Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7717
Peer-review started: March 24, 2021
First decision: May 12, 2021
Revised: May 23, 2021
Accepted: July 23, 2021
Article in press: July 23, 2021
Published online: September 16, 2021
Processing time: 169 Days and 23.8 Hours
Non-alcoholic fatty liver disease has become the most common chronic liver disease worldwide, which originates from the accumulation of triglyceride (TG) in the liver. Patients with type 2 diabetes mellitus (T2DM) are considered to have a predisposition to hepatic steatosis. However, the influencing factors for hepatic fat accumulation in T2DM patients remain unclear.
To investigate the influencing factors for hepatic fat accumulation in T2DM patients.
We enrolled 329 T2DM patients admitted to the Endocrinology Department of the First Affiliated Hospital of Soochow University, who underwent MR mDIXON-Quant examination to quantify the hepatic fat fraction (HFF). According to body mass index (BMI), the patients were divided into normal weight, overweight, and obese groups. The differences in general statistics, biochemical parameters, islet function, and HFF were compared among the three groups. The associations between HFF and other parameters and the influences of various parameters on the severity of hepatic fat accumulation were analyzed.
The HFF of T2DM patients gradually increased in the normal weight, overweight, and obese groups (P < 0.05). Spearman correlation analysis showed that in T2DM patients, HFF was negatively correlated with age and high-density lipoprotein cholesterol (P < 0.05), whereas it was positively correlated with BMI, waist-hip ratio, fasting plasma glucose, alanine aminotransferase (ALT), aspartate aminotransferase, bilirubin, glutamyl transpeptidase, lactate dehydrogenase, albumin (ALB), uric acid (UA), total cholesterol, TG, low-density lipoprotein cholesterol (LDL-C), C-reactive protein, free triiodothyronine, fasting insulin, fasting C-peptide, and homeostasis model assessment of insulin resistance (P < 0.05). Multiple linear regression analysis showed significant positive influences of BMI, ALT, LDL-C, UA, and ALB on HFF in T2DM patients (P < 0.05). Binary logistic regression analysis showed that BMI, ALT, ALB, and LDL-C were independent risk factors for moderate to severe fatty liver in T2DM patients, and obesity increased the risk of being complicated with moderate to severe fatty liver by 4.03 times (P < 0.05).
The HFF of T2DM patients increases with BMI. Higher BMI, ALT, ALB, and LDL-C are independent risk factors for moderate to severe fatty liver in T2DM patients.
Core Tip: Previous studies have showed a bidirectional association between non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM), as the existence of one circumstance aggravates the development of the other. NAFLD originates from the accumulation of triglyceride in hepatocytes, which may consequently lead to further liver injury. This study precisely quantified hepatic fat content by MR mDIXON-Quant imaging, and found that higher body mass index, alanine aminotransferase, albumin, and low-density lipoprotein cholesterol were associated with severer hepatic steatosis in T2DM patients. The MR mDIXON-Quant imaging might play an important role in future research and clinical management of NAFLD.
- Citation: Wu MJ, Fang QL, Zou SY, Zhu Y, Lu W, Du X, Shi BM. Influencing factors for hepatic fat accumulation in patients with type 2 diabetes mellitus. World J Clin Cases 2021; 9(26): 7717-7728
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7717.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7717
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of diseases characterized by hepatic fat accumulation without the excessive alcohol consumption or any other definite secondary pathogenic factors, and it has become the most common chronic liver disease worldwide[1]. In recent years, NAFLD has been considered to be associated closely with a variety of extrahepatic chronic diseases such as metabolic syndrome and its components, coronary heart disease, and chronic kidney diseases[2].
Numerous epidemiological studies have revealed a bidirectional and complex association between NAFLD and type 2 diabetes mellitus (T2DM). Patients with NAFLD have an almost twofold greater risk of developing T2DM than ordinary people[3], and the incidence of T2DM even increases with the severity of NAFLD diagnosed by ultrasound examination[4]. While the prevalence of NAFLD is nearly 25% in the world, the prevalence in T2DM patients is > 72%[5]. Moreover, NAFLD patients with T2DM are more likely to progress from simple hepatic steatosis to steatohepatitis and liver fibrosis[5,6].
According to the classic two-hit theory, the accumulation of triglyceride (TG) in the liver is the first step of NAFLD pathogenesis[7]. MR mDIXON-Quant imaging is a new imaging technique that can directly and precisely measure the hepatic fat content[8], and has been rarely used in the clinical research of NAFLD with T2DM. Therefore, in the present study, this technique was used to measure the hepatic fat fraction (HFF) of T2DM patients. The patients were divided into three groups by their body mass index (BMI), and the parameters of body measurement, biochemical indexes, islet function, and HFF were analyzed to explore the influencing factors for hepatic fat accumulation in T2DM patients.
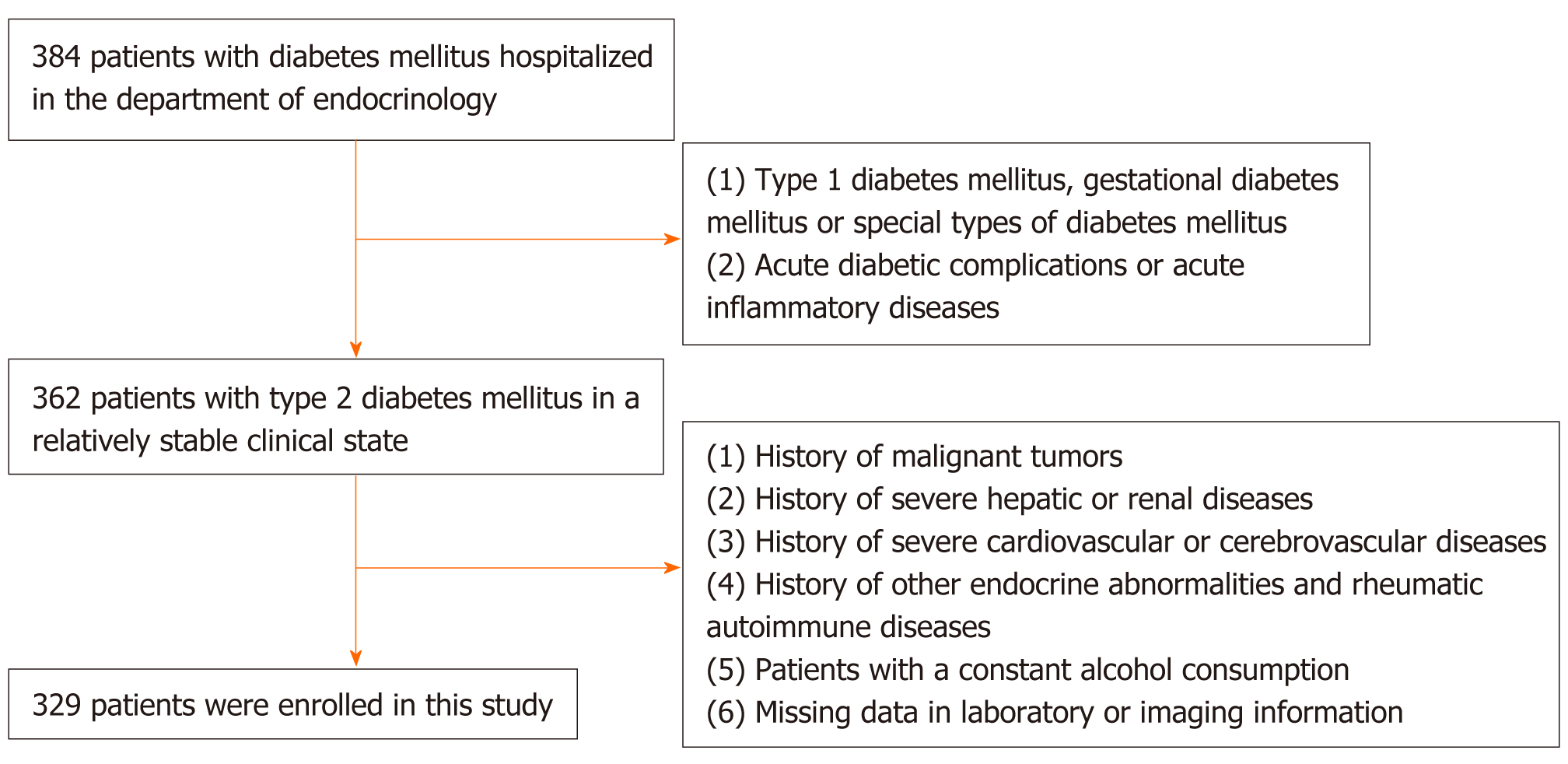
A cross-sectional study was carried out among T2DM patients hospitalized at the Endocrinology Department of the First Affiliated Hospital of Soochow University, Suzhou, China between January 2017 and March 2018. Patients meeting the following criteria were excluded: (1) Type 1 diabetes, gestational diabetes, or special types of diabetes, and patients with acute diabetic complications; (2) History of malignant tumors; (3) Viral hepatitis, drug-induced hepatitis, or other severe liver diseases; (4) Severe renal diseases or a history of unexplained creatinine elevation; (5) Acute infections or other acute inflammatory diseases; (6) Severe cardiovascular and cerebrovascular diseases such as coronary heart diseases, heart failure, and stroke; (7) Hyperthyroidism, hypothyroidism, or any other endocrine abnormalities excluding diabetes mellitus; (8) Autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus; (9) Hemochromatosis or any other congenital liver metabolic diseases; and (10) Alcohol consumption > 30 g/d for men and > 20 g/d for women. A total of 329 T2DM patients were randomly enrolled. The procedure of subject selection is shown in Figure 1.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University, and each patient gave signed informed consent.
Basic information including age, course of diabetes mellitus, complicating diseases, and history of medication were obtained on the day of hospitalization, and body measurements were arranged in the next morning after an overnight fast. Each patient was required to complete measurement of height, weight, waist circumference (WC), and hip circumference (HC) with bare feet and underwear alone. BMI was calculated as weight divided by height squared (kg/m2), and waist to hip ratio (WHR) was calculated as WC divided by HC.
Blood samples from an antecubital vein were collected in a quiet state in the morning after an overnight fast (12 h). The blood samples were placed at room temperature for 30 min, and then partly centrifuged at 3500 r/min for 10 min to extract the serum. Glycosylated hemoglobin (HbA1c) was determined by high performance liquid chromatography (HLC-723G8; Tosoh Company, Japan). The analyses of biochemical parameters including fasting plasma glucose (FPG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), lipase, hydroxybutyrate dehydrogenase, alkaline phosphatase, lactate dehydrogenase (LDH), creatine kinase, γ-glutamyl transpeptidase (GGT), albumin (ALB), globulin, uric acid (UA), creatinine (Cr), total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and C-reactive protein (CRP) were completed with an automated bio
Each subject underwent MR mDIXON-Quant imaging with a Lonwin Magneto 1.5T superconducting magnetic resonance instrument. During examination, the patients were routinely scanned in the sagittal, coronal, and transverse planes, and the data of the axial mLIVE sequence were subsequently collected. The fraction of hepatic TG was presented on the abdominal image reconstructed with the in-built workstation. Specific measuring procedures are as follows. A section of hepatic parenchyma that was far from the edge of the liver and avoiding the large vessels or bile ducts was chosen as the region of interest (ROI). The average HFF of ROI was automatically demonstrated. The average HFF of three ROIs randomly selected from the left, middle and right lobes was taken as the final HFF of each patient. Patients with HFF of 5.5%-10.0%, 10.1%-25.0%, and > 25.0% were diagnosed as having mild, moderate, and severe fatty liver, respectively.
Statistical analyses were performed using SPSS for Windows version 25.0. After normality testing of each continuous variable, variables with a normal distribution are presented as the mean ± SD, while variables with a skewed distribution are presented as median with interquartile range. There was no lack of research data, so during statistical analyses there was no need to deal with the missing values.
In the group comparison, variables with a normal distribution were compared by one-way analysis of variance, and then compared in pairs with the Fisher’s least significant difference test. Variables with a skewed distribution were processed by the logarithm transformation based on 10 to meet a normal distribution, and then compared by the methods above, while those still distributed askew after the logarithmic transformation were compared by the nonparametric Kruskal-Wallis test. Spearman correlation analysis was used to find out the associations between HFF and other variables. Multiple linear regression analysis was used to determine the linear correlations between HFF and other variables. Binary logistic analysis was used to explore the independent influencing factors for moderate to severe fatty liver in patients with T2DM. Before linear regression and logistic regression analyses, collinearity analysis was conducted to screen out variables with a variance inflation factor > 10, under which circumstance only one of the collinear variables would be selected for regression analysis. Univariate regression analysis was performed for variables that were significantly correlated with HFF in Spearman correlation analysis, and variables with P < 0.1 in the univariate regression analysis were further enrolled in the multivariate regression analysis.
All the reported P values were two-tailed, and only those < 0.05 were considered statistically significant.
We enrolled 329 T2DM patients with an average age of 53.19 ± 15.19 years. In accordance with the BMI classification standard for Chinese adults proposed by the Chinese Working Group on Obesity in 2001, the participants were stratified into the following groups: Normal weight group with a BMI of 18.5-23.9 kg/m2, overweight group with a BMI of 24.0-27.9 kg/m2, and obese group with a BMI ≥ 28.0 kg/m2. The normal weight group included 127 patients with a mean age of 54.72 ± 13.40 years, the overweight group included 123 patients with a mean age of 55.98 ± 15.03 years, and the obese group included 79 patients with a mean age of 46.41 ± 16.25 years. Comparisons of general statistics, metabolic parameters, hormones, and HFF among groups are shown in Table 1.
| Variable | Normal weight group | Overweight group | Obese group | P value |
| Cases | 127 | 123 | 79 | - |
| Age (yr) | 54.72 ± 13.40c | 55.98 ± 15.03c | 46.41 ± 16.25a,b | < 0.001 |
| Height (cm) | 164.66 ± 8.36c | 165.78 ± 8.09c | 169.32 ± 9.37a,b | 0.001 |
| Weight (cm) | 60.00 (13.00)b,c | 71.00 (10.00)a,c | 87.00 (16.00)a,b | < 0.001 |
| BMI (kg/mb) | 21.97 (2.63)b,c | 25.81 (1.80)a,c | 29.76 (3.47)a,b | < 0.001 |
| WC (cm) | 83.18 ± 7.81b,c | 92.43 ± 6.47a,c | 103.73 ± 9.20a,b | < 0.001 |
| HC (cm) | 91.52 ± 7.41b,c | 98.96 ± 6.51a,c | 106.89 ± 8.18a,b | < 0.001 |
| WHR | 0.91 (0.06)b,c | 0.95 (0.07)a,c | 0.97 (0.06)a,b | < 0.001 |
| HFF (%) | 7.02 (2.39)b,c | 8.51 (4.91)a,c | 13.03 (11.69)a,b | < 0.001 |
| FPG (mmol/L) | 6.42 (3.55)b,c | 7.59 (2.73)a,c | 8.44 (3.74)a,b | < 0.001 |
| HbA1c (%) | 9.64 ± 2.23 | 9.38 ± 2.10 | 9.48 ± 2.11 | 0.639 |
| FINS (uU/mL) | 9.55 (9.70)b,c | 11.80 (8.40)a | 12.10 (8.60)a | 0.014 |
| FCP (ng/mL) | 0.85 (0.90)b,c | 1.20 (1.10)b,c | 1.60 (1.50)a,b | < 0.001 |
| FGC (pg/mL) | 104.50 (51.90) | 96.50 (51.40) | 97.50 (54.90) | 0.239 |
| HOMA-IR | 2.62 (3.11)b, c | 4.05 (3.31)a | 4.57 (3.01)a | < 0.001 |
| ALT (U/L) | 18.80 (12.30)c | 22.10 (14.50)c | 36.80 (43.00)a, b | < 0.001 |
| AST (U/L) | 18.10 (6.40)c | 20.40 (8.70)c | 27.00 (22.30)a, b | < 0.001 |
| DBIL (mmol/L) | 3.90 (1.90) | 3.90 (2.50) | 4.50 (2.10) | 0.078 |
| IBIL (mmol/L) | 9.60 (4.70)c | 10.30 (6.00) | 10.60 (5.50)a | 0.018 |
| TBIL (mmol/L) | 13.30 (6.10)c | 14.10 (8.10) | 15.50 (8.20)a | 0.031 |
| LPS (U/L) | 30.20 (16.70)c | 29.60 (10.60)c | 27.10 (9.50)a,b | 0.045 |
| HBDH (U/L) | 30.20 (16.70) | 29.60 (10.60) | 27.10 (9.50) | 0.786 |
| ALP (U/L) | 82.70 (33.30) | 82.40 (34.30) | 81.40 (29.30) | 0.487 |
| LDH (U/L) | 165.00 (39.00)c | 169.00 (43.00) | 179.00 (53.00)a | 0.027 |
| CK (U/L) | 70.70 (44.40) | 75.50 (58.10) | 77.60 (51.70) | 0.230 |
| GGT (U/L) | 20.80 (17.50)b,c | 26.30 (33.10)a,c | 37.70 (39.60)a,b | < 0.001 |
| ALB (g/L) | 41.40 (5.30)c | 42.20 (3.90)c | 43.20 (4.00)a,b | 0.007 |
| GLB (g/L) | 25.70 (5.20) | 26.30 (4.60) | 26.50 (4.50) | 0.379 |
| A/G | 1.60 (0.40) | 1.60 (0.40) | 1.60 (0.30) | 0.206 |
| UA (μmol/L) | 280.00 (109.00)b,c | 334.00 (94.00)a,c | 384.00 (137.00)a,b | < 0.001 |
| Cr (μmol/L) | 56.00 (21.00)b | 61.00 (19.00)a | 59.00 (20.00) | 0.016 |
| TC (mmol/L) | 4.40 ± 0.85 | 4.58 ± 1.32 | 4.64 ± 1.18 | 0.273 |
| TG (mmol/L) | 1.06 (0.64)b, c | 1.45 (1.13)a,c | 1.86 (1.46)a,b | < 0.001 |
| LDL-C (mmol/L) | 2.46 (0.95)c | 2.66 (1.15) | 2.89 (1.05)a | 0.006 |
| HDL-C (mmol/L) | 1.31 (0.42)b,c | 1.14 (0.27)a | 1.12 (0.36)a | < 0.001 |
| CRP (mg/L) | 1.07 (2.60)b, c | 1.59 (2.30)a | 2.16 (3.00)a | < 0.001 |
| TSH (mIU/L) | 2.19 (1.58) | 2.50 (1.88) | 2.21 (1.60) | 0.127 |
| FT3 (pmol/L) | 2.42 ± 0.42 | 2.49 ± 0.48 | 2.56 ± 0.46 | 0.109 |
| FT4 (pmol/L) | 1.15 ± 0.29 | 1.12 ± 0.29 | 1.10 ± 0.26 | 0.463 |
In terms of anthropometric data, body weight, WC, HC, WHR, and HFF of T2DM patients gradually increased in the normal weight, overweight, and obese groups (P < 0.01). With regard to metabolic parameters and islet function, FPG increased while HbA1c remained stable with BMI rising among the three groups. FINS, FCP, and HOMA-IR in the normal weight group were significantly lower than those in the other groups (P < 0.05). FCP in the overweight group was significantly lower than that in the obese group (P < 0.01). There were no significant differences in FGC among the three groups (P > 0.05). ALT, AST, GGT, ALB, and UA were significantly higher in the obese group than in the other groups (P < 0.01), and GGT, UA, and Cr were significantly higher in the overweight group than in the normal weight group (P < 0.05). With respect to lipid profile, TG increased gradually in the normal weight, overweight, and obese groups (P < 0.01). HDL-C in the normal weight group was significantly higher than that in the other groups, while LDL-C was significantly lower than that in the obese group (P < 0.01). There were no significant differences in thyroid function among the three groups (P > 0.05).
Table 2 shows that the HFF of T2DM patients was negatively correlated with age and HDL-C (r = -0.136 and -0.251, P < 0.05), and positively correlated with BMI, WHR, FPG, FINS, FCP, HOMA-IR, ALT, AST, DBIL, IBIL, TBIL LDH, GGT, ALB, UA, TC, TG, LDL-C, CRP, and FT3 (r = 0.493, 0.227, 0.281, 0.133, 0.385, 0.247, 0.459, 0.385, 0.154, 0.251, 0.234, 0.126, 0.413, 0.217, 0.305, 0.193, 0.478, 0.269, 0.251, and 0.157, P < 0.05).
| Variable | r | P value |
| Age | 0.136 | 0.013 |
| BMI | 0.493 | < 0.001 |
| WHR | 0.227 | < 0.001 |
| FPG | 0.281 | < 0.001 |
| FINS | 0.133 | 0.016 |
| FCP | 0.385 | < 0.001 |
| HOMA-IR | 0.247 | < 0.001 |
| UA | 0.305 | < 0.001 |
| ALT | 0.459 | < 0.001 |
| AST | 0.385 | < 0.001 |
| DBIL | 0.154 | 0.005 |
| IBIL | 0.251 | < 0.001 |
| TBIL | 0.234 | < 0.001 |
| LDH | 0.126 | 0.022 |
| GGT | 0.413 | < 0.001 |
| ALB | 0.217 | < 0.001 |
| TC | 0.193 | < 0.001 |
| TG | 0.478 | < 0.001 |
| LDL-C | 0.269 | < 0.001 |
| HDL-C | -0.251 | < 0.001 |
| CRP | 0.251 | < 0.001 |
| FT3 | 0.157 | 0.005 |
Multivariate linear regression analysis showed that ALT (β = 0.437, P < 0.001), BMI (β = 0.246, P < 0.001), LDL-C (β = 0.121, P = 0.007), UA (β = 0.108, P = 0.025), and ALB (β = 0.091, P = 0.039) had significant positive linear correlations with HFF in T2DM patients (Table 3).
| Variable | B | SE | β | t | P value | 95%CI |
| ALT | 0.080 | 0.008 | 0.437 | 9.455 | < 0.001 | 0.063-0.096 |
| BMI | 0.409 | 0.083 | 0.246 | 4.925 | < 0.001 | 0.246-0.573 |
| LDL-C | 1.040 | 0.380 | 0.121 | 2.737 | 0.007 | 0.292-1.788 |
| UA | 0.008 | 0.003 | 0.108 | 2.253 | 0.025 | 0.001-0.014 |
| ALB | 0.135 | 0.065 | 0.091 | 2.070 | 0.039 | 0.007-0.263 |
Among 329 T2DM patients, 97 were diagnosed with moderate fatty liver, and 21 with severe fatty liver. In multivariate binary logistic regression analysis, increased BMI was a significant independent risk factor for severer hepatic steatosis. The odds ratio (OR) of moderate to severe fatty liver was 2.29 times higher in overweight patients and 5.03 times higher in obese patients than in patients with normal weight (P < 0.05). Meanwhile, increased ALT (OR = 1.029, P < 0.001), ALB (OR = 1.105, P = 0.006), and LDL-C (OR = 1.710, P = 0.004) were other independent risk factors for moderate to severe fatty liver in T2DM patients (Table 4).
| Variable | B | SE | Wald | P value | OR | 95%CI | |
| BMI | < 24 | ||||||
| 24-27.99 | 0.830 | 0.329 | 6.361 | 0.012 | 2.294 | 1.203-4.373 | |
| ≥ 28 | 1.615 | 0.379 | 18.121 | < 0.001 | 5.027 | 2.390-10.572 | |
| ALT | 0.028 | 0.007 | 15.643 | < 0.001 | 1.029 | 1.014-1.043 | |
| ALB | 0.099 | 0.036 | 7.480 | 0.006 | 1.105 | 1.029-1.186 | |
| LDL-C | 0.537 | 0.185 | 8.389 | 0.004 | 1.710 | 1.189-2.459 |
Among the spectrum of NAFLD, although most patients with non-alcoholic fatty liver (NAFL) are at a low risk of adverse prognosis[9], about 25% of them may progress to nonalcoholic steatohepatitis (NASH)[10], in which case, patients are more likely to develop liver fibrosis, cirrhosis, failure, or even carcinoma[11]. Meanwhile, the ectopic accumulation of fat in the liver leads to increasing gluconeogenesis, decreasing glycogen synthesis, and inhibition of the insulin signaling pathway[12], and finally destroys the homeostasis of glucose metabolism. The increase in hepatic fat content is closely related to the progressive pre-diabetic phase in people without diabetes[13]. Furthermore, glucose metabolic disorder and complications of cardiovascular, retinal, renal, and neurological disorders are more severe in T2DM patients with NAFLD[14]. Therefore, screening and evaluation of NAFL in T2DM patients may help clinical physicians to manage the intensity of metabolic treatment, formulate treatment plan, monitor disease progression, and prevent or delay the development of chronic complications.
In the present study, MR mDIXON-Quant imaging was used to precisely quantify the hepatic fat content in T2DM patients. We found that the HFF of T2DM patients gradually increased in the normal weight, overweight, and obese groups. We also found that lipid metabolism disorder and insulin resistance deteriorated in obese T2DM patients. The multivariate linear regression analysis showed that HFF had a significantly positive linear correlation with BMI, and the binary logistic regression analysis showed that overweight and obesity increased the risk of moderate to severe fatty liver by 2.29 times and 5.03 times, respectively, in T2DM patients. All the above suggest that the increase in BMI played an important role in the aggravation of hepatic fat accumulation, which is consistent with the opinion of obesity serving as a significant risk factor for NAFLD in previous studies. In a retrospective longitudinal study including 4.12 million metabolically healthy people in the UK, the overweight and obese populations had a 3.3 and 6.9 times higher risk of developing NAFLD, respectively, than those with normal weight[15]. Another cohort study including 77425 metabolically healthy individuals in South Korea found a dose-effect relationship between baseline BMI and incidence of NAFLD in both sexes[16]. In the classic double-hit theory, the insulin resistance of adipose tissues leads to imbalance of lipolysis, resulting in excess free fatty acids being transported to the liver[17]. Under normal circumstances, the free fatty acids in the liver are either involved in mitochondrial β-oxidation or esterified to synthesize TG[18]. When the synthesis of TG overbalances its release in the form of very low-density lipoprotein, the rest forms lipid droplets in hepatocytes, which is the first hit in the development of NAFLD. The molecular changes caused by hepatic steatosis may make the liver vulnerable to the second hit, which usually means oxidative stress and cytokine-mediated liver injury[7]. Compared with obese people with normal glucose metabolism, the insulin resistance deteriorates in obese T2DM patients. As a result, strict and precise weight mana
We also found that ALT, ALB, LDL-C, and UA had significantly positive linear correlations with HFF. In binary logistic regression analysis, higher ALT, ALB, and LDL-C were also independent risk factors for moderate to severe fatty liver in T2DM patients. ALT is rich in the hepatic cytoplasm, which is most commonly used to indicate hepatic inflammation and injury, and in some cases is considered to reflect the severity of hepatic fat deposition[19]. Although ALT is not sufficient to serve as a biomarker for the diagnosis of NAFLD and NASH, it may serve as a clinical reference for the severity of NAFLD. ALB is a good indicator of nutritional status in the cli
UA is considered a natural scavenger of free radicals derived from nitrite peroxides in healthy people[24], while when exposed to metabolic disorders, it may convert into a strong oxidant and induce the genesis of reactive oxygen species[25], which exacerbates hepatic steatosis. Moreover, UA can stimulate the synthesis of pro-inflammatory cytokines including interleukin (IL)-1, IL-6, and tumor necrosis factor-α[26], and further stimulate the production of CRP in the liver[27]. Therefore, UA may associate with hepatic fat accumulation by inducing oxidative stress and chronic low-grade inflammation. A domestic cross-sectional study including 183903 people in China who underwent physical examinations found that the prevalence of NAFLD in the non-obese population increased with LDL-C quartiles[28]. In a further cohort study of 16173 people, the cumulative incidence of NAFLD gradually increased with LDL-C quartiles, and the LDL-C level at the upper limit of normal range predicted the risk of developing NAFLD in non-obese individuals[28]. Secretion of very low-density lipoprotein into the circulation increases with synthesis of TG in liver, in which case the proportion of small dense LDL particles also increases in the bloodstream[29]. However, the inferences above are not sufficient to support the predictive influence of LDL-C on the development of NAFLD. In future, more research is needed to determine the relationship between them.
In addition, although insulin resistance is considered a basic characteristic pro
Among the various diagnostic methods for NAFLD, liver biopsy is always a golden standard for quantifying hepatic steatosis. While the pathological features are distributed diffusely in most chronic liver diseases, liver biopsy only assesses 1/50000 of the hepatic parenchyma, which means inevitable sampling differences[33]. Meanwhile, the risks and technical requirements of the invasive procedure limit the clinical application of liver biopsy. Ultrasound examination is the most commonly applied imaging technique for diagnosing NAFL. However, the diagnostic sensitivity decreases among NAFLD patients with HFF < 20%[34] and it is incapable of accurately evaluating the severity of hepatic steatosis in obese individuals. The diagnostic results of ultrasound examination depend on the accuracy of the instruments and professional skills of the operators, which results in poor repeatability. As a semi-quantitative technique for the measurement of NAFL, computed tomography has a high specificity for patients with moderate and severe fatty liver, although the risks of ionizing radiation make it inappropriate for long-term monitoring of NAFLD patients. Magnetic resonance spectroscopy (MRS) is commonly considered the most precise noninvasive method to quantify hepatic TG, but it is time-consuming and requires professional analysis of the results[35]. MR mDIXON-Quant imaging is a burgeoning technique for precisely measuring the hepatic liver content, which is non-invasive and rapid, and has a sensitivity of 95% and specificity of 100% in detecting hepatic steatosis[36]. It is reported that the quantification of hepatic TG by MR mDIXON-Quant imaging is highly correlated with that of MRS or histological analysis[37]. It avoids any invasive injury or radiation threat, takes a shorter period of time, has a high resolution, and diagnoses without any influence from the intensity of the magnetic field[35]. Above all, MR mDIXON-Quant imaging has high clinical value in the diagnosis and grading of NAFL, monitoring of NAFLD progression, and evaluation of treatment and prognosis, and might play an important role in future NAFLD research.
The limitations of this study included the small number of study patients. Therefore, further studies with larger numbers of patients are needed to verify our findings.
This study demonstrated that the severity of hepatic fat accumulation mainly increases with BMI. Higher BMI, ALT, ALB, and LDL-C are significant independent risk factors for moderate to severe fatty liver in T2DM patients. Meanwhile, the MR mDIXON-Quant imaging can precisely measure hepatic TG in T2DM patients, and is important for future research and clinical practice in patients with T2DM and NAFLD.
Non-alcoholic fatty liver disease (NAFLD), the most common hepatic chronic disease worldwide, has been considered to have a complex association with type 2 diabetes mellitus (T2DM). The existence of NAFLD disturbs the homeostasis of glucose metabolism, while patients with T2DM have a higher prevalence of hepatic steatosis, which is the origin of further adverse pathological changes.
In previous clinical studies focused on NAFLD, most diagnoses of hepatic steatosis were conducted using common imaging techniques such as ultrasound and computed tomography, which have limited diagnostic sensitivity and accuracy under certain circumstances. There is an urgent need for a rapid, non-invasive, and inexpensive technique to precisely quantify hepatic fat content and to grade the severity of hepatic steatosis for future NAFLD research.
We aimed to quantify the hepatic triglyceride (TG) content and then investigate the influencing factors for hepatic fat accumulation and the severity of fatty liver in T2DM patients.
A cross-sectional study was conducted at the department of endocrinology, including 329 hospitalized T2DM patients divided into three groups according to their body mass index (BMI). MR mDIXON-Quant imaging was applied to quantify the hepatic fat fraction (HFF) of each patient, and fasting blood samples were obtained for further biochemical analysis. HFF, anthropometric data, and laboratory characteristics were compared among the three groups. Spearman correlation analysis and linear regression analysis were carried out to determine the associations between HFF and other variables, and binary logistic regression analysis was carried out to find out the influencing factors for severer hepatic steatosis in T2DM patients.
The HFF of T2DM patients gradually increased in the normal weight (BMI of 18.5-23.9 kg/m2), overweight weight (BMI of 24.0-27.9 kg/m2), and obese groups (BMI ≥ 28.0 kg/m2). Spearman correlation analysis and multivariate linear regression analysis showed that BMI, alanine aminotransferase (ALT), albumin (ALB), low-density lipoprotein cholesterol (LDL-C), and uric acid had significant positive associations with HFF of T2DM patients. Multivariate binary logistic regression analysis showed that BMI, ALT, ALB, and LDL-C were independent risk factors for moderate to severe fatty liver in T2DM patients. Overweight and obesity increased the odds ratio of severer fatty liver by 2.29 times and 5.03 times, respectively, in T2DM patients.
We quantified hepatic TG content and graded the severity of hepatic steatosis by MR mDIXON-Quant imaging technique. We found that in T2DM patients, hepatic fat accumulation is significantly associated with increasing BMI, ALT, ALB, and LDL-C. Higher BMI, ALT, ALB, and LDL-C are independent risk factors for moderate to severe non-alcoholic fatty liver in T2DM patients.
Considering of the small size of subjects and the limitation of cross-sectional study, prospective studies with larger size of subjects are needed to verify our findings. However, we would like to recommend the MR mDIXON-Quant imaging as an excellent tool for the diagnosis and grading of non-alcoholic hepatic steatosis, which might play an important role in future research and clinical practice of NAFLD.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaewput W S-Editor: Liu M L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Chacko KR, Reinus J. Extrahepatic Complications of Nonalcoholic Fatty Liver Disease. Clin Liver Dis. 2016;20:387-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2138] [Article Influence: 213.8] [Reference Citation Analysis (0)] |
| 3. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 4. | Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 5. | Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, Shu SS, Chan AW, Yeung MW, Chan JC, Kong AP, Wong VW. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 6. | Bazick J, Donithan M, Neuschwander-Tetri BA, Kleiner D, Brunt EM, Wilson L, Doo E, Lavine J, Tonascia J, Loomba R. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care. 2015;38:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 7. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3125] [Article Influence: 115.7] [Reference Citation Analysis (36)] |
| 8. | Zhang Y, Wang C, Duanmu Y, Zhang C, Zhao W, Wang L, Cheng X, Veronese N, Guglielmi G. Comparison of CT and magnetic resonance mDIXON-Quant sequence in the diagnosis of mild hepatic steatosis. Br J Radiol. 2018;91:20170587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1236] [Article Influence: 123.6] [Reference Citation Analysis (0)] |
| 10. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1701] [Article Influence: 243.0] [Reference Citation Analysis (0)] |
| 11. | Pais R, Barritt AS 4th, Calmus Y, Scatton O, Runge T, Lebray P, Poynard T, Ratziu V, Conti F. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016;65:1245-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 326] [Article Influence: 36.2] [Reference Citation Analysis (1)] |
| 12. | Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345-32353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 985] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 13. | Kantartzis K, Machann J, Schick F, Fritsche A, Häring HU, Stefan N. The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia. 2010;53:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | The Branch of Endocrinology of the Chinese Medical Association. The consensus on the diagnosis and treatment of nonalcoholic fatty liver diseases and related metabolic disorders. Lingchuang Gandanbing Zazhi. 2018;34:2103-2108. [DOI] [Full Text] |
| 15. | Vusirikala A, Thomas T, Bhala N, Tahrani AA, Thomas GN, Nirantharakumar K. Impact of obesity and metabolic health status in the development of non-alcoholic fatty liver disease (NAFLD): A United Kingdom population-based cohort study using the health improvement network (THIN). BMC EndocrDisord. 2020;20:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Chang Y, Jung HS, Cho J, Zhang Y, Yun KE, Lazo M, Pastor-Barriuso R, Ahn J, Kim CW, Rampal S, Cainzos-Achirica M, Zhao D, Chung EC, Shin H, Guallar E, Ryu S. Metabolically Healthy Obesity and the Development of Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2016;111:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 17. | Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:1389-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 340] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 18. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 2908] [Article Influence: 415.4] [Reference Citation Analysis (1)] |
| 19. | Chen SC, Tsai SP, Jhao JY, Jiang WK, Tsao CK, Chang LY. Liver Fat, Hepatic Enzymes, Alkaline Phosphatase and the Risk of Incident Type 2 Diabetes: A Prospective Study of 132,377 Adults. Sci Rep. 2017;7:4649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 20. | Cabrerizo S, Cuadras D, Gomez-Busto F, Artaza-Artabe I, Marín-Ciancas F, Malafarina V. Serum albumin and health in older people: Review and meta analysis. Maturitas. 2015;81:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 348] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 21. | Lee HW, Kim BK, Kim SU, Park JY, Kim DY, Ahn SH, Kim KJ, Han KH. Prevalence and Predictors of Significant Fibrosis Among Subjects with Transient Elastography-Defined Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2017;62:2150-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Choi SH, Oh DJ, Kwon KH, Lee JK, Koh MS, Lee JH, Kang HW. A vegetarian diet does not protect against nonalcoholic fatty liver disease (NAFLD): A cross-sectional study between Buddhist priests and the general population. Turk J Gastroenterol. 2015;26:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 416] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 24. | Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol. 2001;38:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 210] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Hayden MR, Tyagi SC. Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. NutrMetab (Lond). 2004;1:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 261] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 26. | Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 600] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 27. | Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2430] [Cited by in RCA: 2481] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 28. | Sun DQ, Wu SJ, Liu WY, Wang LR, Chen YR, Zhang DC, Braddock M, Shi KQ, Song D, Zheng MH. Association of low-density lipoprotein cholesterol within the normal range and NAFLD in the non-obese Chinese population: a cross-sectional and longitudinal study. BMJ Open. 2016;6:e013781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Lewis GF, Murdoch S, Uffelman K, Naples M, Szeto L, Albers A, Adeli K, Brunzell JD. Hepatic lipase mRNA, protein, and plasma enzyme activity is increased in the insulin-resistant, fructose-fed Syrian golden hamster and is partially normalized by the insulin sensitizer rosiglitazone. Diabetes. 2004;53:2893-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, Bass NM; Nonalcoholic Steatohepatitis Clinical Research Network. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 367] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 31. | Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A, Weber MH, Budd JT, Lupi ME, Cusi K. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65:1132-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (1)] |
| 32. | Bril F, Lomonaco R, Orsak B, Ortiz-Lopez C, Webb A, Tio F, Hecht J, Cusi K. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:2178-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T; LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1549] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 34. | Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 478] [Article Influence: 29.9] [Reference Citation Analysis (3)] |
| 35. | Reeder SB, Sirlin CB. Quantification of liver fat with magnetic resonance imaging. MagnReson Imaging Clin N Am. 2010;18:337-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 36. | Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, Hassanein T, Patton HM, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 2009;251:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 271] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 37. | Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, Bettencourt R, Changchien C, Brenner DA, Sirlin C, Loomba R. Utility of magnetic resonance imaging vs histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 438] [Article Influence: 36.5] [Reference Citation Analysis (0)] |