Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7453
Peer-review started: December 28, 2020
First decision: April 29, 2021
Revised: May 9, 2021
Accepted: July 7, 2021
Article in press: July 7, 2021
Published online: September 6, 2021
Processing time: 242 Days and 15 Hours
Plasmacytoma is a rare neoplastic disorder that arises from B-lymphocytes. Solitary bladder plasmacytoma, a type of solitary extramedullary plasmacytoma, is even rarer. Treatments for solitary extramedullary plasmacytoma include surgery, chemotherapy, and radiation. However, there are no clinical trials or guidelines specifying which treatment might represent the gold standard.
We herein report a case of a 51-year-old woman with solitary bladder plasmacy
Radiation is the potential main treatment for SBP. However, surgery is also necessary.
Core Tip: Solitary bladder plasmacytoma is rare. At present, there is no consensus on the optimal treatment for this disease. Herein, we reviewed past case reports on SBP and suggested radiation as its main treatment based on our results. Furthermore, radiation combined with surgery may be better than radiation alone. In addition, close monitoring is as important as treatment, and monoclonal protein is significant to the prognosis of this disease.
- Citation: Cao JD, Lin PH, Cai DF, Liang JH. Successful treatment of solitary bladder plasmacytoma: A case report . World J Clin Cases 2021; 9(25): 7453-7458
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7453.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7453
There are three types of plasmacytomas: Extramedullary plasmacytomas (EMP), solitary plasmacytomas of bone, and multiple myeloma (MM)[1,2]. MM is a common hematological malignancy characterized by the proliferation of clonal plasma cells and the production of monoclonal proteins[3]. Single plasmacytoma and isolated EMP of bone belong to isolated plasmacytoma[1], which refers to localized plasmacytoma occurring in bone (isolated plasmacytoma of bone) or outside bone marrow (single EMP). Less than 5% of plasmacytomas might present as single lesions, whereas extramedullary soft-tissue plasmacytomas are rarer[4]. Most solitary EMP (SEPs)[3] are localized in the head and neck, especially the upper respiratory tract; the second most frequent site is the gastrointestinal tract. Conversely, few rare sites reported include the central nervous system, thyroid, breast, testes, parotid glands, and urinary bladder[5]. Bladder plasmacytoma (BP)[6] is extremely uncommon, with only 22 cases having been reported so far before 2010; 8 had a history of MM, while 5 had lymphadenopathy at presentation, and the most recent one[7] was an asymptomatic solitary bladder plasmacytoma (SBP).
However, solitary plasmacytoma[8,9] can develop into MM, as a more aggressive plasmacytoma associated with shorter progression-free survival and poorer prognosis. Based on the different treatments and prognoses of their malignancies, solitary plasmacytoma should be distinguished from MM.
The diagnostic criteria for isolated plasma cell tumors are: (1) Single extramedullary mass caused by clonal proliferation of plasma cells; (2) Normal morphological examination of bone marrow cells and bone marrow biopsy; (3) Normal skeletal survey including an X-ray examination of the long bones; (4) No anemia, hypercalcemia, or renal failure due to plasma cell disease; and (5) Lack or low levels of monoclonal immunoglobulins in serum or urine. Magnetic resonance imaging) and positron emission tomography-computed tomography (CT) are obviously helpful for determining whether SEP progresses to MM[3].
A 51-year-old woman presented with acute urination pain for 2 wk.
The patient was previously diagnosed with acute urethritis at another institution. After having been unsuccessfully treated, she was transferred to our hospital for further diagnosis and treatment.
The patient had no history of any other illness.
Normal menstruation in the past, but she is menopausal now. She denied any family history.
No abdominal mass was palpated, no pain was elicited upon pressing the bladder area, and no obvious positive findings were found.
The results such as routine hematological testing, blood sedimentation rate, serum carbohydrate antigen (CA)199, CA125, CA153, alpha-fetoprotein, and carcinoembryonic antigen and so on were normal.
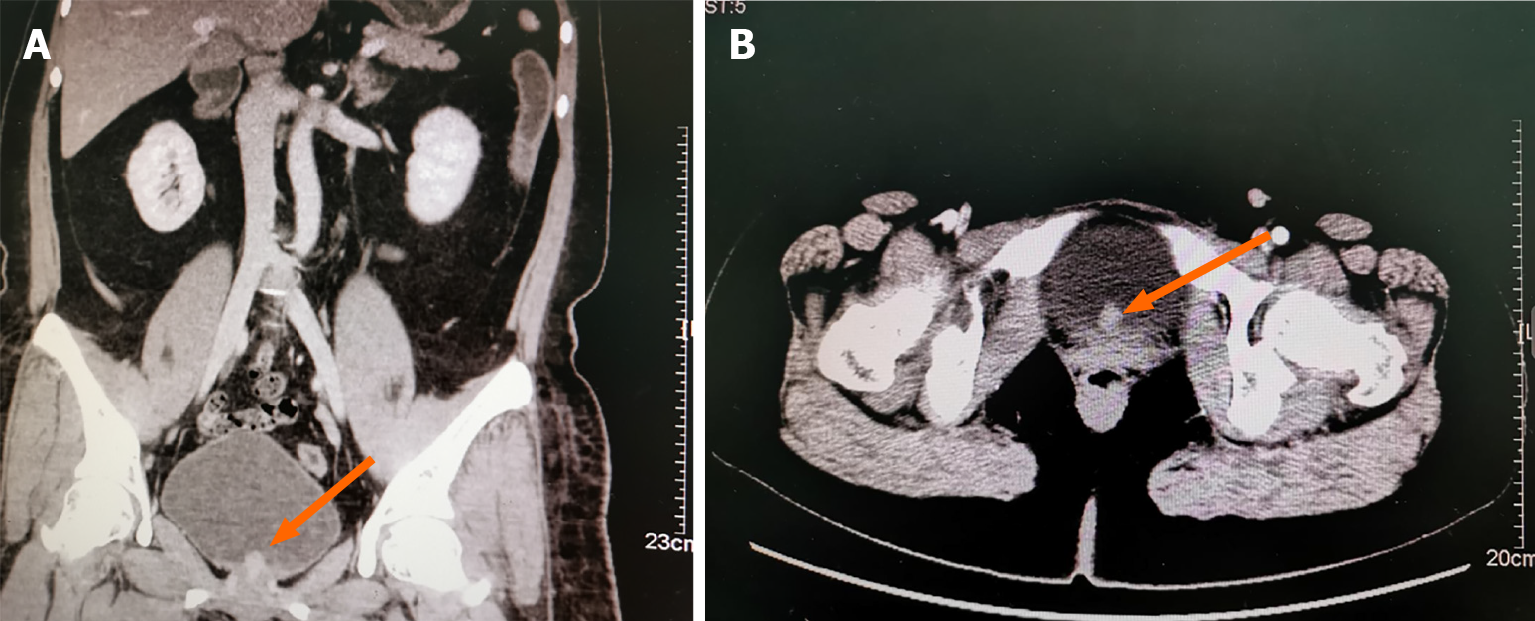
A renal color-Doppler ultrasonography detected solid bladder nodules localized at the inner surface of the urinary bladder close to the urethral orifice. Then, the patient was transferred to our hospital for more specialized treatment. We performed contrast-enhanced CT scan of both kidneys and the pelvis. It indicated posterior bladder occupation: A nodule on the posterior surface of the bladder, measuring about 15 mm × 11 mm. The CT attenuation value of plain scan was about 26 Hu, and the enhancement was obvious, showing progressive enhancement of about 80 Hu. Neoplastic lesions were considered, and bladder cancer was not excluded (Figure 1).
Solitary bladder plasmacytoma.
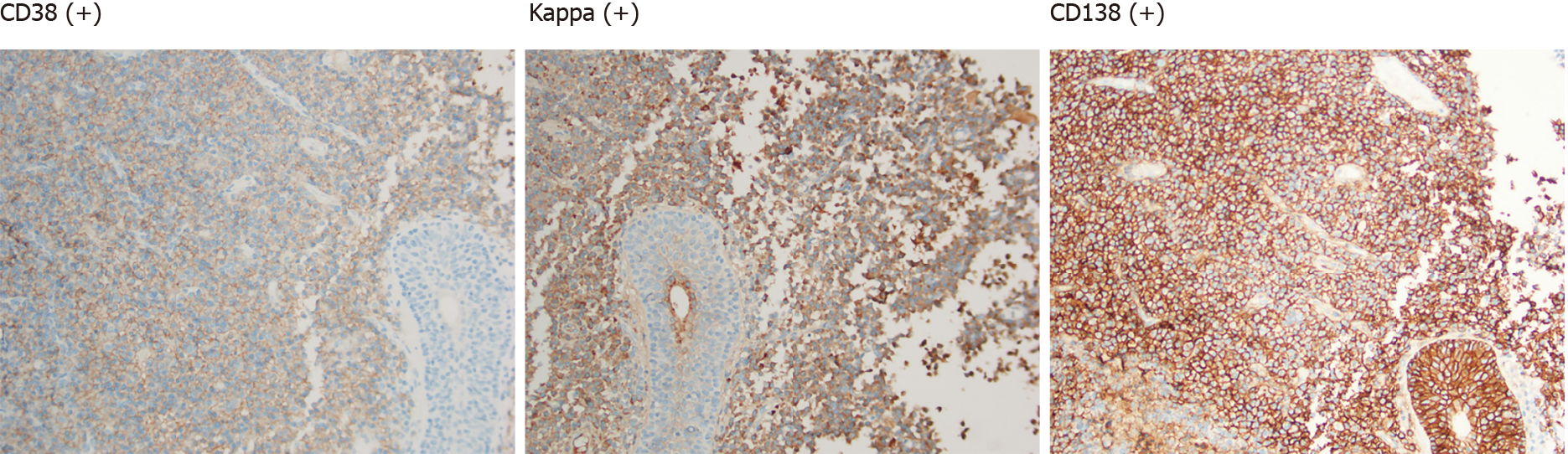
The patient consented to undergo transurethral resection of bladder tumor. Pathological examination of the resected specimen suggested bladder tumor: Diffuse infiltration of tumor cells of the same size in the lamina propria were present, the nucleus was offset, and it looked like plasma cells. Mitosis was rare, and plasmacytoma was highly suspected. Immunohistochemistry results revealed: P63 (-), cytokeratin 20 (-), P53 (-), Ki67 (2%+), GATA binding protein 3 (-), and cytokeratin (-). B-cell lymphoma clonal gene rearrangement test results were: immunoglobulin heavy chain (+) and immunoglobulin light chain (+). Supplemental immunohistochemical results were: CD38 (+), CD138 (+), Kappa (+), and Lambda (-) (Figure 2).
Thereafter, as we recommended, the patient underwent postoperative radiation therapy of 50 Gy/25 F. Further bone marrow examination revealed that the ratio of bone marrow hematopoietic tissue to fat cells was about 4:6 under microscope; three lines of hematopoietic cells could be seen. In addition, the proportion of granulocytes and erythrocytes was also slightly elevated. Erythroblasts were the most identified cells in the erythroid. Myelocytes, metamyelocytes, and mature granulocytes were the most identified cells in the granulocyte series. No obvious abnormalities in the morphology and number of megakaryocytes were observed. No significant increase in the number of plasma cells was seen. Immunohistochemical results were: CD20 (Scattered decimal+), CD3 (Scattered decimal+), CD138 (Scattered+), CD38 (Scattered+), K (few+), L (Scattered+), epithelial membrane antigen (-), multiple myeloma oncogene 1 (-), CD56 (-), and myeloperoxidase (part+).
Postoperative positron emission tomography-CT indicated: (1) Normal changes after resection of the posterior tumor of the bladder; a close follow–up was recommended; (2) The trunk axis bone metabolism was slightly increased diffusely; thus, it was necessary to pay attention to the possibility of developing myeloma, and bone marrow biopsy was recommended; (3) There were multiple slightly enlarged lymph nodes in level II of the bilateral neck space, and the metabolism was slightly increased, suggesting lymph node inflammatory hyperplasia; (4) The soft tissues around the shoulder joints were slightly thickened, and the metabolic symmetry was slightly increased indicating inflammatory changes; and (5) No clear abnormally high metabolic lesions in the rest of the body were detected. Laboratory test results including serum calcium and hemoglobin levels were normal. Levels of serum immunoglobulins: Kappa and lambda and Bence-Jones protein were also normal. After completing postoperative radiotherapy of 50 Gy/25 F, a 7-mo follow-up showed no obvious symptoms.
Based on our findings, radiotherapy might be the main treatment of SBP. However, surgery is also necessary. Close collaboration between the hematology department, radiotherapy department, and surgeons is essential to formulate an appropriate treatment plan. Radical radiotherapy is recommended for SBP as the first choice. The tumor irradiation range should be at least 2 cm outside the edge detection of the magnetic resonance imaging view. The dose used was 40 Gy, given in 20 doses. When SEP is > 5 cm, the recommended dose should be 50 Gy, given in 25 divided doses. Evaluation of the response after radiotherapy depends on changes in monoclonal protein levels, progression or elimination of symptoms, and appearance of new lesions on imaging examinations. Patients whose monoclonal protein disappears after treatment indicate a high probability of cure. However, patients whose paraproteins persist after 1 year of treatment will progress to MM. The role of adjuvant chemotherapy has not yet been elucidated. Adding chemotherapy to radiotherapy has advantages in improving local control and preventing or delaying progression to MM. Patients with SBP need to be closely monitored for developing MM and should be followed up every 6 wk for 6 mo[2].
Regarding prognosis, patients with systemic diseases have a poor prognosis, and 10% of patients experience local recurrence[7]. However, there is currently a lack of reports on specific long-term follow-up data of bladder plasmacytoma[10].Therefore, we reviewed nine case reports of SBP and summarized them in Table 1.
| Ref. | Age | Sex | Main symptoms | Treatment | Prognosis |
| Takahashi et al[8] | 28 | Female | Hematuria (after renal transplantation) | ED combined with chemotherapy including vincristine, doxorubicin, and dexamethasone | Died after 1 yr (developed to MM) |
| Cormio et al[9] | 74 | Female | No urinary symptoms (complicated with liver cirrhosis) | Transurethral resection of bladder tumor | No recurrence was found after 4 mo (but died of liver failure) |
| Wadhwa et al[10] | 69 | Male | Hematuria (complicated with bladder urothelial carcinoma) | Transurethral resection of bladder tumor and radiotherapy | No recurrence (still following up) |
| Mokhtar et al[11] | 95 | Female | Hematuria | Radiotherapy | No supply data |
| Yang et al[12] | 47 | Male | Dysuria, hematuria, low back pain | Cyclophosphamide and radiotherapy | No recurrence was found after 13 yr |
| Yang et al[12] | 68 | Female | Hematuria | Systemic chemotherapy (melphalan) | No recurrence was found after 1 yr |
| Ho et al[13] | 74 | Female | Dysuria | Radiotherapy and cystectomy | No recurrence was found after 4 yr |
| Gorfain[14] | 39 | Male | Hematuria | Partial cystectomy and radiotherapy | No recurrence was found after 1 yr |
| Miller et al[15] | 61 | Male | Hematuria | Radiotherapy | No recurrence in a short term (still following up) |
Most of the cases reviewed were treated with radiotherapy, and some cases underwent adjuvant chemotherapy or surgery. Two of these 9 cases were reported dead. Only 1 case developed MM and died, and there were no signs of recurrence. Therefore, the recurrence rate of SBP is low, and prognosis is significantly better than that of MM. However, SBP is still possible to progress to MM.
SBP is a very rare bladder malignancy. At present, there is still a lack of optimally sufficient treatment and prognostic data on SBP. Treatment of SBP is mainly based on the treatment of SEP. In terms of prognosis, the recurrence rate and survival rate of SBP are still unclear, and it may progress to MM. However, the overall prognosis of SBP is significantly better than that of MM.
Radiation is the potential main treatment of SBP. Moreover, radiation combined surgery may be better than radiation alone. In addition, close monitoring and follow-up are as important as treatment, and monoclonal protein is a significant laboratory examination for the prognosis of this disease.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pessoa WFB, Xavier-Elsas P S-Editor: Liu M L-Editor: Filipodia P-Editor: Guo X
| 1. | Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 574] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 2. | Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, Samson D; Guidelines Working Group of the UK Myeloma Forum; British Committee for Standards in Haematology; British Society for Haematology. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Haematol. 2004;124:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12186] [Article Influence: 1523.3] [Reference Citation Analysis (3)] |
| 4. | Moulopoulos LA, Dimopoulos MA, Weber D, Fuller L, Libshitz HI, Alexanian R. Magnetic resonance imaging in the staging of solitary plasmacytoma of bone. J Clin Oncol. 1993;11:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 137] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Wirk B, Wingard JR, Moreb JS. Extramedullary disease in plasma cell myeloma: the iceberg phenomenon. Bone Marrow Transplant. 2013;48:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Kondo H, Kainuma O, Itami J, Minoyama A, Nakada H. Extramedullary plasmacytoma of maxillary sinus with later involvement of the gall bladder and subcutaneous tissues. Clin Oncol (R Coll Radiol). 1995;7:330-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Khaliq W, Uzoaru I, Konchanin RP, Sapiente RA, Egner JR. Solitary extramedullary plasmacytoma of the bladder: a case report and literature. Oncology (Williston Park). 2010;24:832-835. [PubMed] |
| 8. | Takahashi R, Nakano S, Namura K, Yamada N, Uchida R, Fuchida S, Okano A, Okamoto M, Ochiai N, Shimazaki C. Plasmacytoma of the urinary bladder in a renal transplant recipient. Int J Hematol. 2005;81:255-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Cormio L, Mancini V, Calò B, Selvaggio O, Mazzilli T, Sanguedolce F, Carrieri G. Asymptomatic solitary bladder plasmocytoma: A case report and literature review. Medicine (Baltimore). 2017;96:e9347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Wadhwa K, Singh R, Solomon LZ. Bladder extramedullary plasmacytoma and synchronous bladder urothelial transitional cell carcinoma: A case report and review of the literature. Open Access J Urol. 2011;3:25-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Mokhtar GA, Yazdi H, Mai KT. Cytopathology of extramedullary plasmacytoma of the bladder: a case report. Acta Cytol. 2006;50:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Yang C, Motteram R, Sandeman TF. Extramedullary plasmacytoma of the bladder: a case report and review of literature. Cancer. 1982;50:146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Ho DS, Patterson AL, Orozco RE, Murphy WM. Extramedullary plasmacytoma of the bladder: case report and review of the literature. J Urol. 1993;150:473-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Gorfain AD. Extramedullary plasmacytoma of the bladder with local metastasis. Calif Med. 1949;71:147. [PubMed] |
| 15. | Miller DV, McClure RF, Crawford BG, Zeldenrust SR, Leibovich BC, Sebo TJ. Histiocytes containing immunoglobulin crystals in the urine of a patient with IgA kappa plasmacytoma of the bladder. Diagn Cytopathol. 2004;31:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |