Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7614
Peer-review started: March 16, 2021
First decision: April 24, 2021
Revised: May 12, 2021
Accepted: August 17, 2021
Article in press: August 17, 2021
Published online: September 16, 2021
Processing time: 177 Days and 16.9 Hours
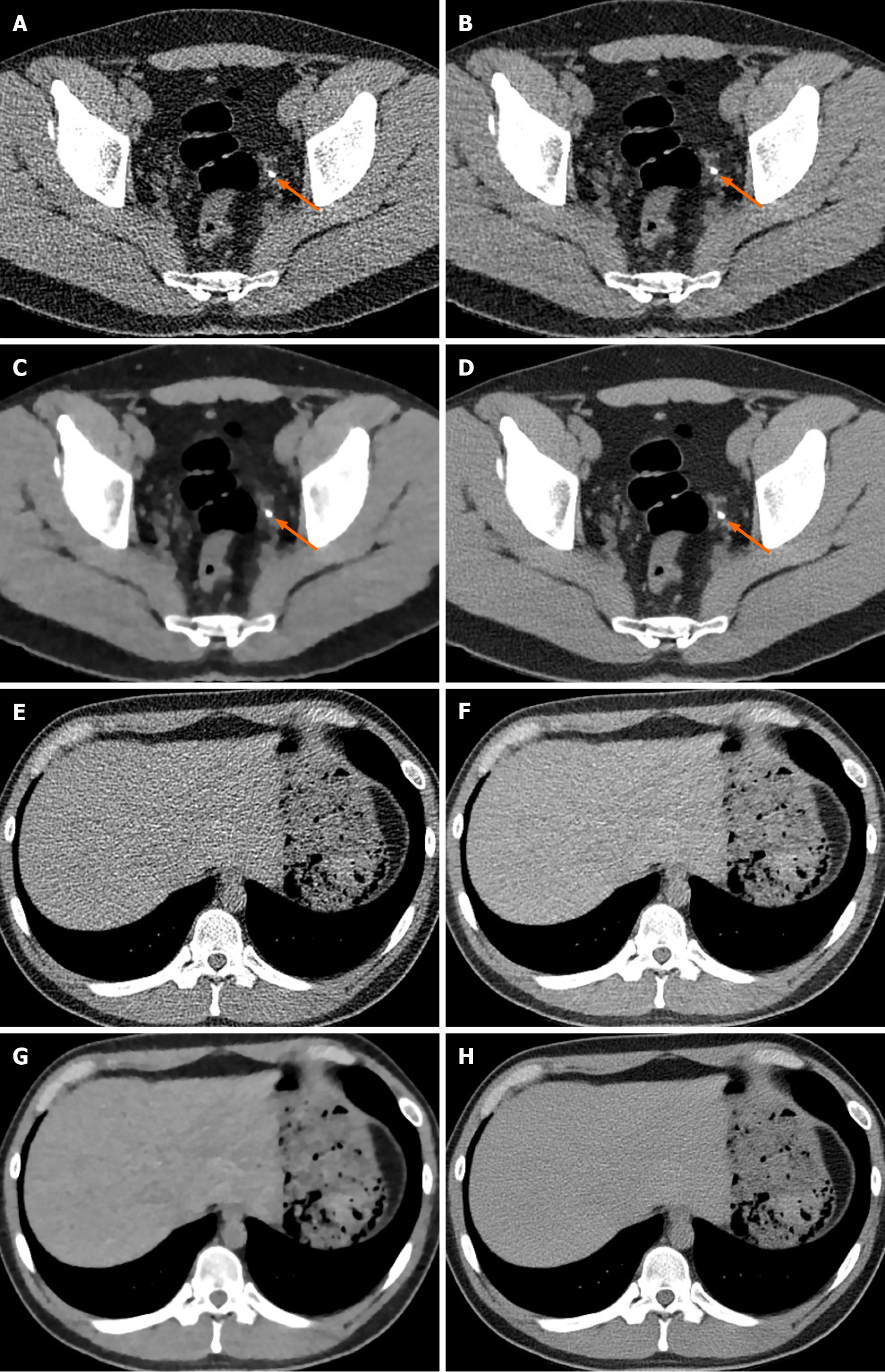
Computed tomography (CT) has seen a rapid increase in use in recent years. Radiation from CT accounts for a significant proportion of total medical radiation. However, given the known harmful impact of radiation exposure to the human body, the excessive use of CT in medical environments raises concerns. Concerns over increasing CT use and its associated radiation burden have prompted efforts to reduce radiation dose during the procedure. Therefore, low-dose CT has attracted major attention in the radiology, since CT-associated x-ray radiation carries health risks for patients. The reduction of the CT radiation dose, however, compromises the signal-to-noise ratio, which affects image quality and diagnostic performance. Therefore, several denoising methods have been developed and applied to image processing technologies with the goal of reducing image noise. Recently, deep learning applications that improve image quality by reducing the noise and artifacts have become commercially available for diagnostic imaging. Deep learning image reconstruction shows great potential as an advanced re
Core Tip: Early application of deep learning techniques have shown success in the denoising of computed tomography (CT) images, especially low-dose CT images, and future advances are expected to provide additional benefit.
- Citation: Park SB. Advances in deep learning for computed tomography denoising. World J Clin Cases 2021; 9(26): 7614-7619
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7614.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7614
In radiography, decreases in radiation dosage result in the debasement of image quality, basically due to an increment in image noise[1]. Increased image noise can compromise the diagnostic performance of computed tomography (CT) images. Hen
CT has seen a rapid increase in use in recent years[2,7,8]. Radiation from CT accounts for a significant proportion of total medical radiation. However, given the known harmful impact of radiation exposure to the human body, the excessive use of CT in medical environments raises concerns[7]. Concerns over increasing CT use and its associated radiation burden have prompted efforts to reduce radiation dose during the procedure[9-11].
Recently, LDCT has attracted significant interest in the radiography community[8,12,13]. Several approaches can be used to reduce radiation exposure as follows: avoiding unnecessary examinations and superfluous acquisitions; optimizing CT acquisition parameters (i.e., lowering tube voltage or current and pitch); routinely using size adaptation techniques, such as automatic tube current modulation; and progressing the postprocessing and reconstruction of CT images[2]. In any case, the diminishment of radiation dosage increases noise and presents artifacts in reconstructed images[7,8]. In other words, reductions in radiation dose lead to decreases in image quality, which may adversely affect diagnosis using LDCT images[1,8]. Therefore, much exertion has been made to plan better image processing techniques that can further diminish image noise after image capture[1].
Image noise reduction, generally called “image denoising,” is an important but cha
Noise reduction algorithms for LDCT can be categorized into three types: (1) Handling the raw data gotten from sinogram (projection space denoising); (2) Iterative reconstruction (IR) strategies; and (3) Handling reconstructed CT image (image space denoising)[2,8,13].
In projection space denoising, the noise expulsion algorithm is connected to the CT sinogram information gotten from low-dose CT. These strategies join system physics and photon statistics to diminish both image noise and artifacts. In any case, this process uses an algorithm supplied by the vendor. These strategies too require get to sinogram information, which isn’t accessible for commercial CT scanners. At last, these procedures ought to be actualized within the scanner reconstruction system, which increments the taken a toll of denoising[2].
IR strategies refer to another group of techniques to make strides the image quality of LDCT. For the past 30 years, filtered back-projection (FBP) has been the prevailing strategy of reconstruction due to its computational proficiency and precision. FBP requires noteworthy amounts of high-quality projection data to get precise recon
In contrast to these first two strategies, image space denoising algorithms don’t require raw projection information. They work straightforwardly on the reconstructed CT images and are by and large quick, free of the scanner vendor and can be effe
Recently, promising results in low-dose CT denoising have been achieved using DL-based algorithms, especially convolutional neural networks (CNNs) and generative adversarial network architectures[7,8]. With the rapid development of CNNs, de
Currently, two available vendor-specific DLIR technologies, TrueFidelity (GE Heal
ClariCT (ClariPi, Seoul, South Korea) is based on the CNN algorithm noise re
The crossover algorithm in ClariCT is completed taking after a special handle. The primary stage is forward projection of CT image from FBP to form a synthesized sinogram, which is similar to other crossover reconstruction technologies. The ge
Although DLIR algorithms appear to be exceedingly compelling for moving forward image quality, there are a few issues or obstacles to be talked about. Firstly, further external validation of DLIR is vital. Secondly, actual radiation dose reduction in the clinical practice because of altering procurement parameters whereas perfor
The use of DLIR methods employing deep CNNs has been proposed to encourage dose reduction whereas keeping up the image quality and diagnostic performance of CT imaging.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karavaş E S-Editor: Ma YJ L-Editor: A P-Editor: Yuan YY
| 1. | Hong JH, Park EA, Lee W, Ahn C, Kim JH. Incremental Image Noise Reduction in Coronary CT Angiography Using a Deep Learning-Based Technique with Iterative Reconstruction. Korean J Radiol. 2020;21:1165-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Ehman EC, Yu L, Manduca A, Hara AK, Shiung MM, Jondal D, Lake DS, Paden RG, Blezek DJ, Bruesewitz MR, McCollough CH, Hough DM, Fletcher JG. Methods for clinical evaluation of noise reduction techniques in abdominopelvic CT. Radiographics. 2014;34:849-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Chen H, Zhang Y, Zhang W, Liao P, Li K, Zhou J, Wang G. Low-dose CT via convolutional neural network. Biomed Opt Express. 2017;8:679-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 364] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 4. | Du W, Chen H, Wu Z, Sun H, Liao P, Zhang Y. Stacked competitive networks for noise reduction in low-dose CT. PLoS One. 2017;12:e0190069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Kang E, Min J, Ye JC. A deep convolutional neural network using directional wavelets for low-dose X-ray CT reconstruction. Med Phys. 2017;44:e360-e375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 352] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 6. | Chen H, Zhang Y, Kalra MK, Lin F, Chen Y, Liao P, Zhou J, Wang G. Low-Dose CT With a Residual Encoder-Decoder Convolutional Neural Network. IEEE Trans Med Imaging. 2017;36:2524-2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 710] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 7. | Lee D, Choi S, Kim HJ. High quality imaging from sparsely sampled computed tomography data with deep learning and wavelet transform in various domains. Med Phys. 2019;46:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Shan H, Zhang Y, Yang Q, Kruger U, Kalra MK, Sun L, Cong W, Wang G. 3-D Convolutional Encoder-Decoder Network for Low-Dose CT via Transfer Learning From a 2-D Trained Network. IEEE Trans Med Imaging. 2018;37:1522-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 9. | Kalra MK, Becker HC, Enterline DS, Lowry CR, Molvin LZ, Singh R, Rybicki FJ. Contrast Administration in CT: A Patient-Centric Approach. J Am Coll Radiol. 2019;16:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Singh R, Szczykutowicz TP, Homayounieh F, Vining R, Kanal K, Digumarthy SR, Kalra MK. Radiation Dose for Multiregion CT Protocols: Challenges and Limitations. AJR Am J Roentgenol. 2019;213:1100-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Singh R, Digumarthy SR, Muse VV, Kambadakone AR, Blake MA, Tabari A, Hoi Y, Akino N, Angel E, Madan R, Kalra MK. Image Quality and Lesion Detection on Deep Learning Reconstruction and Iterative Reconstruction of Submillisievert Chest and Abdominal CT. AJR Am J Roentgenol. 2020;214:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 12. | Park SB, Kim YS, Lee JB, Park HJ. Knowledge-based iterative model reconstruction (IMR) algorithm in ultralow-dose CT for evaluation of urolithiasis: evaluation of radiation dose reduction, image quality, and diagnostic performance. Abdom Imaging. 2015;40:3137-3146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Gholizadeh-Ansari M, Alirezaie J, Babyn P. Deep Learning for Low-Dose CT Denoising Using Perceptual Loss and Edge Detection Layer. J Digit Imaging. 2020;33:504-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Higaki T, Nakamura Y, Tatsugami F, Nakaura T, Awai K. Improvement of image quality at CT and MRI using deep learning. Jpn J Radiol. 2019;37:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Kim I, Kang H, Yoon HJ, Chung BM, Shin NY. Deep learning-based image reconstruction for brain CT: improved image quality compared with adaptive statistical iterative reconstruction-Veo (ASIR-V). Neuroradiology. 2021;63:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Kim JH, Yoon HJ, Lee E, Kim I, Cha YK, Bak SH. Validation of Deep-Learning Image Reconstruction for Low-Dose Chest Computed Tomography Scan: Emphasis on Image Quality and Noise. Korean J Radiol. 2021;22:131-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 17. | Padole A, Ali Khawaja RD, Kalra MK, Singh S. CT radiation dose and iterative reconstruction techniques. AJR Am J Roentgenol. 2015;204:W384-W392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 18. | Wolterink JM, Leiner T, Viergever MA, Isgum I. Generative Adversarial Networks for Noise Reduction in Low-Dose CT. IEEE Trans Med Imaging. 2017;36:2536-2545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 482] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 19. | Lee S, Choi YH, Cho YJ, Lee SB, Cheon JE, Kim WS, Ahn CK, Kim JH. Noise reduction approach in pediatric abdominal CT combining deep learning and dual-energy technique. Eur Radiol. 2021;31:2218-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Lim WH, Choi YH, Park JE, Cho YJ, Lee S, Cheon JE, Kim WS, Kim IO, Kim JH. Application of Vendor-Neutral Iterative Reconstruction Technique to Pediatric Abdominal Computed Tomography. Korean J Radiol. 2019;20:1358-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Arndt C, Güttler F, Heinrich A, Bürckenmeyer F, Diamantis I, Teichgräber U. Deep Learning CT Image Reconstruction in Clinical Practice. Rofo. 2021;193:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |