerging as viable alternatives in a subset of patients. In clinical practice, irradiation as an adjuvant to cystectomy, preoperative radiation therapy, or postoperative radiation therapy is often used. In the past, radical EBRT was widely used as a single modality for T2 to T4 bladder cancers, particularly in Europe. In the United States, however, the use of irradiation was frequently limited to patients whose medical comorbidities made them poor candidates for surgical monotherapy.
However, currently, there is no CIRT protocol for bladder cancer worldwide. The main reason is that the bladder volume and shape are constantly changing, so it is difficult to ensure the accuracy of treatment and spare OARs. In our clinical experience with applying carbon ion treatment for prostate cancer and cervical cancer, we adopted a closed thoracic drainage bottle to control the pressure and volume of the bladder to ultimately summarize the "galloping scheme" and found that this approach could perfectly resolve the issue of changes in bladder volume and shape.
The present patient had inoperable cancer, was elderly, and was not even a candidate for surgery or chemotherapy. From our clinical experience, for both bladder cancer and other cancers, we found that CIRT was effective in controlling hemorrhage. For this patient, after six fractions of CIRT, the patient's gross hematuria disappeared. Studies investigating EBRT for bladder-related bleeding (follow-up) have reported a 10%-100% efficacy. A few retrospective studies have reported the efficacy of different radiotherapy schedules as palliative treatment for bladder invasion-related gross hematuria, with a hemostatic control rate of 16%-100%. Even though the studies were very heterogeneous in terms of doses and techniques, all of them consistently showed a high rate of HC with low toxicity rates. All patients presented hematuria related to bladder invasion.
For muscle invasive bladder cancer, a single radiotherapy modality is no longer sufficient, and comprehensive treatment is often required. This patient was unable to receive comprehensive treatment due to the presence of many complications, which may have affected the efficacy of treatment in this patient.
Carbon ion beams can provide ideal dose distributions and improved biological efficacy, and their efficacy does not depend on the cell cycle stage.
In summary, the present study shows that CIRT is feasible, even in very elderly patients with comorbidities. CIRT had good tolerance, with few acute or late severe toxic events. As shown in this study, CIRT deserves further investigation in trials. Nevertheless, this is the first report of CIRT for bladder carcinoma, and we achieved a great success. Thus, we want to report our results to help more patients around the world.
The management of this patient population, which includes our patient, is challenging, and CIRT might be an effective and safe option worthy for further evaluation due to its higher relative biological effectiveness associated with greater linear energy transfer; this may lead to a higher probability of achieving tumor control with a lower rate of post radiation morbidities.
We reported a case of bladder cancer treated by CIRT. To the best of our knowledge, this is the first study to use CIRT for bladder cancer. Currently, the patient has achieved relapse-free survival. The favorable clinical course and outcome suggest that CIRT might allow for avoiding resection and was well tolerated with curative outcomes.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Siracusano S S-Editor: Yan JP L-Editor: Filipodia P-Editor: Xing YX
| 1. | Roghmann F, Breyer J, Kriegmair M, Wezel F, Burger M, Noldus J, Bolenz C; Bladder Cancer Research Initiative for Drug Targets Germany (BRIDGE) Consortium e. V. [Quality assessment of radical cystectomy-opportunities, risks, challenges]. Urologe A. 2021;60:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Aminoltejari K, Black PC. Radical cystectomy: a review of techniques, developments and controversies. Transl Androl Urol. 2020;9:3073-3081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Domínguez Escrig JL. [Muscle invasive bladder cancer and bladder preservation protocols. Where are we? Arch Esp Urol. 2020;73:986-995. [PubMed] |
| 4. | Álvarez-Maestro M, Guerrero-Ramos F, Rodríguez-Faba O, Domínguez-Escrig JL, Fernández-Gómez JM. Current treatments for BCG failure in non-muscle invasive bladder cancer (NMIBC). Actas Urol Esp (Engl Ed). 2021;45:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Liu Z, Zhang X, Wu B, Zhao Y, Bai S. Development and Validation of a Model for Predicting Urethral Recurrence in Male Patients with Muscular Invasive Bladder Cancer After Radical Cystectomy Combined with Urinary Diversion. Cancer Manag Res. 2020;12:7649-7657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Inamoto T, Ibuki N, Komura K, Juri H, Yamamoto K, Fujita K, Nonomura N, Narumi Y, Azuma H. Can bladder preservation therapy come to the center stage? Int J Urol. 2018;25:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Brummelhuis ISG, Wimper Y, Witjes-van Os HGJM, Arends TJH, van der Heijden AG, Witjes JA. Long-Term Experience with Radiofrequency-Induced Hyperthermia Combined with Intravesical Chemotherapy for Non-Muscle Invasive Bladder Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Mascarenhas F, Maes K, Marques F, Formoso R, Antunes T. Robot-assisted brachytherapy of the bladder with long distance support using video conferencing. J Contemp Brachytherapy. 2017;9:375-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Kulkarni GS, Hermanns T, Wei Y, Bhindi B, Satkunasivam R, Athanasopoulos P, Bostrom PJ, Kuk C, Li K, Templeton AJ, Sridhar SS, van der Kwast TH, Chung P, Bristow RG, Milosevic M, Warde P, Fleshner NE, Jewett MAS, Bashir S, Zlotta AR. Propensity Score Analysis of Radical Cystectomy Versus Bladder-Sparing Trimodal Therapy in the Setting of a Multidisciplinary Bladder Cancer Clinic. J Clin Oncol. 2017;35:2299-2305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 10. | Tiwana MS, Ni LH, Saini S, Verma SK, Doddamani D, Jain N, Biswas M, Gupta M, Saini M, Chauhan N. Radiation therapy outcomes in muscle invasive urinary bladder cancer: A single institution experience. Indian J Cancer. 2016;53:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Ploussard G, Daneshmand S, Efstathiou JA, Herr HW, James ND, Rödel CM, Shariat SF, Shipley WU, Sternberg CN, Thalmann GN, Kassouf W. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol. 2014;66:120-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 12. | Coraggio G, Husheng S, Loganadane G, Ghith S, Grellier N, Hervé ML, To NH, Colson Durand L, Jouhaud A, Fayolle Campana M, Nourieh M, Vordos D, Belkacemi Y. Hemostatic radiotherapy for bladder cancer-related hematuria in patients unfit for surgery: is there any impact of fractionation schedule? J BUON. 2020;25:2092-2096. [PubMed] |
| 13. | Lacarrière E, Smaali C, Benyoucef A, Pfister C, Grise P. The efficacy of hemostatic radiotherapy for bladder cancer-related hematuria in patients unfit for surgery. Int Braz J Urol. 2013;39:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Dirix P, Vingerhoedt S, Joniau S, Van Cleynenbreugel B, Haustermans K. Hypofractionated palliative radiotherapy for bladder cancer. Support Care Cancer. 2016;24:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Mohamad O, Tabuchi T, Nitta Y, Nomoto A, Sato A, Kasuya G, Makishima H, Choy H, Yamada S, Morishima T, Tsuji H, Miyashiro I, Kamada T. Risk of subsequent primary cancers after carbon ion radiotherapy, photon radiotherapy, or surgery for localised prostate cancer: a propensity score-weighted, retrospective, cohort study. Lancet Oncol. 2019;20:674-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Wang WH, Tsuji H, Ishikawa H, Tsujii H, Kamada T, Mizoe J, Li YX. [Comparison of treatment planning by carbon ion radiotherapy and by intensity-modulated radiotherapy for prostatic adenocarcinoma]. Zhonghua Zhong Liu Za Zhi. 2006;28:836-839. [PubMed] |
| 17. | Wang L, Wang X, Zhang Q, Ran J, Geng Y, Feng S, Li C, Zhao X. Is there a role for carbon therapy in the treatment of gynecological carcinomas? Future Oncol. 2019;15:3081-3095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Okonogi N, Fukahori M, Wakatsuki M, Ohkubo Y, Kato S, Miyasaka Y, Tsuji H, Nakano T, Kamada T. Dose constraints in the rectum and bladder following carbon-ion radiotherapy for uterus carcinoma: a retrospective pooled analysis. Radiat Oncol. 2018;13:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Okonogi N, Wakatsuki M, Kato S, Shiba S, Kobayashi D, Kiyohara H, Karasawa K, Ohno T, Nakano T, Kamada T, Shozu M; WORKING GROUP OF GYNECOLOGICAL TUMORS. Long-term Outcomes of Carbon-ion Radiotherapy for Locally Advanced Squamous Cell Carcinoma of the Uterine Cervix. Anticancer Res. 2018;38:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Shiba S, Wakatsuki M, Kato S, Ohno T, Okonogi N, Karasawa K, Kiyohara H, Tsujii H, Nakano T, Kamada T, Shozu M; The Working Group of the Gynecological Tumor. Carbon-ion radiotherapy for locally advanced cervical cancer with bladder invasion. J Radiat Res. 2016;57:684-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |