Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7818
Peer-review started: February 23, 2021
First decision: June 15, 2021
Revised: June 28, 2021
Accepted: July 28, 2021
Article in press: July 28, 2021
Published online: September 16, 2021
Processing time: 198 Days and 14.8 Hours
T-cell large granular lymphocytic leukemia (T-LGLL) is a rare type of aplastic anemia with diverse clinical manifestations. Concomitant diseases are often present at the first manifestation. We describe the treatment of a patient with CD57-negative γδT-LGLL with pure red cell aplasia (PRCA).
A 34-year-old woman with a 20-year history of anemia visited our hospital owing to severe dizziness and was admitted. Her condition was diagnosed as CD57-negative γδT-LGLL with PRCA through bone marrow cytology, bone marrow pathology, bone marrow flow cytometry, bone marrow multiplex polymerase chain reaction combined with fluorescent fragment analysis, and other tests. Treatment with prednisone, methotrexate, and subcutaneous erythropoietin did not significantly change her hemoglobin level. After treatment with oral cyclophosphamide for 3 mo, her hemoglobin level increased to approximately 100 g/L. After 5 mo of treatment, the patient could perform activities of daily living independently.
The treatment of CD57-negative γδT-LGLL with PRCA with cyclophosphamide helps to improve prognosis.
Core Tip: This case report presents a rare case of γδ T-cell large granular lymphocytic leukemia with pure red cell aplasia. A 34-year-old woman was admitted to our hospital with a 20-year history of anemia. Upon investigation, it was discovered that our patient had an atypical immunophenotype. Cyclophosphamide was added to her treatment, and other drugs, such as prednisone, methotrexate, and erythropoietin, were discontinued. Her hemoglobin level increased to 100 g/L within 3 mo. We believe that our study makes a significant contribution to the literature because we report on a favorable prognosis in our patient.
- Citation: Xiao PP, Chen XY, Dong ZG, Huang JM, Wang QQ, Chen YQ, Zhang Y. Treatment for CD57-negative γδ T-cell large granular lymphocytic leukemia with pure red cell aplasia: A case report. World J Clin Cases 2021; 9(26): 7818-7824
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7818.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7818
T-cell large granular lymphocytic leukemia (T-LGLL) is classified into two types, αβ-LGLL and γδ-LGLL, based on the T-cell receptors (TCR). Of these, αβ-LGLL is common and γδT-LGLL is rare, accounting for approximately 5% of all T-LGLL cases[1]. Approximately 8%–19% of T-LGLL patients have pure red cell aplasia (PRCA), 25% have thrombocytopenia, and 10%–18% have rheumatoid arthritis[2]. The typical immunophenotype of γδT-LGLL is CD57-positive, which mostly results in indolent disease. However, we present a case of CD57-negative γδT-LGLL involving progre
A 34-year-old woman with a 20-year history of anemia was admitted to our hospital. She experienced severe dizziness, which led her to visit the hospital.
The patient reported that a diagnosis of mild anemia was made 20 years ago; however, no treatment followed the diagnosis. Gradually, the anemia worsened. Two years ago, the patient visited a hospital for bone marrow cytology and received a diagnosis of PRCA. She was treated with oral cyclosporine A (CSA) 125 mg bid for 2 years. Her condition worsened, and approximately every 2 wk, she received an infusion of red blood cells after her diagnosis (until this admission).
The patient had no other remarkable medical history.
The patient reported an unremarkable personal and family history.
The patient presented with the features of severe anemia, including pale skin and lips. There was no palpable swelling of superficial lymph nodes and no tenderness in the sternum. The body temperature was 36.7 °C; heart rate, 92 beats/min; blood pressure, 110/76 mmHg; respiratory rate, 20 breaths/min, and oxygen saturation, 98%. The lung sounds were normal, and the spleen and liver were not enlarged.
Complete blood count showed the following results: White blood cell count, 2.56 × 109/L; neutrophil level, 0.87 × 109/L; red blood cell count, 1.16 × 1012/L; hemoglobin (Hb) level, 35 g/L; platelet count, 241 × 109/L; and reticulocyte count, 0.98%. Biochemical test results were normal, and direct and indirect antiglobulin test results were negative. Further, the following findings were noted: Antinuclear antibody/ extractable nuclear antigen antibody, negative; antineutrophil cytoplasmic antibody, negative; anticentromere antibody, negative; humoral immunity, normal; rheumatoid factor, normal; cyclic citrullinated peptide antibody, negative; human leukocyte antigen-B27, negative; erythrocyte sedimentation rate, 21.0 mm/h; ferritin, > 1500 ng/mL; Vit B12, > 1117.75 pmol/L; C-reactive protein, normal range; Coombs test, negative; flow cytometry for paroxysmal nocturnal hemoglobinuria, negative; thyroid function, normal, and anti-human T-cell lymphotropic virus antibody, negative.
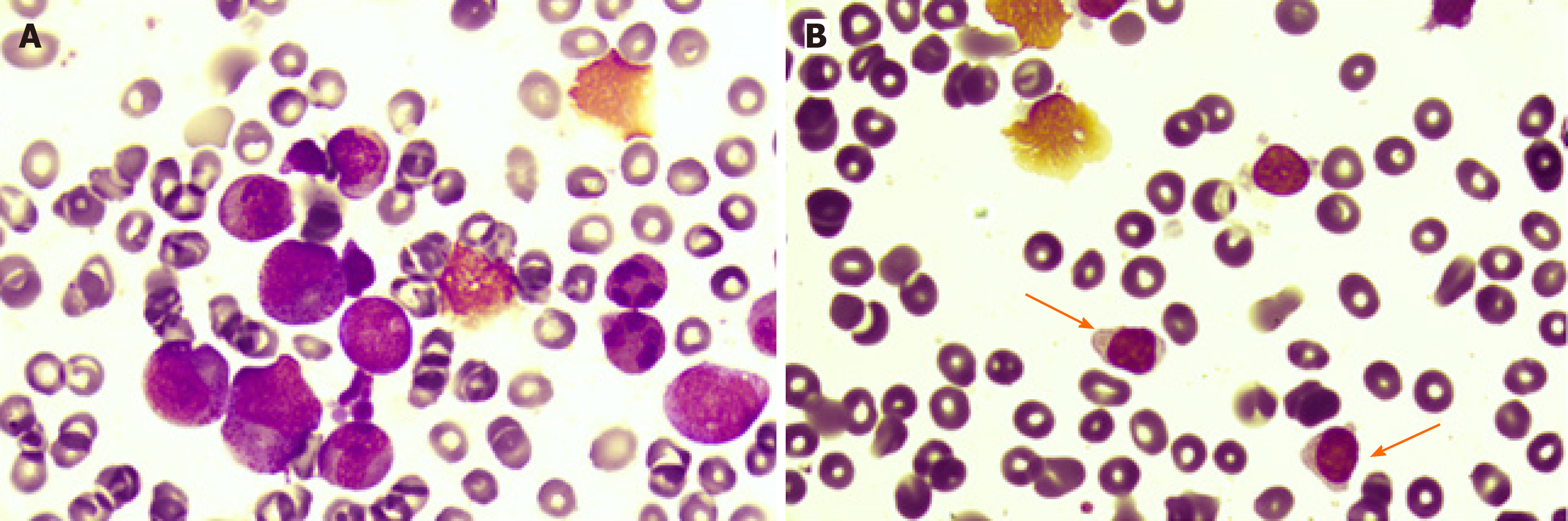
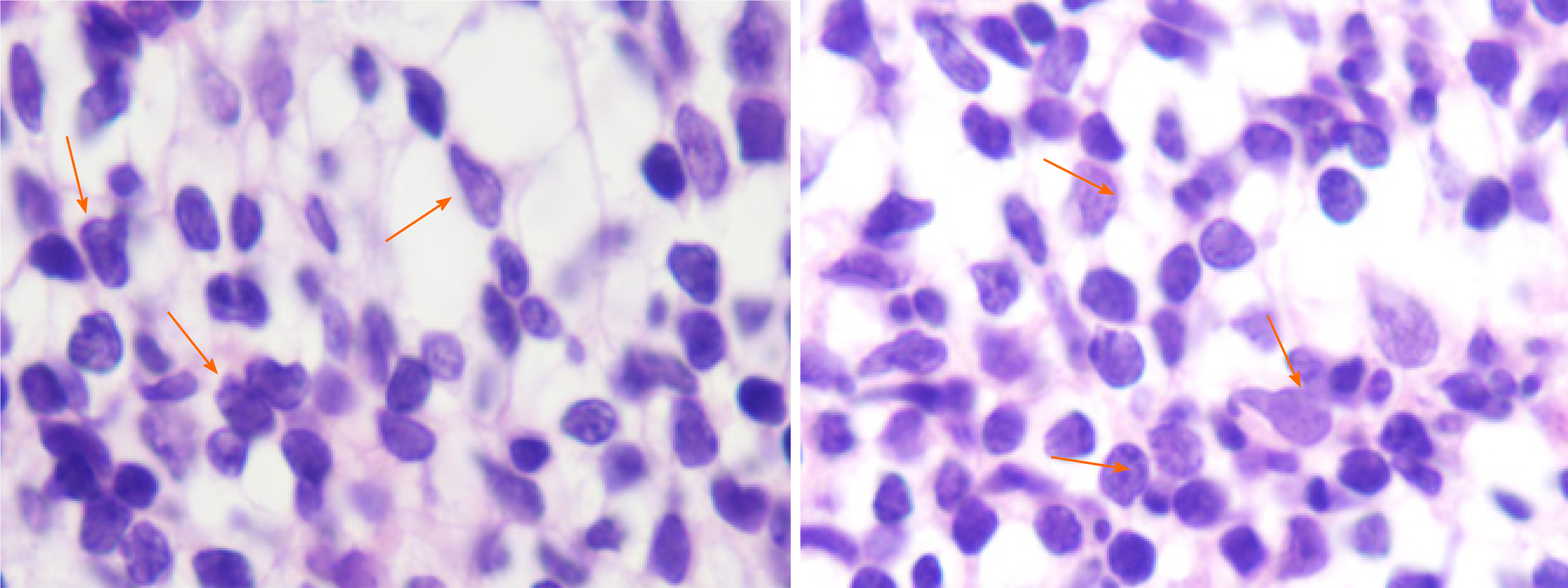
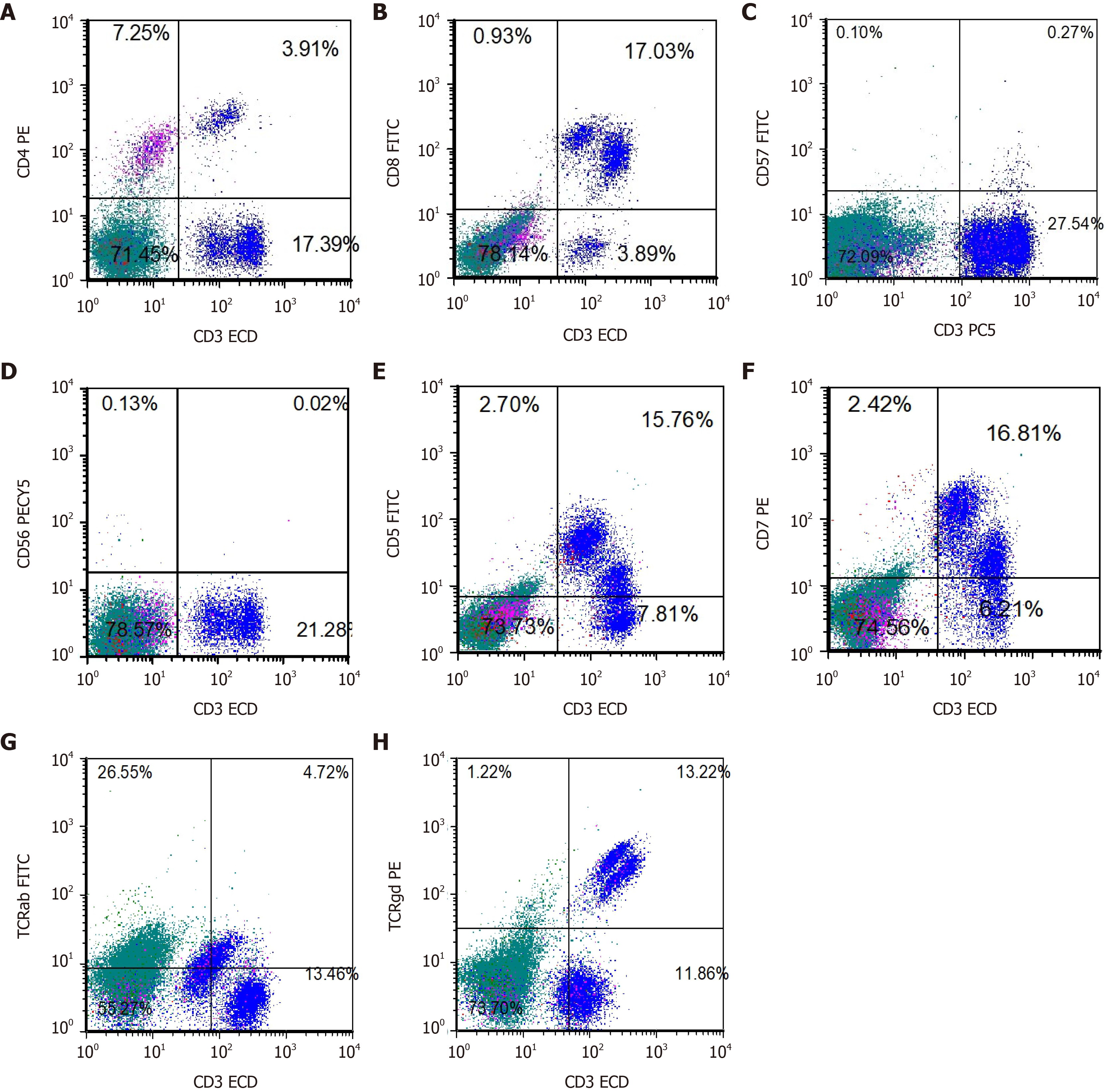
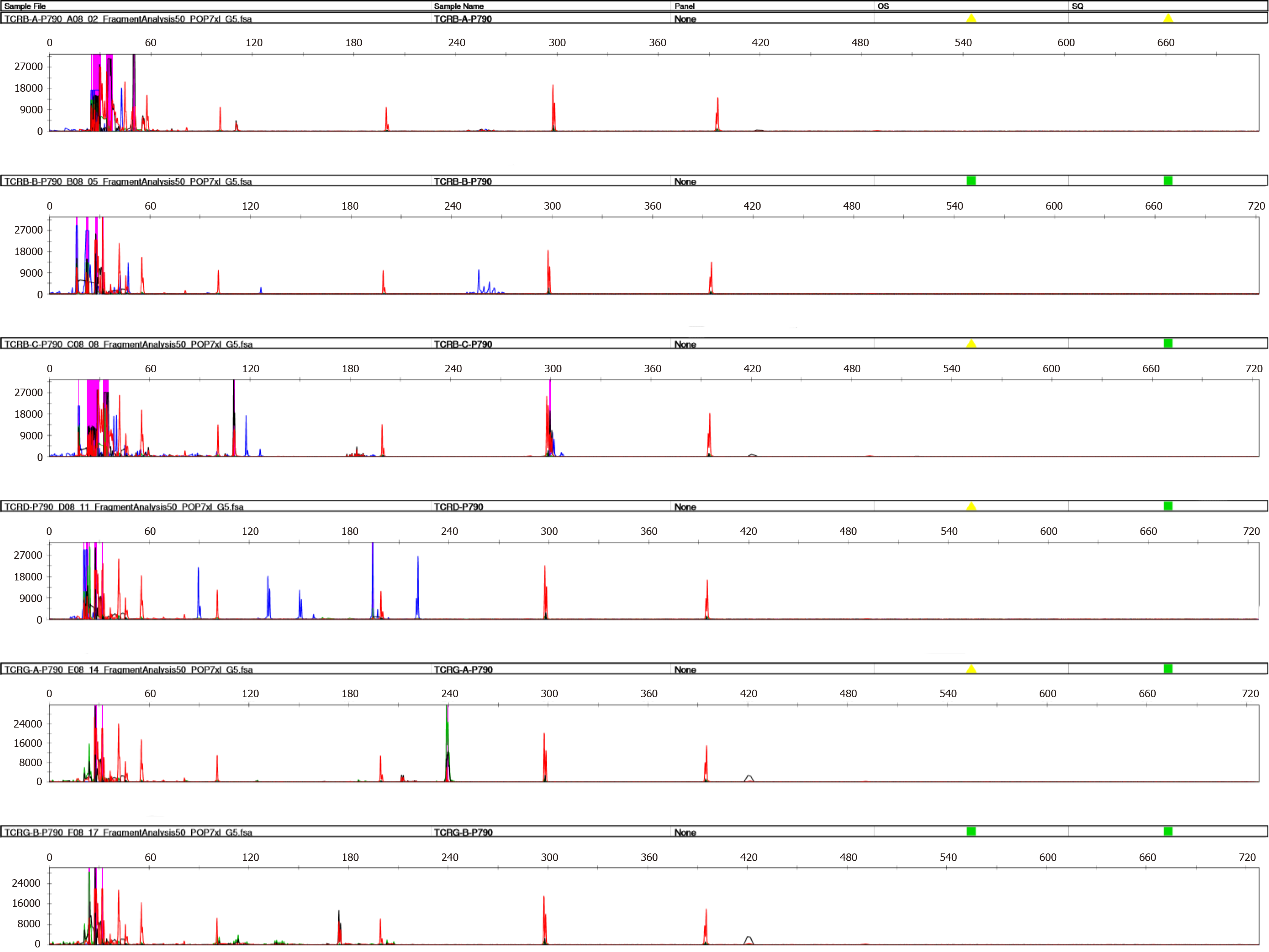
Bone marrow cytology revealed PRCA as well as large granular lymphocytes (Figure 1). Bone marrow pathology revealed the presence of few erythroid cells and a relatively higher T lymphocyte count (Figure 2). A sinus rhythm was noted on performing electrocardiography. The result of chromosome karyotype examination was normal. Erythroid progenitor cell culture resulted in 60/105 bone marrow-derived mononuclear cells. Bone marrow flow cytometry revealed the presence of 13.22% of γδ T lymphocytes with the immunophenotype CD3++, CD8+, TCRγδ+, CD2 part+, CD5 part+, CD4−, CD57−, CD34−, CD33−, CD117−, CD56−, and TCRαβ− (Figure 3). Bone marrow multiplex polymerase chain reaction combined with fluorescent fragment analysis found a Dβ-Jβ rearrangement in TCRβ (299 bp) at 194 bp and a Vδ-Jδ rearrangement in TCRδ at 221 bp. The monoclonal rearrangement was detected in the Vγlf interval, Vγ10-Jγ interval (238 bp), and Vγ9 and Vγ11-Jγ intervals in TCRγ at 174 bp (Figure 4).
Chest computed tomography showed two notable findings. First, the patient had decreased heart density, which is in line with the diagnosis of anemia. Second, the density of the liver parenchyma was high, which might signify splenomegaly. Using hepatobiliary and spleen color Doppler ultrasound, splenomegaly was confirmed. The patient showed a normal sinus rhythm on echocardiography.
This patient’s condition was diagnosed as CD57-negative γδ T-LGLL combined with PRCA.
Two units of red blood cells were transfused in our patient after admission. Then, the patient was administered oral methotrexate (MTX; 15 mg, weekly) and prednisone (50 mg, daily) and a subcutaneous injection of erythropoietin (3000 IU, three times weekly). After 1 mo, the Hb level was maintained at 40–50 g/L, and it did not increase. Therefore, the prednisone and MTX doses were gradually tapered until each drug was completely discontinued; erythropoietin was also discontinued. Cyclophosphamide (CTX) 200 mg bid was administered as the only treatment. The Hb level increased gradually up to approximately 100 g/L after 3 mo.
With the current diagnosis and 5 mo of treatment, the patient has been able to perform activities of daily living independently.
T-LGLL is a relatively rare disorder; only a few clinical trials have been conducted, and few experiences have been published regarding this disease. Therefore, simply relying on traditional diagnostic methods, such as examination of bone marrow morphology, may lead to misdiagnosis and missed diagnosis. Liu et al[3] retrospectively analyzed 10 cases of T-LGLL. Among them, two cases of T-LGLL involved PRCA. Zhu et al[4] analyzed 17 cases of γδT-LGLL, of which 11 cases involved PRCA[4]. γδ- and αβT-LGLL show very similar clinico-biological behaviors as well as antigen-driven T-cell lymphoproliferation[5]. The typical immunophenotype of γδT-LGLL is CD2+/CD3+/CD4−/CD7+/CD8-/CD16−/CD56−/CD57+/ TCRαβ−/TCRγδ+.
This report describes a case of γδT-LGLL with PRCA (immunophenotype CD57−), which is atypical for γδT-LGLL. Zhang et al[6] presented one case of CD57– γδT-LGLL with multiple organ failure. In their case, which involved a chromosomal abnormality, three courses of chemotherapy including CTX, fludarabine, and granulocyte-colony stimulating factor were administered for hemophagocytic syndrome, but the patient died of an infection. Sylvia et al[7] also presented one case of CD57− and CD56− γδT-LGLL, but no related treatments or prognoses were reported.
At present, the treatment for T-LGLL mainly focuses on immunosuppressive therapy, including MTX, CTX, and CSA, as well as purine drugs, combined chemotherapy, and splenectomy. Second- and third-line treatments such as targeted drugs are available[8,9], but there is no consensus as to whether immunosuppressive agents should be used as a single agent or in combination. Our patient had a medical history of anemia for more than 20 years. She showed no improvement in the Hb level following CSA treatment for PRCA. Frequent red blood cell transfusions caused iron overload, and her condition gradually worsened. After the diagnosis of T-LGLL was confirmed, prednisone combined with MTX and erythropoietin was administered. Combination of prednisone with first-line immunosuppressive agents (MTX, CSA, or CTX) may lead to rapid hematologic improvement[10]. MTX has anti-inflammatory and anti-proliferative effects, in part by inducing the apoptosis of activated T-cells[11]. However, there was no significant improvement in the Hb level of our patient. The Hb level increased to a value close to normal after 3 mo of treatment with CTX alone. The patient no longer required regular red blood cell transfusion, which further confirmed the therapeutic potential of CTX for T-LGLL combined with PRCA. Sanikommu et al[12] presented the cases of 118 LGLL patients who received CSA, MTX, or CTX. CTX had the highest initial response rate and the shortest response duration. In atypical CD57-negative T-LGLL cases, the effect of CTX is particularly significant. The mechanism of CTX in T-LGLL combined with PRCA is still unknown. One possible mechanism is that CTX reduces the count of cytotoxic T lymphocytes (CTLs) that damage antibody-bound erythroblasts directly. Another possible mechanism is that CTX reduces the cytotoxic T lymphocytes-mediated damage to hematopoietic progenitors, which leads to anemia[13]. In our case, combined chemotherapy, splenectomy, and other treatments that pose a greater risk of side effects were avoided.
We describe our experience regarding the treatment of CD57-negative γδT-LGLL with PRCA and provide a reference for evaluating prognosis in this patient group. Our patient responded well to CTX and resumed routine activities. The mechanism of CTX in CD57-negative T-LGLL with PRCA still needs to be explored.
We thank our patient who agreed to the publication of this case report and provided information.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Patel VJ, Sultana N S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Sandberg Y, Almeida J, Gonzalez M, Lima M, Bárcena P, Szczepañski T, van Gastel-Mol EJ, Wind H, Balanzategui A, van Dongen JJ, Miguel JF, Orfao A, Langerak AW. TCRgammadelta+ large granular lymphocyte leukemias reflect the spectrum of normal antigen-selected TCRgammadelta+ T-cells. Leukemia. 2006;20:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Zhu Y, Gao Q, Hu J, Liu X, Guan D, Zhang F. Clinical features and treatment outcomes in patients with T-cell large granular lymphocytic leukemia: A single-institution experience. Leuk Res. 2020;90:106299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Liu AQ, Zhou L, Li YH, Jing Y, Wang SH, Mei JH, Dou LP, Wang LL, Yu L. [Analysis of Clinical Characteristics in 10 Patietns with T Large Granular Lymphocytic Leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016;24:693-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Zhu YM, Gao QY, Hu J, Liu X, Guan DR, Zhang FK. [Clinical and laboratory analysis of 17 patients with γδT-cell large granular lymphocyte leukemia]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:112-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Bourgault-Rouxel AS, Loughran TP Jr, Zambello R, Epling-Burnette PK, Semenzato G, Donadieu J, Amiot L, Fest T, Lamy T. Clinical spectrum of gammadelta+ T cell LGL leukemia: analysis of 20 cases. Leuk Res. 2008;32:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Zhang L, Ramchandren R, Papenhausen P, Loughran TP, Sokol L. Transformed aggressive γδ-variant T-cell large granular lymphocytic leukemia with acquired copy neutral loss of heterozygosity at 17q11.2q25.3 and additional aberrations. Eur J Haematol. 2014;93:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Sylvia MT, Jacob SE, Basu D, Amalnath D, Dutta TK. CD56 Negative Aggressive T Cell Large Granular Lymphocytic Leukemia. Indian J Hematol Blood Transfus. 2016;32:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Fujishima N, Sawada K, Hirokawa M, Oshimi K, Sugimoto K, Matsuda A, Teramura M, Karasawa M, Arai A, Yonemura Y, Nakao S, Urabe A, Omine M, Ozawa K; PRCA Collaborative Study Group. Long-term responses and outcomes following immunosuppressive therapy in large granular lymphocyte leukemia-associated pure red cell aplasia: a Nationwide Cohort Study in Japan for the PRCA Collaborative Study Group. Haematologica. 2008;93:1555-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Go RS, Li CY, Tefferi A, Phyliky RL. Acquired pure red cell aplasia associated with lymphoproliferative disease of granular T lymphocytes. Blood. 2001;98:483-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Lamy T, Loughran TP Jr. How I treat LGL leukemia. Blood. 2011;117:2764-2774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 11. | Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 307] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Sanikommu SR, Clemente MJ, Chomczynski P, Afable MG 2nd, Jerez A, Thota S, Patel B, Hirsch C, Nazha A, Desamito J, Lichtin A, Pohlman B, Sekeres MA, Radivoyevitch T, Maciejewski JP. Clinical features and treatment outcomes in large granular lymphocytic leukemia (LGLL). Leuk Lymphoma. 2018;59:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Qiu ZY, Qin R, Tian GY, Wang Y, Zhang YQ. Pathophysiologic Mechanisms And Management Of Large Granular Lymphocytic Leukemia Associated Pure Red Cell Aplasia. Onco Targets Ther. 2019;12:8229-8240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |