Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7551
Peer-review started: February 28, 2021
First decision: July 5, 2021
Revised: July 12, 2021
Accepted: July 19, 2021
Article in press: July 19, 2021
Published online: September 6, 2021
Processing time: 183 Days and 10.7 Hours
Polycythemia vera (PV) is a chronic myeloproliferative disorder characterized by an increase in red blood cells in the peripheral blood. Previous work has reported the occurrence of thrombosis or hemorrhage arising in the cerebral vasculature secondary to PV. However, hemorrhagic transformation after PV-associated acute ischemic stroke has not been previously described.
We herein present two cases of PV where hemorrhagic transformation occurred after an acute ischemic stroke. Case 1 was a 57-year-old woman with a history of hypertension who was admitted for left-sided weakness. Case 2 was a 68-year-old man who was admitted for a 10-d sudden left arm weakness. Imaging examinations for the two patients revealed hemorrhagic transformation after acute ischemic stroke. Both patients had JAK-2-V617F mutation and received antiplatelet therapy. Both of them had a good prognosis during the follow-up.
This report suggested that hemorrhagic transformation may occur in acute ischemic stroke caused by PV. Antiplatelet drugs do not seem to influence the long-term outcomes in such patients. Future research should focus on establishing a standard antiplatelet treatment strategy for this condition.
Core Tip: Polycythemia vera (PV) is a rare, myeloproliferative disorder. Ischemic stroke is the most frequent neurologic manifestation of PV. However, hemorrhagic transformation after acute ischemic stroke caused by PV has not been previously reported. Here, we present two patients with PV who developed hemorrhagic transformation after sustaining an acute ischemic stroke. Antiplatelet drugs did not lead to poor long-term outcomes in our patients.
- Citation: Cao YY, Cao J, Bi ZJ, Xu SB, Liu CC. Hemorrhagic transformation after acute ischemic stroke caused by polycythemia vera: Report of two case. World J Clin Cases 2021; 9(25): 7551-7557
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7551.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7551
Polycythemia vera (PV) is a rare, myeloproliferative disorder characterized by the overproduction of erythrocytes, granulocytes, and megakaryocytes. It has been widely recognized that PV increases the incidence of thrombotic events, especially cerebral infarctions and transient ischemic attacks. Previous studies have revealed that ischemic stroke is the most frequent neurologic manifestation of PV[1]. Although not as common as thrombosis, hemorrhagic stroke has also been associated with PV[2,3]. To our knowledge, hemorrhagic transformation after acute ischemic stroke caused by PV has not been previously reported. Here, we present two patients with PV who developed hemorrhagic transformation after sustaining an acute ischemic stroke.
Case 1: A 57-year-old woman presented with a chief complaint of left-side weakness for 5 d (Supplementary Figure 1).
Case 2: A 68-year-old man was admitted to our hospital with left arm weakness for 10 d (Supplementary Figure 2).
Case 1: The patient had hypertension for 3 years.
Case 2: The patient was a smoker with an otherwise unremarkable medical history.
Case 1: The patient had hypertension for 3 years.
Case 2: The patient was a smoker with an otherwise unremarkable medical history.
Case 1: The patient had hypertension for 3 years.
Case 2: The patient was a smoker with an otherwise unremarkable medical history.
Case 1: On admission, the patient’s blood pressure was 154/93 mmHg. Physical examination revealed obvious redness on her face and hands, as well as left hemiplegia with a muscle strength grade of 3/5.
Case 2: Neurological examination showed the patient to be healthy, although he experienced left hemiplegia of 0/5 as assessed by the manual muscle test.
Case 1: Laboratory testing revealed a leukocyte level of 9.3 × 1012/L, hemoglobin level of 19.8 g/dL, and hematocrit of 66.7%. Inflammation, coagulation, and autoimmunity markers were all negative. In addition, an ultrasound examination of her lower extremities detected left intermuscular venous thrombosis. Abdominal ultrasound revealed mild splenomegaly (spleen thickness: 43 mm). Bone marrow biopsy and positive JAK-2-V617F mutation tests provided strong evidence for PV.
Case 2: Routine blood testing highlighted that the patient possessed an erythrocyte count of 10.6 × 1012/L, hematocrit of 71.7%, hemoglobin 22 g/dL, and platelet count of 794 × 109/L. Other blood sample tests revealed no obvious abnormalities. Abdominal ultrasound revealed splenomegaly (spleen thickness: 46 mm). A diagnosis of PV was suspected and a subsequent bone marrow biopsy and a positive JAK-2-V617F mutation confirmed this diagnosis.
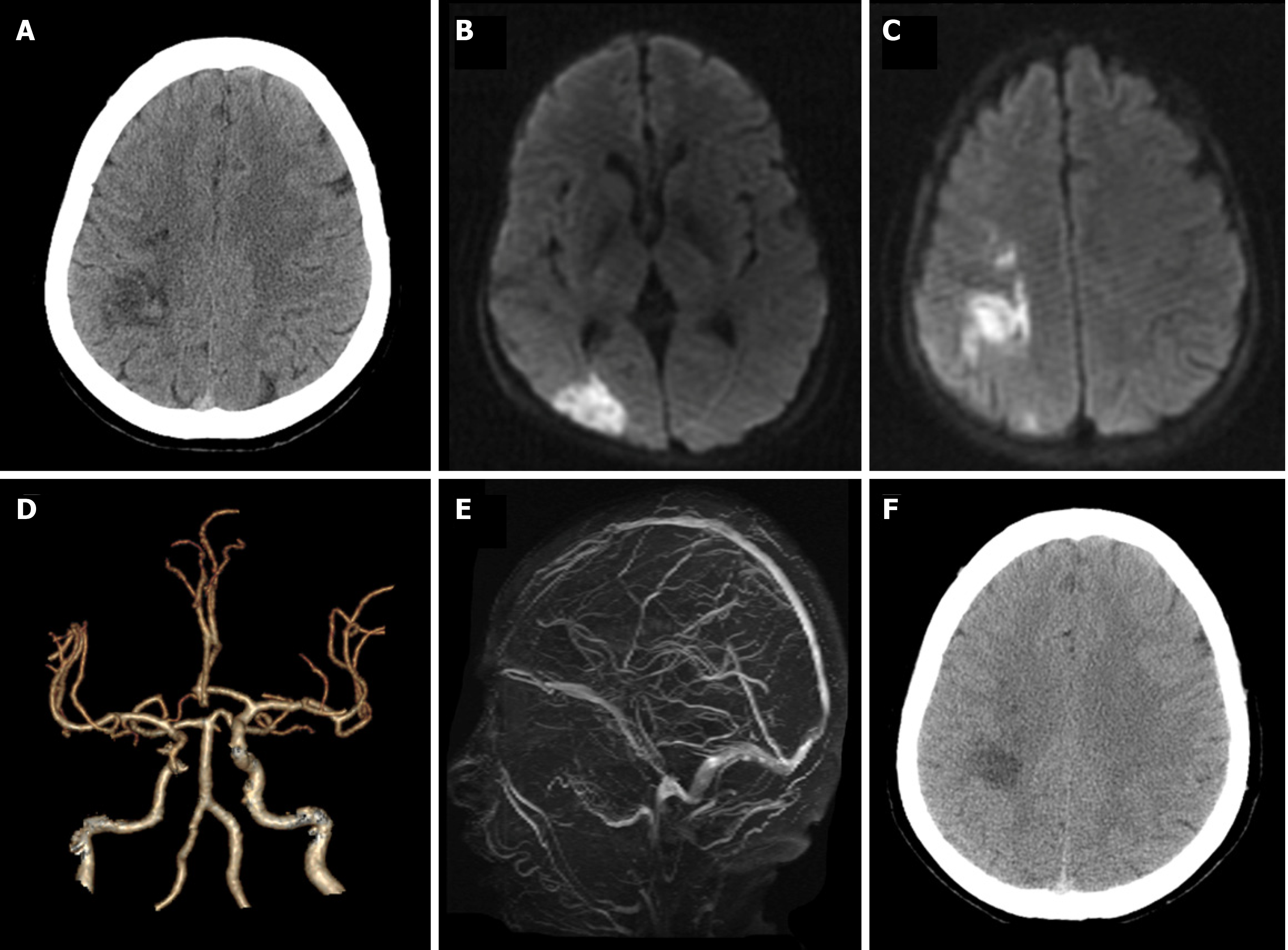
Case 1: Brain computed tomography (CT) showed patchy high-density changes in right parietal low-density lesions (Figure 1A). Magnetic resonance imaging confirmed an acute cerebral infarct in the right parietal and occipital lobes with restricted diffusion (Figure 1B and C). CT-angiography imaging revealed only mild atherosclerosis (Figure 1D). A magnetic resonance venogram (MRV) was unremarkable (Figure 1E).
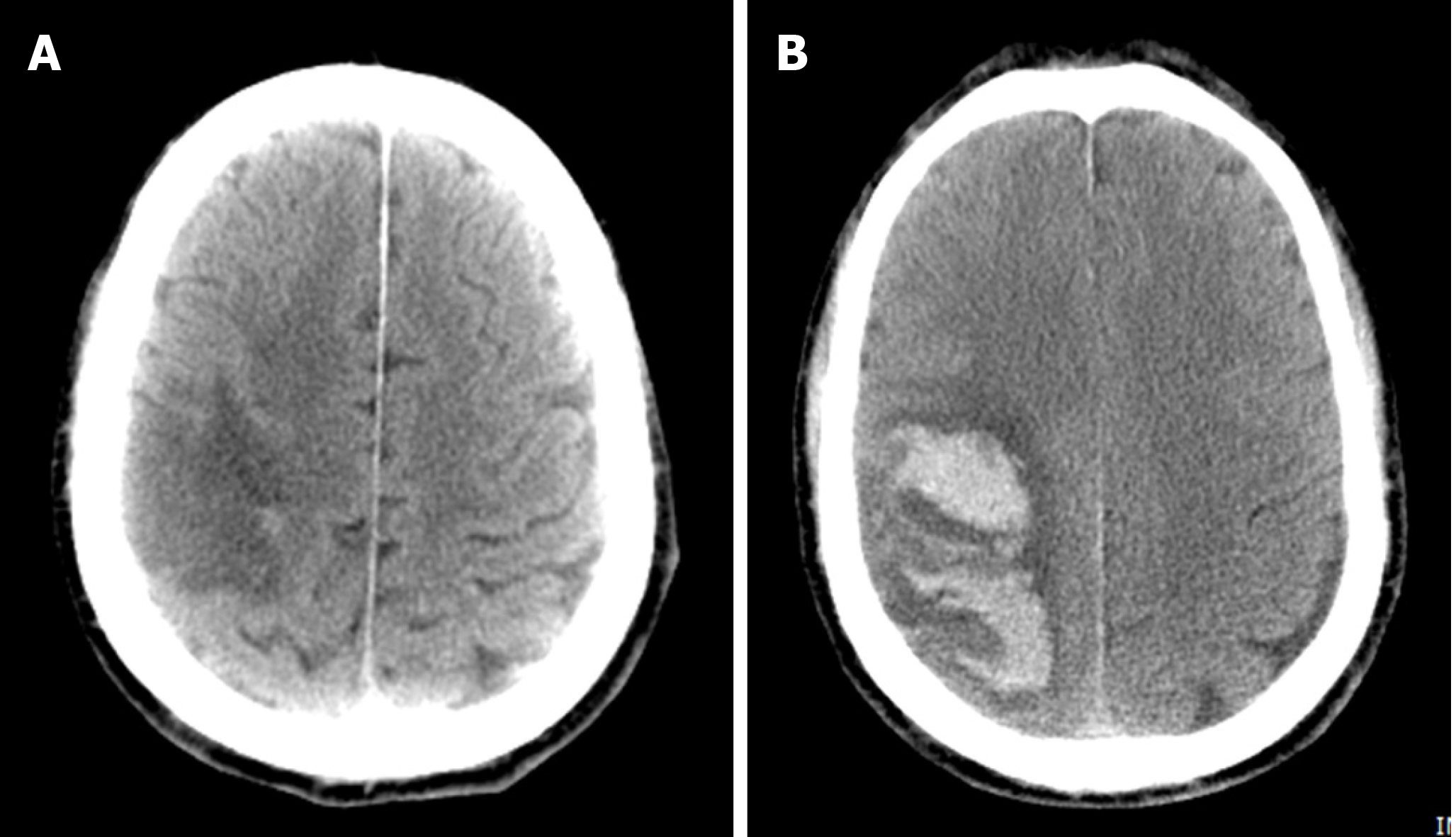
Case 2: On admission, brain CT revealed infarctions in the right parietal and temporal lobes (Figure 2A). Three days later, a brain CT re-examination revealed an infarct lesion in the right cerebral hemisphere with a right parietal hematoma (Figure 2B). Brain magnetic resonance angiography, MRV, cardiac ultrasonography, and 24-h dynamic electrocardiogram revealed no obvious abnormalities.
Case 1: The patient was diagnosed with hemorrhagic infarction in addition to PV.
Case 2: The presence of post-ischemic stroke parenchymal hematomas was concluded to be derived from the patient’s PV.
Case 1: The patient received hydroxyurea and hydration treatment, as well as antip
Case 2: The patient was consequently given antiplatelet therapeutics, hydration, and cytoreductive therapy. However, 3 d later, the patient suffered a hemiplegia deterioration, accompanied by left-side weakness and drowsiness.
Case 1: At the 6-mo follow-up, the patient retained slight weakness and mild disability (modified Rankin Scale score, 1). She was stable in hematology and neurology outpatient follow-ups without any recurrence of vascular complications.
Case 2: Three months after disease onset, the patient improved to ambulatory care and was provided a brace (modified Rankin Scale score, 3). He was referred for hematology outpatient monitoring with chemotherapy with hydroxyurea follow-ups. To prevent secondary strokes, a regimen of low-dose aspirin (100 mg daily) was reinitiated 6 mo after symptom onset. Currently, he is recovering well with aspirin and hydroxyurea.
Stroke is a prevalent disorder in neurology clinics. Its common causes include atherosclerosis, cardiogenic embolism, and small vessel disease among others. By contrast, hematological diseases rarely underly a stroke, accounting for less than 1% of cases[4,5]. PV, as a chronic hematological disorder, is typically associated with an increased incidence of stroke[1,6]. Our cases further suggest that in PV patients, hemorrhagic transformation can occur after an acute ischemic stroke, a clinical manifestation that has yet to be reported.
Ischemic stroke is considered to be the first presenting symptom in more than 15% of PV patients[4]. The peak incidence of PV occurs between 50–70 years of age, and these ages coincide with a high incidence of stroke[7]. However, the etiology behind increased thrombotic events in PV patients is not fully understood. The mechanisms are likely multifactorial. First, increased hematocrit and blood viscosity may decrease cerebral blood flow and form a prothrombotic state for patients with PV[1]. Second, JAK2 V617F mutations, which occur in 95% of PV cases[8], cause endothelial damage to systematic vessels, and confer a differentiation advantage towards megakaryocytes, thereby increasing intrinsic platelet reactivity[9,10]. Activated platelets will adhere and aggregate on ruptured plaques, resulting in thrombosis formation. Coincidingly, endothelial dysfunction in vessels facilitates leukocyte migration, which in turn triggers an inflammatory cascade in a similar way to atherosclerotic lesions[11]. Moreover, malignant cells can release cytokines and other mediators and provoke an inflammatory response in the vascular endothelial cells. Third, an increase in erythrocytes could obstruct small vessels and form an embolic infarct in the brain[12]. Consistent with this mechanism, microembolic signals on transcranial doppler sonography have been detected in patients with PV, indicative of cardiac embolism[2]. Finally, the hemodynamic infarct is common in PV. Previous work has shown that erythrocytosis decreases plasma volume and leads to a relative thrombocytosis, consequently accelerating thrombogenesis to form a hemodynamic infarct[13].
Hemorrhagic transformation is a frequent complication of acute ischemic stroke, especially after tissue-plasminogen activator intravenous thrombolysis or mechanical thrombectomy[14]. Moreover, massive cerebral infarction and cardioembolic stroke are associated with an increased risk of hemorrhagic transformation[15,16]. The mechanism behind hemorrhage in PV patients, an unusual presentation, has rarely been explored.
Consistent across all causes of ischemic stroke, and present in our first case, infarction is always accompanied by brain edema, which leads to compression of the peripheral vasculature. Increased permeability of the vascular wall caused by vascular compression enhances the risk of hemorrhagic transformation[17]. Furthermore, PV increases blood volume, and this, in turn, brings high pressure to the vessel wall, resulting in vessel overfilling and microaneurysm formation[18]. Once vessel rupture and microaneurysms occur, hemorrhage may follow. Moreover, spontaneous hemorrhages in PV patients may be also associated with platelet dysfunction caused by abnormal proliferation of bone marrow cells[19]. In our second case, we further speculate a role for antiplatelet therapy in causing hemorrhage. However, although neither of our cases developed cerebral venous thrombosis, we cannot exclude that impaired venous drainage, caused by PV, may have also contributed to hemorrhagic infarction[20].
There is a high risk of thrombotic events in PV patients[21]. Antiplatelet therapy can be useful for decreasing the risk of thrombosis and related morbidities. The European Collaboration on Low-Dose Aspirin in PV study confirmed that low-dose aspirin can safely prevent thrombotic complications in PV patients without contraindications[22]. A meta-analysis also suggested that the use of low-dose aspirin in patients with PV is associated with a reduced risk of all-cause mortality, and does not increase the risk of bleeding[23]. For both of our cases, aspirin was also the main preventive treatment for long-term adverse vascular events. For our patients, hemorrhagic transformation as a contraindication existed in the acute phase of stroke. After the resolution of the hemorrhage, reinitiating antiplatelet drug did not overtly cause long-term negative outcomes. Future research should focus on the treatment of this condition.
Our case report suggests that hemorrhagic transformation can occur following acute ischemic stroke in PV patients. Antiplatelet drugs do not lead to poor long-term outcomes in such patients.
We are grateful to the patients for their contributions to the study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Belen Fernandez A, Eyituoyo HO S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li JH
| 1. | Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood. 2002;100:4272-4290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 298] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, Marilus R, Villegas A, Tognoni G, Barbui T. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 516] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 3. | Chen L, Xiao H, Hu Z. Cerebral Hemorrhage of a 50-Year-Old Female Patient with Polycythemia Vera. J Stroke Cerebrovasc Dis. 2019;28:e110-e112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Hart RG, Kanter MC. Hematologic disorders and ischemic stroke. A selective review. Stroke. 1990;21:1111-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 129] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Ferro JM, Infante J. Cerebrovascular manifestations in hematological diseases: an update. J Neurol. 2021;268:3480-3492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Frederiksen H, Szépligeti S, Bak M, Ghanima W, Hasselbalch HC, Christiansen CF. Vascular Diseases In Patients With Chronic Myeloproliferative Neoplasms - Impact Of Comorbidity. Clin Epidemiol. 2019;11:955-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Zoraster RM, Rison RA. Acute embolic cerebral ischemia as an initial presentation of polycythemia vera: a case report. J Med Case Rep. 2013;7:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Kilpivaara O, Levine RL. JAK2 and MPL mutations in myeloproliferative neoplasms: discovery and science. Leukemia. 2008;22:1813-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Falanga A, Marchetti M, Evangelista V, Vignoli A, Licini M, Balicco M, Manarini S, Finazzi G, Cerletti C, Barbui T. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood. 2000;96:4261-4266. [PubMed] |
| 10. | Fleischman AG, Tyner JW. Causal role for JAK2 V617F in thrombosis. Blood. 2013;122:3705-3706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Hobbs CM, Manning H, Bennett C, Vasquez L, Severin S, Brain L, Mazharian A, Guerrero JA, Li J, Soranzo N, Green AR, Watson SP, Ghevaert C. JAK2V617F leads to intrinsic changes in platelet formation and reactivity in a knock-in mouse model of essential thrombocythemia. Blood. 2013;122:3787-3797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Fiermonte G, Aloe Spiriti MA, Latagliata R, Petti MC, Giacomini P. Polycythaemia vera and cerebral blood flow: a preliminary study with transcranial Doppler. J Intern Med. 1993;234:599-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Harrison MJ. Influence of haematocrit in the cerebral circulation. Cerebrovasc Brain Metab Rev. 1989;1:55-67. [PubMed] |
| 14. | Wang W, Li M, Chen Q, Wang J. Hemorrhagic Transformation after Tissue Plasminogen Activator Reperfusion Therapy for Ischemic Stroke: Mechanisms, Models, and Biomarkers. Mol Neurobiol. 2015;52:1572-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Hornig CR, Bauer T, Simon C, Trittmacher S, Dorndorf W. Hemorrhagic transformation in cardioembolic cerebral infarction. Stroke. 1993;24:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenillas JF, Alvarez-Sabín J. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Michiels JJ, Berneman Z, Schroyens W, Koudstaal PJ, Lindemans J, Neumann HA, van Vliet HH. Platelet-mediated erythromelalgic, cerebral, ocular and coronary microvascular ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemia vera: a distinct aspirin-responsive and coumadin-resistant arterial thrombophilia. Platelets. 2006;17:528-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | McMahon B, Stein BL. Thrombotic and bleeding complications in classical myeloproliferative neoplasms. Semin Thromb Hemost. 2013;39:101-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Garaci FG, Gandini R, Romagnoli A, Fasoli F, Varrucciu V, Simonetti G. Hepatic artery pseudoaneurysm in von Willebrand's disease. Eur Radiol. 2003;13:1913-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Martin K. Risk Factors for and Management of MPN-Associated Bleeding and Thrombosis. Curr Hematol Malig Rep. 2017;12:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, De Stefano V, Elli E, Iurlo A, Latagliata R, Lunghi F, Lunghi M, Marfisi RM, Musto P, Masciulli A, Musolino C, Cascavilla N, Quarta G, Randi ML, Rapezzi D, Ruggeri M, Rumi E, Scortechini AR, Santini S, Scarano M, Siragusa S, Spadea A, Tieghi A, Angelucci E, Visani G, Vannucchi AM, Barbui T; CYTO-PV Collaborative Group. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 580] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 22. | Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, Barbui T; European Collaboration on Low-Dose Aspirin in Polycythemia Vera Investigators. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 672] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 23. | Squizzato A, Romualdi E, Passamonti F, Middeldorp S. Antiplatelet drugs for polycythaemia vera and essential thrombocythaemia. Cochrane Database Syst Rev. 2013;CD006503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |