Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7484
Peer-review started: January 16, 2021
First decision: April 29, 2021
Revised: May 12, 2021
Accepted: July 16, 2021
Article in press: July 16, 2021
Published online: September 6, 2021
Processing time: 226 Days and 23.4 Hours
Octreotide is widely used for the treatment of acromegaly, neuroendocrine tumors, and secretory diarrhea. However, long-term octreotide treatment can increase the incidence of gallstones. Vicarious contrast medium excretion (VCME) through the hepatobiliary system is well known. However, few studies have reported octreotide-induced acute gallstones following VCME.
A 69-year-old man presented with left lower back pain and hematuria caused by a fall. The patient had a history of polycystic kidney disease. VCME occurred following renal artery embolization for a ruptured polycystic kidney. After 5 d of treatment with octreotide, the patient developed acute gallstones and intrahepatic cholestasis which further induced pancreatitis and cholangitis. He was discharged after hemodialysis, antibiotics, and supportive treatments.
For patients with a high-risk of VCME, octreotide should be cautiously admini
Core Tip: Vicarious contrast medium (CM) excretion (VCME) through the hepatobiliary system is well known. Long-term octreotide treatment can increase the incidence of gallstones. In this case, acute gallstones may have been induced by octreotide after VCME through the hepatobiliary system. When the CM was excreted into the hepatobiliary system, which was retained for a long time and concentrated by octreotide, it might change the physicochemical properties of bile and decreased nucleation time, finally resulting in the formation of acute gallstones.
- Citation: Han ZH, He ZM, Chen WH, Wang CY, Wang Q. Octreotide-induced acute life-threatening gallstones after vicarious contrast medium excretion: A case report. World J Clin Cases 2021; 9(25): 7484-7489
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7484.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7484
Contrast medium (CM) is widely used in intravenous pyelography, computed tomography (CT), and angiography examinations. The incidence of adverse reactions due to CM is low, especially when using modern non-ionic CMs. Vicarious CM excretion (VCME) is a well-recognized entity, of which excretion through the hepatobiliary system accounts for the majority of cases, and is asymptomatic[1]. Octreotide is widely used for the treatment of acromegaly, neuroendocrine tumors, and secretory diarrhea, and long-term treatment significantly increases the incidence of gallstones, most of which are asymptomatic[2].
We report a patient who developed acute gallstones following treatment with octreotide after VCME.
A 69-year-old Chinese male complained of yellow discoloration of the skin and urine with abdominal distension.
The patient was admitted to the emergency department of our hospital in the afternoon of June 11, 2020 complaining of left lower back pain and hematuria caused by a fall 6 h previously. His blood pressure was 81/46 mmHg at admission, and laboratory tests revealed a hemoglobin level of 80 g/L, blood urea nitrogen of 7.9 mmol/L, creatinine of 105 μmol/L, and pH 7.30. Ultrasonography (US) and contrast-enhanced CT (60 mL, 270 mg of iodine/mL; Yangtze River Pharmaceutical Group, Taizhou, China) revealed bilateral polycystic kidney with rupture of the left kidney, a huge hematoma, and multiple liver cysts. The gallbladder and pancreas were normal. Emergency renal artery embolization (RAE, 150 mL iodixanol) was performed, after which his blood pressure promptly returned to normal and hematuria decreased. On the second day after RAE (d1-post-RAE), the patient complained of abdominal distension with absence of the passage of both flatus and stool. Paralytic intestinal obstruction was diagnosed together with absence of bowel sounds. He was treated with fasting, gastrointestinal decompression, fluid replacement, and octreotide (100 mg, once daily; Novartis Pharma Schweiz AG, Risch-Rotkreuz, Switzerland). On d5-post-RAE, the patient resumed passage of both flatus and stool, and the above treatments were discontinued.
He had a history of polycystic kidney disease for 40 years, but had no other illnesses.
He denied a history of similar diseases in close relatives.
Physical examination revealed that the skin and sclera were slightly jaundiced, and a mass 16 cm × 12 cm in size on the left flank was observed, which was soft and tender with percussion pain in the left renal region. There were no abnormal liver and gallbladder findings.
On d6-post-RAE, the patient’s sclera and skin were slightly yellow, and was worse the following day. Laboratory tests showed that the levels of bilirubin, alkaline phosphatase (AKP) and gamma-glutamyl transpeptidase (γ-GT) were significantly increased (Table 1), but transaminases were normal. Urinalysis showed that urinary bilirubin was positive and urobilinogen was negative.
| Date | TB | DB | AKP | γ-GT |
| μmol/L | μmol/L | μ/L | μ/L | |
| 6.12 | 13.4 | 6.1 | 58 | 23.2 |
| D11 | 10.0 | 5.1 | 77 | 20.3 |
| D2 | 11.4 | 5.7 | 83 | 18.8 |
| D3 | 17.8 | 9.0 | 75 | 19.3 |
| D7 | 214.1 | 166.8 | 874 | 366.3 |
| D9 | 230.9 | 177.6 | 1092 | 447.5 |
| D10 | 309.3 | 232.3 | 1489 | 563.7 |
| D11 | 367.6 | 289.5 | 1759 | 734.5 |
| D12 | 422.7 | 319.5 | 1936 | 782.1 |
| D14 | 453.8 | 355.0 | 2147 | 805.5 |
| D16 | 466.9 | 363.1 | 2318 | 868.3 |
| D17 | 413.7 | 320.7 | 2045 | 774.6 |
| D17 | 374.8 | 305.7 | 1871 | 689.1 |
| D17 | 226.7 | 194.0 | 1137 | 462.3 |
| D17 | 206.4 | 174.3 | 1055 | 448.6 |
| D18 | 285.3 | 226.1 | 1365 | 532.7 |
| D19 | 245.4 | 198.0 | 1194 | 457.4 |
| D19 | 142.1 | 117.9 | 658 | 249.3 |
| D19 | 120.5 | 99.3 | 601 | 213.9 |
| D21 | 168.3 | 136.9 | 746 | 335.1 |
| D22 | 142.1 | 115.4 | 641 | 253.6 |
| D23 | 109.0 | 95.1 | 408 | 175.2 |
| D25 | 82.3 | 70.9 | 325 | 136.6 |
| D27 | 58.9 | 50.2 | 265 | 102.1 |
| D30 | 50.9 | 41.5 | 198 | 98.1 |
| D34 | 35.3 | 29.1 | 153 | 77.6 |
| M12 | 17.2 | 5.9 | 92 | 37.5 |
| M3 | 13.6 | 5.4 | 51 | 22.3 |
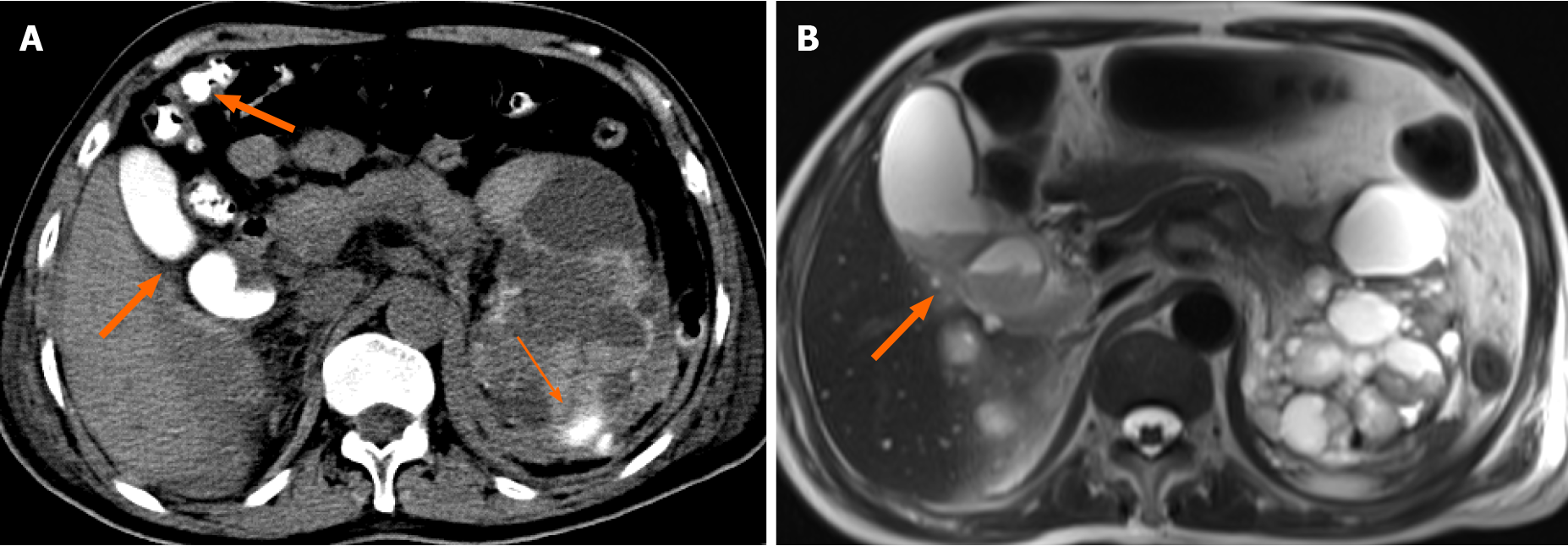
On d3-post-RAE, non-contrast CT showed high density in the gallbladder and colon, which was considered to be due to VCME, while in the upper pole of the left kidney CM had spilled out of the renal artery (Figure 1A). On d7-post-RAE, repeat US revealed a large amount of sludge in the gallbladder, but no dilation of intrahepatic and extrahepatic bile ducts.
Final diagnoses were octreotide-induced acute gallstones and VCME.
On the evening of d7-post-RAE, the patient complained of upper abdominal pain. Laboratory tests revealed an amylase level of 640 U/L and lipase level of 1198 U/L, resulting in a diagnosis of acute pancreatitis, which dropped to normal following treatment for 5 d. However, the levels of bilirubin, AKP and γ-GT continuously increased (Table 1). On d8-post-RAE, endoscopic retrograde cholangiopancreatography (ERCP) (10 mL ioversol, 320 mg of iodine/mL; Hengrui Medicine, Jiangsu, China) was conducted and revealed that the duodenal papilla was plump, and brown-black bile and sludge were observed, a nasobiliary catheter was then placed for the drainage of bile. A non-contrast CT on d10-post-RAE showed that the liver was normal except for multiple cysts, while the CM still remained in the gallbladder and colon. Tests for hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis E virus, hemolysis and autoimmune hepatitis were negative, and upper abdominal magnetic resonance imaging was normal except for multiple cysts and sludge in the gallbladder (Figure 1B). Antibiotics were upgraded to imipenem-cilastatin sodium as bile culture showed multi-drug resistant Escherichia coli. However, dynamic monitoring of the levels of bilirubin, AKP and γ-GT continued to increase with skin and sclera becoming more yellow (Table 1). The patient developed transient lethargy at night on d16-post-RAE, and then recovered after treatment with the double plasma molecular adsorption system (DPMAS) for 4 h the following day. Dynamic laboratory tests showed that the levels of bilirubin, AKP and γ-GT had declined, but increased again the next day (Table 1). DPMAS was performed again to reduce bilirubin on d19-post-RAE, and to achieve the previous similar changes in laboratory tests (Table 1). Repeat bile culture showed multi-drug resistant Klebsiella pneumoniae subsp, and the antibiotics were changed to sulbactam-cefoperazone followed by a multidisciplinary consultation. Surprisingly, dynamic laboratory tests showed that the levels of bilirubin, AKP, γ-GT, and the white blood cell count gradually began to decrease (Table 1) together with a decrease in the patient's fever, and yellowing of the sclera and skin.
The patient recovered and was discharged after 2 wk of treatment. After 1 and 3 mo, repeated laboratory tests showed that blood cell counts, liver and kidney function were all within normal limits (Table 1).
VCME refers to the excretion of water-soluble CM through a route other than renal secretion, and is a well-known phenomenon, although the exact mechanism is still not fully understood. According to previous studies, these authors believed that possible factors promoting the heterotopic biliary (vicarious) excretion of CM included prolonged recirculation of the CM due to impaired renal function, hypovolemia and hypotension, and increased protein binding of CM in the presence of acidosis[1,3]. Higher doses, higher molecular weight and lower osmotic pressure of CM are also thought to be factors contributing to VCME[1,3]. Although VCME can also occur in patients with normal renal function, studies have shown that patients with renal insufficiency have a higher incidence of VCME[1]. An analysis of our patient showed that some of the above-mentioned high-risk factors were present, which may have led to VCME.
Long-term treatment with octreotide can lead to a significant increase in the incidence of gallstones, which is reported to be 10%-63%, but these patients are often asymptomatic[2]. Many previous studies have shown that octreotide not only inhibits meal-stimulated cholecystokinin release from the small intestine and gallbladder contraction, but it also directly promotes gallbladder absorption, which may act synergistically to increase the concentration of prolithogenic factors in bile and facilitate nucleation and stone growth[4]. Moreover, octreotide induces lithogenic changes in bile composition and physical chemistry such as supersaturated bile, excess biliary cholesterol transport in vesicles, a high vesicular cholesterol: phospholipids molar ratio, and abnormally rapid nucleation of cholesterol microcrystals[5]. Some studies have shown that octreotide prolonged intestinal transit leads to increased deoxycholic acid absorption from the colon and thereby the risk of gallstone formation[6,7], which was consistent with the long-term retention of CM in the gallbladder and colon in this case. In addition, octreotide inhibits the usual prandial relaxation of the sphincter of Oddi, thus creating physical conditions favoring microcrystal precipitation and stone formation[6]. The development of stones occurs after an average period of 3 years, and increases with the dose of medication and duration of treatment[8]. In this patient, the gallbladder sediment-like stones developed after treatment with octreotide for only 5 d, which was inconsistent with previous studies. Parasher et al[9] demonstrated that ERCP CMs have crystals that can mimic calcium bilirubinate granules (pseudomicrolithiasis). In our patient, we speculate that the cause of intrahepatic cholestasis was calculi in the intrahepatic biliary tract induced by octreotide after VCME through the hepatobiliary system, as autoimmune liver disease, hemolytic and hepatocellular jaundice were excluded, and the intrahepatic bile duct did not dilate. When the CM was excreted into the hepatobiliary system, which was retained for a long time and concentrated by octreotide, this may have changed the physicochemical properties of bile and decreased nucleation time, resulting in the formation of acute gallstones. Similar changes might have occurred simultaneously in the intrahepatic biliary tree, which was the cause of poor bile excretion, and led to intrahepatic cholestasis and jaundice. When these gallstones were eliminated and passed through the duodenal papilla, they were embedded and induced acute pancreatitis. Ju et al[10] demonstrated that the growth of gallbladder endothelial cells was significantly inhibited by CM and was positively correlated with osmotic pressure. Unfortunately, analysis of gallstones composition was not performed. In our patient, the causes of cholangitis may have been cholestasis, long-term indwelling nasobiliary catheter, and CM damage to the bile duct epithelium. Gallstones, cholestasis, and cholangitis caused severe jaundice, which can induce bilirubin encephalopathy.
Although symptomatic VCME and octreotide-induced gallstones are relatively rare, with the widespread use of CM, the frequency of this rare complication is expected to increase and careful observation of patients is required in order not to miss the opportunity of treatment especially when the patient is at high risk of ectopic excretion. As we did not have data on the composition of the gallstones to confirm our hypothesis, future research is required.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Piozzi GN, Sakaguchi Y S-Editor: Fan JR L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Yamazaki H, Oi H, Matsushita M, Teshima T, Murayama S, Nose T, Koizumi T, Tanaka E. Gallbladder opacification 12-24 h after angiography by CT examination: a multivariate analysis. Abdom Imaging. 1996;21:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Bergeron E, Bensoussan M. Massively distended, necrotic and hemorrhagic gallbladder in a long-term octreotide-treated patient with added everolimus. Int J Surg Case Rep. 2019;61:107-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Ku MC, Kok VC, Lee MY, Hsu SM, Lee PY, Chang CW, Tyan YS, Juan CW. Clinical analysis of contributors to the delayed gallbladder opacification following the use of water-soluble contrast medium. Ther Clin Risk Manag. 2016;12:1357-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Moschetta A, Stolk MF, Rehfeld JF, Portincasa P, Slee PH, Koppeschaar HP, Van Erpecum KJ, Vanberge-Henegouwen GP. Severe impairment of postprandial cholecystokinin release and gall-bladder emptying and high risk of gallstone formation in acromegalic patients during Sandostatin LAR. Aliment Pharmacol Ther. 2001;15:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Hussaini SH, Murphy GM, Kennedy C, Besser GM, Wass JA, Dowling RH. The role of bile composition and physical chemistry in the pathogenesis of octreotide-associated gallbladder stones. Gastroenterology. 1994;107:1503-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Hofmann AF. Increased deoxycholic acid absorption and gall stones in acromegalic patients treated with octreotide: more evidence for a connection between slow transit constipation and gall stones. Gut. 2005;54:575-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Thomas LA, Veysey MJ, Murphy GM, Russell-Jones D, French GL, Wass JA, Dowling RH. Octreotide induced prolongation of colonic transit increases faecal anaerobic bacteria, bile acid metabolising enzymes, and serum deoxycholic acid in patients with acromegaly. Gut. 2005;54:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Brighi N, Lamberti G, Maggio I, Manuzzi L, Ricci C, Casadei R, Santini D, Mosconi C, Lisotti A, Ambrosini V, Pantaleo MA, Campana D. Biliary stone disease in patients receiving somatostatin analogs for neuroendocrine neoplasms. A retrospective observational study. Dig Liver Dis. 2019;51:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 9. | Parasher VK, Romain K, Sukumar R, Jordan J. Can ERCP contrast agents cause pseudomicrolithiasis? Gastrointest Endosc. 2000;51:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ju YM, Kim MH, Lee SK, Seo DW, Min YI, Kim JY. Comparative cytotoxicity of low-osmolar nonionic and high-osmolar ionic contrast media to dog gallbladder epithelial cells. Gastrointest Endosc. 2002;55:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |