Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7319
Peer-review started: May 25, 2021
First decision: June 21, 2021
Revised: July 7, 2021
Accepted: July 26, 2021
Article in press: July 26, 2021
Published online: September 6, 2021
Processing time: 97 Days and 17.8 Hours
Heyde’s syndrome is an under reported systemic disease of gastrointestinal and cardiac manifestation in older adults. It is characterized by a triad of aortic stenosis, angiodysplasia with bleeding and acquired von Willebrand syndrome. It is characterized by proteolysis of high molecular weight multimers of von Willebrand Factor and loss of platelet mediated homeostasis. Heyde’s syndrome is a treatable condition in most cases, especially in the current era of evolution in interventional cardiology and gastroenterology. There are currently no established guidelines in the management of this condition due to paucity of high quality studies, which warrant future trials. High index of suspicion and increasing the awareness of the syndrome among the general practitioners and sub-specialists will improve the diagnostic potential of Heyde’s syndrome. Future studies may change the management aspect of Heyde's syndrome and pave a path for drawing specific guidelines and algorithms. The aim of our review article is to summarize the basic pathophysiology, diagnostics and management of Heyde’s syndrome with a special attention to Transcatheter aortic valve replacement.
Core Tip: We summarize the literature on the aortic valve replacement in aortic stenosis and angiodysplasia (Heyde's syndrome). This is a very attractive area of interest for interventional gastroenterologists and cardiologists. Future studies may change the management aspect of Heyde's syndrome and pave a path for drawing specific guidelines and algorithms.
- Citation: Lourdusamy D, Mupparaju VK, Sharif NF, Ibebuogu UN. Aortic stenosis and Heyde’s syndrome: A comprehensive review. World J Clin Cases 2021; 9(25): 7319-7329
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7319.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7319
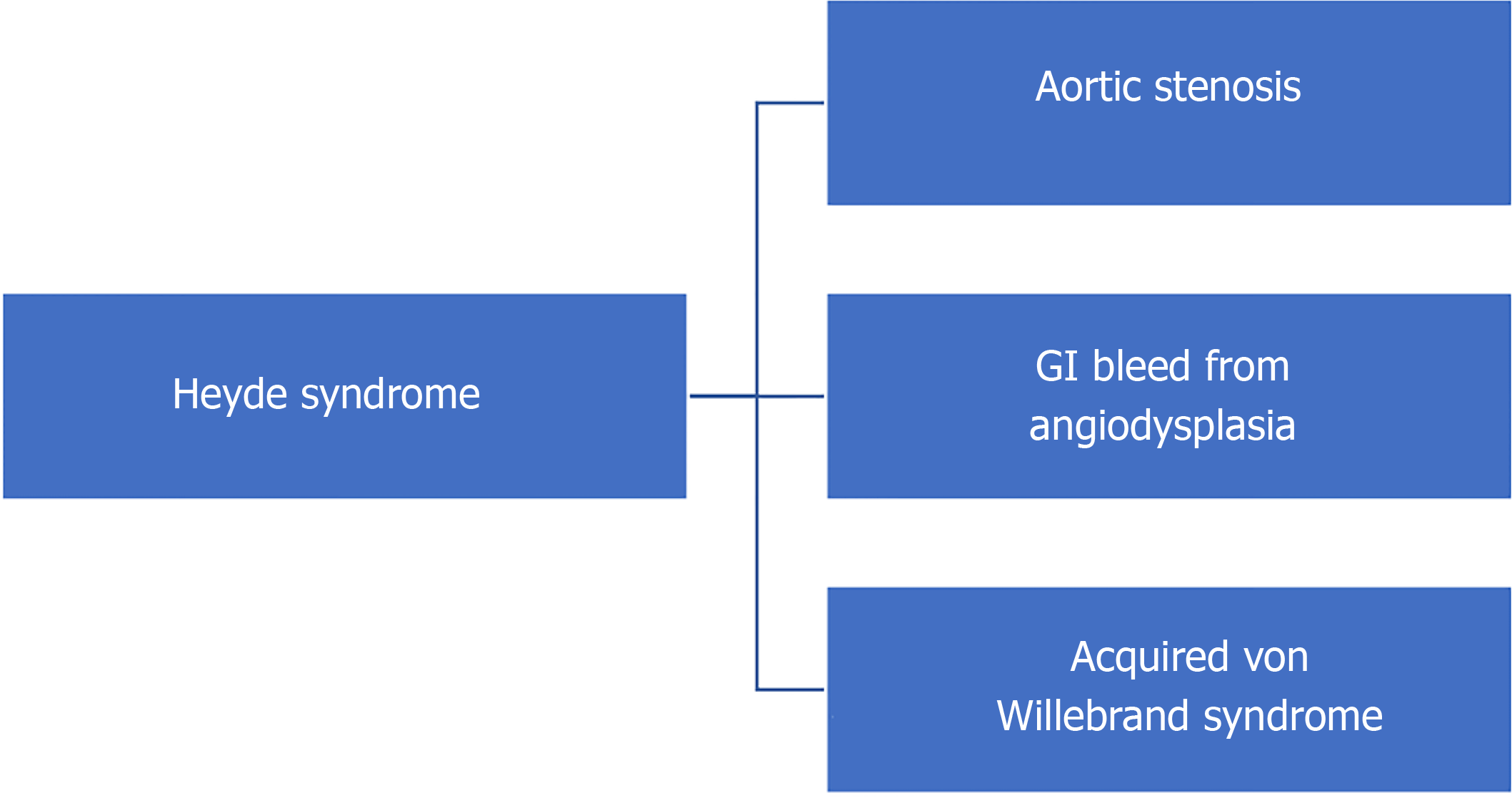
Heyde’s syndrome is a multi-system disorder of the cardiovascular, gastrointestinal (GI) and hematological system that is commonly described as a triad of aortic stenosis, gastrointestinal bleeding from angiodysplasia and acquired von Willebrand syndrome (Figure 1). Dr. Edward Heyde first described the association between aortic stenosis and gastrointestinal (GI) bleeding in 1958[1]. However, it was late in the 1980s to early 1990s that the role of coagulopathy in the form of acquired von Willebrand Disease was hypothesized to be a pathophysiologic mechanism of Heyde’s syndrome[2,3]. Angiodysplasia is characterized by abnormal and tortuous small blood vessels in the mucosal and submucosal layers of GI tract (upper GI, small bowel, colon). These are pathologically dilated communications between veins and capillaries[4]. Angiodysplasia is the second leading cause of lower gastrointestinal bleeding in the elderly[5]; accounts for 4%-7% of upper GI bleed and is the most common cause of obscure lower GI bleed in up to 50% of cases[6]. The terms angiodysplasia, arteriovenous malformations (AVMs), vascular ectasia have been used interchangeably. Aortic stenosis is the most common degenerative valvular heart disease in the elderly. Prevalence of aortic stenosis in patients with bleeding angiodysplasia has been shown to be between 7% to 41%[7-9]. The contrary is also true in that, patients with aortic stenosis have a greater potential for gastrointestinal (GI) bleeding. Clinically significant GI bleeding is estimated to occur in 1%-3% of patients with moderate to severe aortic stenosis[10-12]. Controversies exist in this regard, as to whether these two conditions occurring together in elderly, is a mere coincidence or if there is a causal relationship. However, improvement in understanding of the pathophysiology of acquired von Willebrand disease (as described later) and observation of resolution of GI bleed in patients with angiodysplasia who underwent aortic valve replacement strongly support the existence of Heyde’s syndrome[13-15]. In this article, we review the epidemiology, pathogenesis and management of Heyde’s syndrome in the era of interventional cardiology and gastroenterology.
Heyde’s syndrome predominantly occurs in the elderly population (> 65 years) and is probably under reported. The prevalence of aortic stenosis is around 7% in population aged 75 years or older and increases to 10% in those over 80 years[16,17]. The severity of aortic stenosis also increases with age, with about 1.8% of population over 75 years having moderate to severe aortic stenosis[18,19]. A retrospective study from Cleveland clinic which looked into the association between GI Arteriovenous malformations (AVMs) and Aortic stenosis, showed a 31.7% prevalence of Aortic stenosis in patients with AVMs[8]. Another large single center study from Germany[20] involving a cohort of aortic stenosis patients with transcatheter aortic valve replacement (TAVR) showed that GI bleeding existed in 11% with endoscopically proven bleeding from angiodysplasia in 3% of the study population before TAVR. Angiodysplasia predominantly occurs in people over 60 years[6,21]. Although the true prevalence of angiodysplasia is difficult to estimate, a report from pooled prospective studies showed a prevalence of 0.83% of colonic angiodysplasia in population of healthy asymptomatic adults > 50 years[21]. However, its prevalence is thought to be higher in patients with GI bleed, end stage renal disease and von Willebrand disease[22,23]. Angiodysplasia can also be an incidental finding in healthy older adults without GI bleed during screening endoscopic procedures or procedures done for other purposes. It is also important to note that while the risk of bleeding in incidentally diagnosed angiodysplasia is not well established, a bleeding angiodysplasia is at increased risk for subsequent bleeding[24].
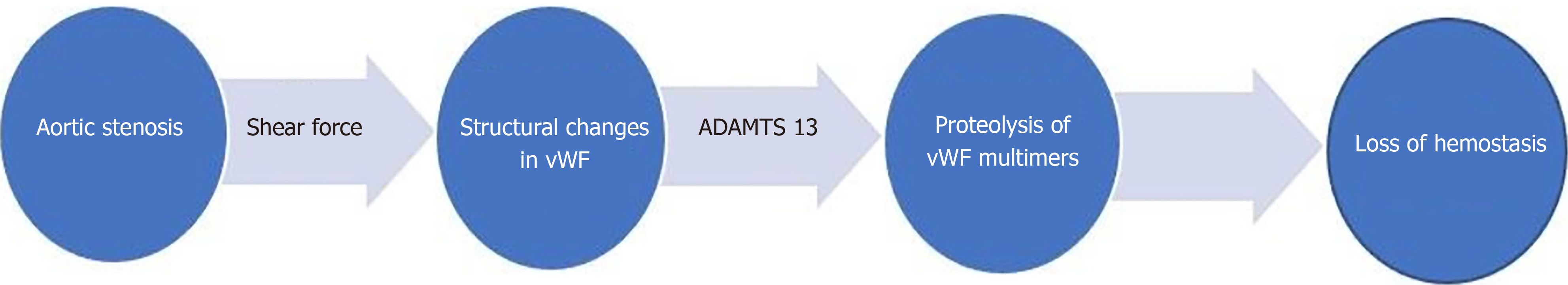
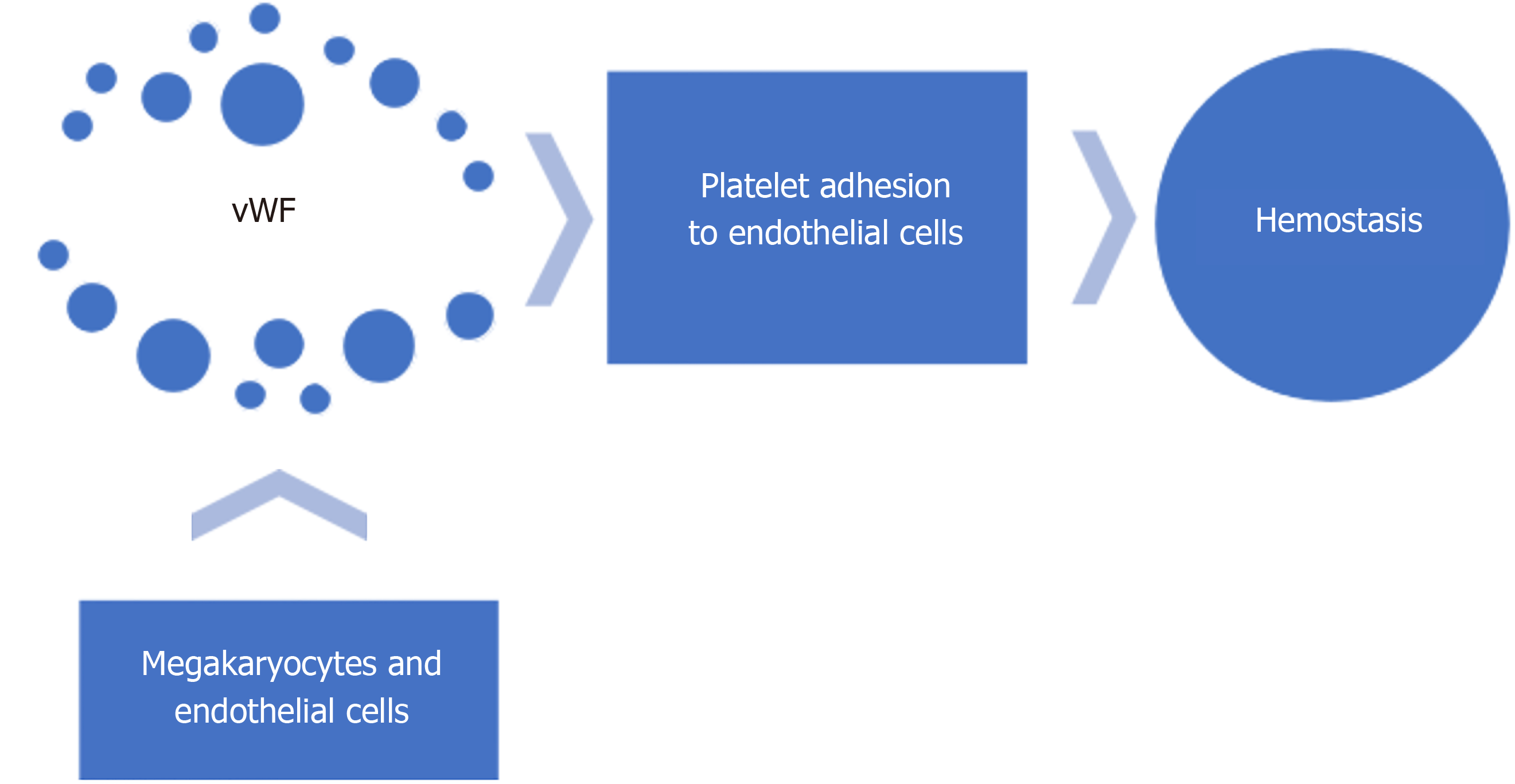
Acquired von Willebrand syndrome (vWS) is a key pathogenetic factor in Heyde’s syndrome. Acquired vWS encompasses a broad category of syndromes caused by shear induced proteolysis; antibodies to vWF, aberrant vWF binding to tumor cells; decreased vWF synthesis; or drug related[25,26]. vWF is a large glycoprotein, synthesized in megakaryocytes and endothelial cells, and plays a vital role in hemostasis (Figure 2) by mediating platelet adhesion to the sub endothelium and stabilizing factor VIII[27]. Heyde’s syndrome is characterized by the loss of the largest multimers of vWF[12,25,28,29]. Shear forces induce structural changes in the vWF while passing through the stenotic aortic valve, making it more sensitive to the action of a specific von Willebrand protease (ADAMTS 13)[2]. This results in proteolysis of high molecular weight multimers of vWF and loss of platelet mediated hemostasis (acquired von Willebrand syndrome vWS)[12,30] (Figure 3). Similar pathophysiology involving shear forces has been observed in hypertrophic cardiomyopathy, Left ventricular Assist Device (LVAD) placement, extra corporeal life support and severe mitral regurgitation[31-33]. Acquired vWS is common in patients with severe aortic stenosis. Decrease in high molecular weight vWF multimers has been shown in up to 68%-79% of cases with severe aortic stenosis[12,34]. vWF is also thought to play a role in suppressing angiogenesis[35,36] through integrin mediated signaling and vascular endothelial growth factor (VEGF) signaling[37,38]. Loss of vWF multimers leads to increased angiogenesis. This is hypothesized to be another mechanism of bleeding from angiodysplasia through increase in angiogenesis with acquired vW syndrome.
In general, the triad of aortic stenosis, bleeding angiodysplasia and evidence of acquired vWS should be sought for while evaluating suspected Heyde’s syndrome. Isolated aortic valve stenosis or GI bleeding in the setting of alternative etiologies including peptic ulcer disease, malignancies, diverticular bleed , etc., are the other differentials to be considered. History and physical exam in a patient with suspected Heyde’s syndrome should focus on evidence of aortic stenosis, GI bleeding and impaired hemostasis suggestive of acquired vWS (Table 1). In addition to a complete medical history, prior episodes and etiologies of GI bleeding, use of concomitant drugs that can accentuate GI bleed including NSAIDS, anti-coagulants, aspirin and other anti-platelet agents should be taken into account. Echocardiogram will provide insight into the severity of aortic stenosis (ventricular-aortic gradient and the valve area) and will direct treatment options.
| Due to | Symptoms | Signs |
| GI bleed | Hematemesis; Hematochezia; Melena; Abdominal pain | Pallor; Blood on rectal exam; Orthostasis |
| Aortic stenosis | Dyspnea on exertion; Syncope; Fatigue; Exertional chest pain | Low volume slow rising; carotid pulse; Ejection systolic murmur; Absence of physiologic S2 split |
| Acquired vWS | Easy bruisability; Mucosal bleeding; Heavy menstrual bleeding | Hemarthosis; Hematoma |
Patients with GI bleed can present with a wide range of clinical symptoms, from asymptomatic to hematemesis, hematochezia, melena, abdominal pain, pallor or blood on digital rectal examination. Acute bleeding with hypotension is rare in Heyde’s syndrome. Orthostasis may be present. Bleeding if present is typically painless, and usually chronic or recurrent. Fecal occult blood testing is a useful diagnostic tool in asymptomatic and occult GI blood loss. Symptoms can stem from aortic valve disease. Dyspnea on exertion, syncope, fatigue and exertional chest pain can occur. Physical examination should include complete cardiac exam with special note pertinent to features of aortic stenosis such as low volume and slow-rising carotid pulse, a loud mid-to late-peaking systolic murmur in the right intercostal space and a single second heart sound (absence of physiologic splitting of S 2). Easy bruisability and hemarthrosis are suggestive of impaired hemostasis (acquired vWS).
Labs including complete blood count (CBC) with platelet count and coagulation testing including a prothrombin time (PT) and an activated partial thromboplastin time (aPTT) should be obtained. Metabolic panel and test for fecal occult blood should be included as well. In individuals with acquired vWS, prolonged PTT may be observed as a result of low factor VIII levels. However normal PTT levels do not rule out acquired vWS. Platelets counts are not affected in acquired vWS, however there may be a coexisting condition or drug use that has to be accounted for.
Reduced levels of large HMW multimers of VWF by gel electrophoresis is a sensitive test for acquired vWS, but is time consuming (7-10 d) and expensive. Another commercially available test of primary hemostasis platelet function assay (PFA)-100 to quantify primary hemostasis (closure time to collagen-ADP) is a useful screening test. VWF antigen and ristocetin cofactor activities are usually normal. PFA is often used as an initial screening test for acquired vWS, as it is completed in a few hours. If abnormal, then VWF multimer assay can be done as a confirmatory test.
Management of Heyde’s syndrome basically includes management of the GI bleeding and appropriate consideration for aortic valve repair. In many cases it would mean a multi-team approach coordinated by cardiology, gastroenterology and primary care physician or inpatient hospitalist or geriatrician. There are no specific guidelines drawn for the management of Heyde’s syndrome and is rather based on expert consensus. vWF replacement therapies including vWF, factor VIII or octreotide/ desmopressin therapy are not found be of benefit in the management of Heyde’s syndrome[39].
Initial approach to GI bleeding in suspected Heyde’s syndrome does not vary from a general approach for any case of GI bleed. Initial resuscitative measures should include intravenous fluids and appropriate blood transfusion to ensure hemodynamic stability prior to identifying the source of bleed or endoscopic interventions. As previously noted, bleeding angiodysplasia is the second most common cause of lower GI bleed in the elderly, however not without diagnostic difficulties or uncertainties. In cases of obscure GI bleeding caused by small bowel angiodysplasia, conventional techniques of upper and lower GI endoscopy might not be sufficient and warrant deep enteroscopy or video capsule studies. CT Angiography may be preferred in the setting of active bleeding, followed by angiography and embolization after localization. Rarely surgical resection or intraoperative enteroscopy might have to be undertaken in life threatening bleed. Actively bleeding angiodysplasia identified during endoscopy should be treated. Argon plasma coagulation (APC) is the most commonly employed non contact technique utilizes energy from ionized argon. Bipolar cauterization can also be effective albeit with a low risk of perforation. Mechanical hemostasis using endoscopic clips, injection sclerotherapy and radio frequency ablation are the other less commonly employed techniques. Non-bleeding angiodysplasia in the setting of occult bleed or severe iron deficiency anemia, unexplained by other etiology should be treated as well. Incidentally found angiodysplasia in asymptomatic patients (without GI bleed or iron deficiency anemia) are usually thought to be low risk for bleeding and are not treated, and they don't fall under Heyde’s syndrome.
Aortic stenosis, if severe can be an independent factor for valve replacement despite the presence or absence of GI bleeding. Cardiology guidelines on management of Aortic stenosis should nevertheless be employed in all cases. Comprehensive echocardiographic evaluation should be performed to assess the disease severity. There is no robust data on aortic valve correction in Heyde’s syndrome based on the existing literature. There is a lack of data on randomized trials comparing conservative management vs aortic valve correction in suspected or proven Heyde’s syndrome. Most of the literature supporting aortic valve replacement in Heyde’s syndrome is from case reports, case series and retrospective studies[13,15,40-48] (Table 2). Recur
| Ref. | Year | Type of study | No of patients | Type of aortic valve replacement | Outcome (Positive) | Outcome (negative) |
| Love et al[13] | 1982 | Case series | 3 | Surgical | Complete resolution of GI bleed | |
| Scheffer et al[15] | 1986 | Case report | 1 | Surgical-porcine | No GI bleeding during 9 mo follow up | |
| Cappell et al[40] | 1986 | Case series | 2 | Surgical | No further bleeding with negative stool guaiac in one pt at 18 mo followup; Disappearance of angiodysplasia by endoscopy with no further bleeding in 2 nd patient at 15 mo follow up | |
| Abi-akar et al[41] | 2011 | Case report | 1 | Surgical- bio prosthetic | No bleeding or blood transfusion at 9 mo follow up | |
| Thompson et al[42] | 2012 | Retrospective review (1971-2001) | 57 | surgical bioprosthetic(47); mechanical(10) | 45 patients (79%) had no recurrence of GI bleeding in 15 year followup | 12 patients had persistent GI bleeding post AV replacement |
| Kadkhodayan et al[43] | 2012 | Case report | 1 | TAVR | No further episodes of GI bleeding post discharge | |
| Balbo et al[44] | 2016 | Case report | 1 | TAVR | No GI bleeding at 3 and 6 mo of follow up post TAVR | |
| Alshuwaykh et al[45] | 2018 | Case report | 1 | TAVR | No further bleeding with stable Hemoglobin at 6 mo follow up | Melena requiring blood transfusion at 2 wk follow up |
| Garcia et al[46] | 2019 | Case report | 1 | Surgical, mechanical | No new episodes of GI bleeding post valve replacemet | |
| Famularo et al[47] | 2020 | Case report | 1 | TAVR | No GI bleeding at 3 follow up post TAVR | |
| Godino et al[48] | 2012-13 | Retrospective (2007-2012) | 7 | TAVR | During a mean follow-up interval of 22 ± 15 mo, 6 patients (86%) had no recurrence of GI bleeding | One patient had TAVR failure with re hospitalization and blood trasnfusion |
The severity of vWF abnormality is noted to directly correlate with the severity of aortic stenosis measured by transvalvular gradient[12,49,50]. Correction of valve abnormalities or other conditions associated with high shear force (e.g., ventricular assist device) leads to resolution of GI bleeding[13,14,15,48]. Nevertheless, the hemostatic abnormalities and therefore bleeding can recur if there is a mismatch between the patient and prosthesis, (e.g., paravalvular leak)[12]. TAVR (Transcatheter aortic valve replacement) has also been shown to be effective in this regard, especially in surgically high risk patients, with resolution of GI bleeding[48], and recovery of high molecular weight vWF multimers after successful TAVR[20,48,51].
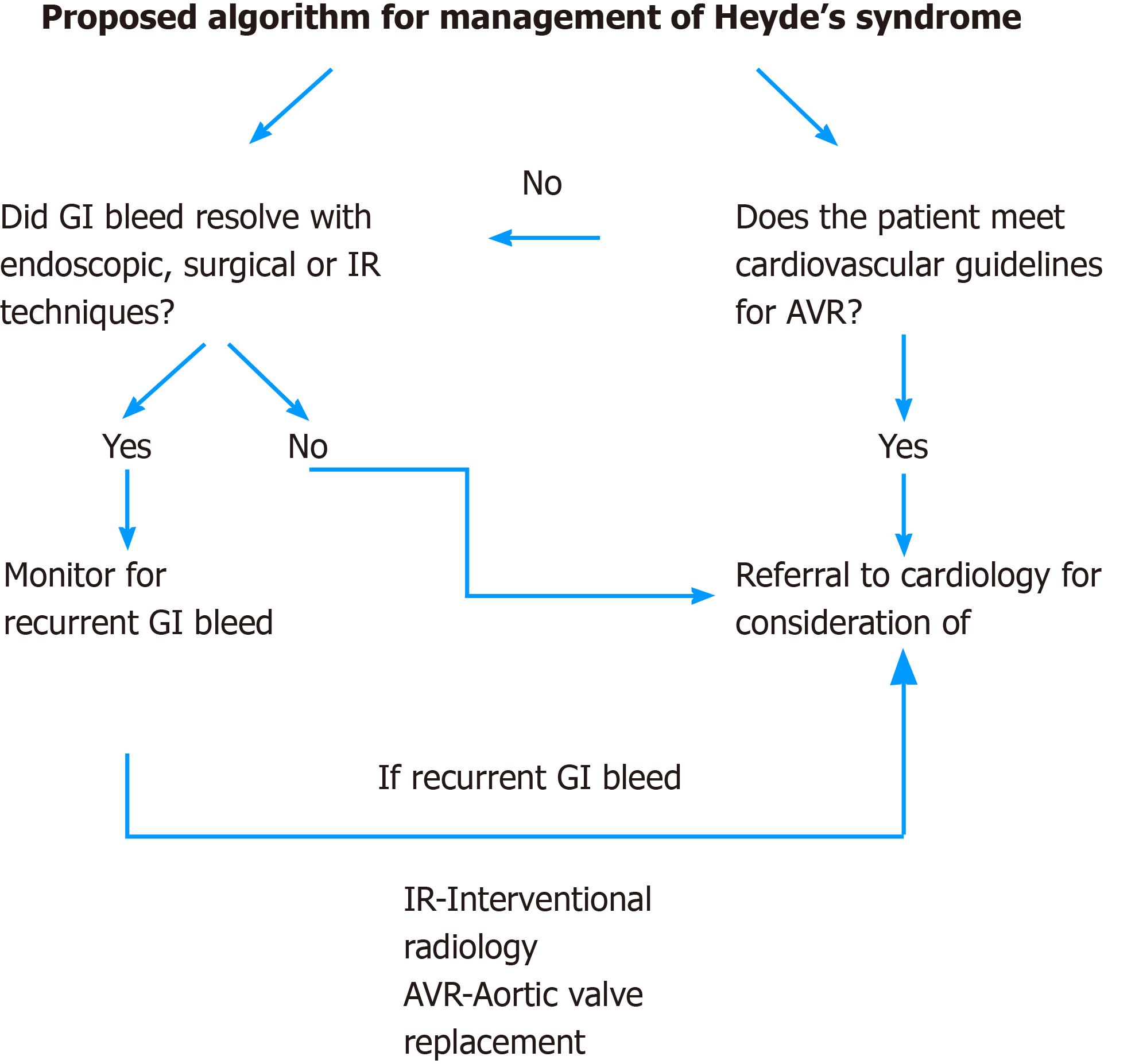
Aortic valve stenosis is corrected either by surgical techniques (bio-prosthetic or mechanical) or by TAVR. Bio-prosthetic valve may have an upper hand over mechanical valve in preventing recurrent GI bleed theoretically, as the latter will need anticoagulation. In a retrospective analysis of Heyde’s syndrome and surgical aortic valve replacement, recurrent GI bleed was shown to be significantly lower with bioprosthetic when compared to mechanical prosthesis (15% vs 50%)[42]. The two most commonly used scores to determine the candidacy for surgical vs TAVR are the STS (Society of Thoracic Surgeons)[52] risk score and the EuroScore[53]. TAVR is currently the treatment of choice in patients with intermediate[52] to high risk for the conventional surgical valve replacement. In a recent randomized control trial with intermediate-risk patients, TAVR was similar to surgical aortic-valve replacement with respect to the primary end point of death or disabling stroke with a significantly lower rate of life threatening bleeding at 30 d in TAVR group (10.4% vs 43.4%)[54]. Recent studies have shown that TAVR is non-inferior to surgical valve replacement even in low risk surgical patients[55,56].TAVR (in the context of angiodysplasia associated GI bleeding) has been shown to have fewer peri-operative complications including myocardial infarction and stroke and decreased rates of blood transfusion than surgical valve replacement[57]. Overall, TAVR is becoming a popular and alternative method to surgery for valvular heart diseases. While it may appear reasonable to consider TAVR or aortic valve surgery for Heyde’s syndrome, one cannot make a strong recommendation based on the current available literature. We also proposed an algorithm for management for Heyde’s syndrome (Figure 4).
Bleeding and thromboembolic complications are the major concerns in the post TAVR period. Several factors can determine the use of anti-platelets and /or anti-coagulants including history of coronary artery disease, cardiac stent, atrial fibrillation, stroke, etc. Attempts should be made to minimize the use and/or duration of anti-coagulants and anti-platelet agents in patients with suspected or proven Heyde’s syndrome in appropriate situations. Current guidelines recommend a dual anti-platelet therapy (DAPT) of 3-6 mo after TAVR[58,59]. Recent randomized control trials in TAVR have questioned the use of DAPT against single anti platelet therapy (SAPT), with less bleeding risk with SAPT and no difference in thromboembolic events[60-63]. In a recent randomized trial on TAVR in low risk patients, the use of low dose aspirin plus warfarin did not increase short-term bleeding (30 d)[64]. The option of SAPT with TAVR seems to be promising, especially in the setting of Heyde’s syndrome.
Several factors may determine the outcomes of GI bleeding post aortic valve replacement including the duration and number of anti-platelet agents/anticoagulants, age, co-morbidities, frailty and type of aortic valve replacement. Peri-procedural bleeding can also be related to access site bleeding. Theoretically, correction of acquired Von Willebrand syndrome with Aortic valve replacement (as described in above section) can lead to control or resolution of GI bleeding. Current data on the outcomes of GI bleeding post aortic valve replacement in Heyde’s syndrome is mostly from case reports and show favorable outcomes[13,15,40,41,43-47] (Table 2). There are a few retrospective studies[42,47] which show favorable outcomes with no recurrence of GI bleed in 79%-86% of patients post TAVR. Thompson et al[42], in a retrospective analysis of 57 patients with Heyde’s syndrome who underwent AVR, found that in a 15 year follow-up, 79% had no recurrence of GI bleeding.
GI bleeding may complicate TAVR regardless of its existence pre TAVR, and the rates of GI bleeding vary according to literature (1.4%-11.8%). Spiewak et al[65], in a retrospective analysis of 482 patients hospitalized for TAVR showed that GI bleed was only 1.4% in the immediate post TAVR period, with 40.6% of the population on DAPT and 29% on oral anticoagulation[65]. However, the risk of GI bleeding post TAVR is may be significantly higher (up to 10 fold, 11.8%) in the setting of triple therapy with DAPT and oral anticoagulant use as shown in a large cohort study by Stanger et al[66]. A large retrospective analysis on readmission rates for late GI bleeding following TAVR vs surgical aortic valve replacement showed that it was higher in TAVR cohort (3.3%) than surgical cohort (1.5%) with average time to readmission similar in both groups (approximately 90 d)[67]. Another large retrospective study from France, involving a cohort of 372 patients receiving TAVR, showed that major GI bleeding occurred in up to 11.3% of population with a median follow-up of 383 d[68]. For patients with high CHADS2-VASc score and clinically high risk for bleeding (HAS-BLED score)[69] with anti-coagulants or anti-platelet agents, WATCHMAN device (left atrial appendage closure) is another option. WATCH-TAVR is a prospective, multi-center, randomized controlled trial currently enrolling patients in 32 centers in United States, aiming at the prevention of stroke and bleeding in patients with atrial fibrillation undergoing TAVR by using WATCHMAN device.
Heyde’s syndrome, a complex multi system disorder is often overlooked and under reported, especially in the growing era of geriatric population. Heyde’s syndrome should be suspected in patients with intestinal bleeding and aortic stenosis. A high index of suspicion will improve the diagnosis. With evolution in interventional cardiology and gastroenterology the prognosis of this syndrome appears to be excellent. Future randomized controlled trials comparing clinical outcomes with and without aortic valve correction in Heyde’s syndrome will help formulate guidelines for aortic valve correction in Heyde’s syndrome.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kawaratani H S-Editor: Ma YJ L-Editor: A P-Editor: Xing YX
| 1. | Heyde EC. Gastrointestinal bleeding in aortic stenosis. N Engl J Med. 1958;259:196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 2. | Warkentin TE, Moore JC, Morgan DG. Aortic stenosis and bleeding gastrointestinal angiodysplasia: is acquired von Willebrand's disease the link? Lancet. 1992;340:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 186] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Greenstein RJ, McElhinney AJ, Reuben D, Greenstein AJ. Colonic vascular ectasias and aortic stenosis: coincidence or causal relationship? Am J Surg. 1986;151:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Boley SJ, Sammartano R, Adams A, DiBiase A, Kleinhaus S, Sprayregen S. On the nature and etiology of vascular ectasias of the colon. Degenerative lesions of aging. Gastroenterology. 1977;72:650-660. [PubMed] |
| 5. | Regula J, Wronska E, Pachlewski J. Vascular lesions of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2008;22:313-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Dodda G, Trotman BW. Gastrointestinal angiodysplasia. J Assoc Acad Minor Phys. 1997;8:16-19. [PubMed] |
| 7. | Tedesco FJ, Griffin JW Jr, Khan AQ. Vascular ectasia of the colon: clinical, colonoscopic, and radiographic features. J Clin Gastroenterol. 1980;2:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Batur P, Stewart WJ, Isaacson JH. Increased prevalence of aortic stenosis in patients with arteriovenous malformations of the gastrointestinal tract in Heyde syndrome. Arch Intern Med. 2003;163:1821-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Meyer CT, Troncale FJ, Galloway S, Sheahan DG. Arteriovenous malformations of the bowel: an analysis of 22 cases and a review of the literature. Medicine (Baltimore). 1981;60:36-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 126] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Cody MC, O'Donovan TP, Hughes RW Jr. Idiopathic gastrointestinal bleeding and aortic stenosis. Am J Dig Dis. 1974;19:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Undas A, Natorska J. Bleeding in patients with severe aortic stenosis in the era of transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015;8:701-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, Bauters A, Decoene C, Goudemand J, Prat A, Jude B. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 580] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 13. | Love JW. The syndrome of calcific aortic stenosis and gastrointestinal bleeding: resolution following aortic valve replacement. J Thorac Cardiovasc Surg. 1982;83:779-783. [PubMed] |
| 14. | Warkentin TE, Moore JC, Morgan DG. Gastrointestinal angiodysplasia and aortic stenosis. N Engl J Med. 2002;347:858-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Scheffer SM, Leatherman LL. Resolution of Heyde's syndrome of aortic stenosis and gastrointestinal bleeding after aortic valve replacement. Ann Thorac Surg. 1986;42:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. the Tromsø study. Heart. 2013;99:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 434] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 17. | Bhatia N, Basra SS, Skolnick AH, Wenger NK. Aortic valve disease in the older adult. J Geriatr Cardiol. 2016;13:941-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 18. | Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3079] [Cited by in RCA: 3318] [Article Influence: 174.6] [Reference Citation Analysis (0)] |
| 19. | Kanwar A, Thaden JJ, Nkomo VT. Management of Patients With Aortic Valve Stenosis. Mayo Clin Proc. 2018;93:488-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 20. | Spangenberg T, Budde U, Schewel D, Frerker C, Thielsen T, Kuck KH, Schäfer U. Treatment of acquired von Willebrand syndrome in aortic stenosis with transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015;8:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Foutch PG, Rex DK, Lieberman DA. Prevalence and natural history of colonic angiodysplasia among healthy asymptomatic people. Am J Gastroenterol. 1995;90:564-567. [PubMed] |
| 22. | Chalasani N, Cotsonis G, Wilcox CM. Upper gastrointestinal bleeding in patients with chronic renal failure: role of vascular ectasia. Am J Gastroenterol. 1996;91:2329-2332. [PubMed] |
| 23. | Alhumood SA, Devine DV, Lawson L, Nantel SH, Carter CJ. Idiopathic immune-mediated acquired von Willebrand's disease in a patient with angiodysplasia: demonstration of an unusual inhibitor causing a functional defect and rapid clearance of von Willebrand factor. Am J Hematol. 1999;60:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Raju GS, Gerson L, Das A, Lewis B; American Gastroenterological Association. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1697-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 25. | Federici AB, Rand JH, Bucciarelli P, Budde U, van Genderen PJ, Mohri H, Meyer D, Rodeghiero F, Sadler JE; Subcommittee on von Willebrand Factor. Acquired von Willebrand syndrome: data from an international registry. Thromb Haemost. 2000;84:345-349. [PubMed] |
| 26. | Nichols WL, Rick ME, Ortel TL, Montgomery RR, Sadler JE, Yawn BP, James AH, Hultin MB, Manco-Johnson MJ, Weinstein M. Clinical and laboratory diagnosis of von Willebrand disease: a synopsis of the 2008 NHLBI/NIH guidelines. Am J Hematol. 2009;84:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Denis CV, Lenting PJ. von Willebrand factor: at the crossroads of bleeding and thrombosis. Int J Hematol. 2012;95:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Gill JC, Wilson AD, Endres-Brooks J, Montgomery RR. Loss of the largest von Willebrand factor multimers from the plasma of patients with congenital cardiac defects. Blood. 1986;67:758-761. [PubMed] |
| 29. | Veyradier A, Balian A, Wolf M, Giraud V, Montembault S, Obert B, Dagher I, Chaput JC, Meyer D, Naveau S. Abnormal von Willebrand factor in bleeding angiodysplasias of the digestive tract. Gastroenterology. 2001;120:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Sugimoto M, Matsui H, Mizuno T, Tsuji S, Miyata S, Matsumoto M, Matsuda M, Fujimura Y, Yoshioka A. Mural thrombus generation in type 2A and 2B von Willebrand disease under flow conditions. Blood. 2003;101:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Blackshear JL. Heyde Syndrome: Aortic Stenosis and Beyond. Clin Geriatr Med. 2019;35:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Heilmann C, Geisen U, Beyersdorf F, Nakamura L, Benk C, Trummer G, Berchtold-Herz M, Schlensak C, Zieger B. Acquired von Willebrand syndrome in patients with extracorporeal life support (ECLS). Intensive Care Med. 2012;38:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 33. | Klovaite J, Gustafsson F, Mortensen SA, Sander K, Nielsen LB. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J Am Coll Cardiol. 2009;53:2162-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 34. | Tamura T, Horiuchi H, Imai M, Tada T, Shiomi H, Kuroda M, Nishimura S, Takahashi Y, Yoshikawa Y, Tsujimura A, Amano M, Hayama Y, Imamura S, Onishi N, Tamaki Y, Enomoto S, Miyake M, Kondo H, Kaitani K, Izumi C, Kimura T, Nakagawa Y. Unexpectedly High Prevalence of Acquired von Willebrand Syndrome in Patients with Severe Aortic Stenosis as Evaluated with a Novel Large Multimer Index. J Atheroscler Thromb. 2015;22:1115-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10:2428-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 36. | Starke RD, Ferraro F, Paschalaki KE, Dryden NH, McKinnon TA, Sutton RE, Payne EM, Haskard DO, Hughes AD, Cutler DF, Laffan MA, Randi AM. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 37. | Franchini M, Mannucci PM. Von Willebrand disease-associated angiodysplasia: a few answers, still many questions. Br J Haematol. 2013;161:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Randi AM, Laffan MA. Von Willebrand factor and angiogenesis: basic and applied issues. J Thromb Haemost. 2017;15:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 39. | Warkentin TE, Moore JC, Anand SS, Lonn EM, Morgan DG. Gastrointestinal bleeding, angiodysplasia, cardiovascular disease, and acquired von Willebrand syndrome. Transfus Med Rev. 2003;17:272-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Cappell MS, Lebwohl O. Cessation of recurrent bleeding from gastrointestinal angiodysplasias after aortic valve replacement. Ann Intern Med. 1986;105:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Abi-Akar R, El-Rassi I, Karam N, Jassar Y, Slim R, Jebara V. Treatment of Heyde's Syndrome by Aortic Valve Replacement. Curr Cardiol Rev. 2011;7:47-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Thompson JL 3rd, Schaff HV, Dearani JA, Park SJ, Sundt TM 3rd, Suri RM, Blackshear JL, Daly RC. Risk of recurrent gastrointestinal bleeding after aortic valve replacement in patients with Heyde syndrome. J Thorac Cardiovasc Surg. 2012;144:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Kadkhodayan K, Anklesaria A, Khorshidi I, Dendi VS, Rahmani R. Resolution of Recurrent Gastrointestinal Bleeding after Transcatheter Aortic Valve Replacement in a Patient with Heyde's Syndrome. Am J Gastroenterol. 2012;107:S363-S364. [DOI] [Full Text] |
| 44. | Balbo CP, Seabra LP, Galoro VG, Caputi G, Palma JH, Buffolo Ê. Heyde's Syndrome and Transcatheter Aortic Valve Implantation. Arq Bras Cardiol. 2017;108:378-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Alshuwaykh O, Krier MJ. A Case of Heyde Syndrome with Resolution of Gastrointestinal Bleeding Two Weeks After Aortic Valve Replacement. Am J Case Rep. 2018;19:924-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Garcia LR, Garzesi AM, Tripoli G, Campos NLKL, Martins AS, Felicio ML. Heyde Syndrome Treated by Conventional Aortic Valve Replacement. Braz J Cardiovasc Surg. 2019;34:630-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Famularo G, Marrollo M. Of aortic valve and bleeding: Heyde's syndrome. Am J Emerg Med. 2020;38:2493.e1-2493.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Godino C, Lauretta L, Pavon AG, Mangieri A, Viani G, Chieffo A, Galaverna S, Latib A, Montorfano M, Cappelletti A, Maisano F, Alfieri O, Margonato A, Colombo A. Heyde's syndrome incidence and outcome in patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;61:687-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Natorska J, Bykowska K, Hlawaty M, Marek G, Sadowski J, Undas A. Increased thrombin generation and platelet activation are associated with deficiency in high molecular weight multimers of von Willebrand factor in patients with moderate-to-severe aortic stenosis. Heart. 2011;97:2023-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Blackshear JL, Wysokinska EM, Safford RE, Thomas CS, Stark ME, Shapiro BP, Ung S, Johns GS, Chen D. Indexes of von Willebrand factor as biomarkers of aortic stenosis severity (from the Biomarkers of Aortic Stenosis Severity [BASS] study). Am J Cardiol. 2013;111:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Van Belle E, Rauch A, Vincentelli A, Jeanpierre E, Legendre P, Juthier F, Hurt C, Banfi C, Rousse N, Godier A, Caron C, Elkalioubie A, Corseaux D, Dupont A, Zawadzki C, Delhaye C, Mouquet F, Schurtz G, Deplanque D, Chinetti G, Staels B, Goudemand J, Jude B, Lenting PJ, Susen S. Von Willebrand factor as a biological sensor of blood flow to monitor percutaneous aortic valve interventions. Circ Res. 2015;116:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 52. | O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP; Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg. 2009;88:S23-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 935] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 53. | Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur Heart J. 2003;24:881-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 781] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 54. | Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; PARTNER 2 Investigators. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3232] [Cited by in RCA: 3809] [Article Influence: 423.2] [Reference Citation Analysis (0)] |
| 55. | Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL 3rd, Forrest JK, Tchétché D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ; Evolut Low Risk Trial Investigators. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2802] [Cited by in RCA: 2655] [Article Influence: 442.5] [Reference Citation Analysis (0)] |
| 56. | Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR; PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3668] [Cited by in RCA: 3480] [Article Influence: 580.0] [Reference Citation Analysis (0)] |
| 57. | Desai R, Parekh T, Singh S, Patel U, Fong HK, Zalavadia D, Savani S, Doshi R, Sachdeva R, Kumar G. Alarming Increasing Trends in Hospitalizations and Mortality With Heyde's Syndrome: A Nationwide Inpatient Perspective (2007 to 2014). Am J Cardiol. 2019;123:1149-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1738] [Cited by in RCA: 1884] [Article Influence: 235.5] [Reference Citation Analysis (0)] |
| 59. | Otto CM, Kumbhani DJ, Alexander KP, Calhoon JH, Desai MY, Kaul S, Lee JC, Ruiz CE, Vassileva CM. 2017 ACC Expert Consensus Decision Pathway for Transcatheter Aortic Valve Replacement in the Management of Adults With Aortic Stenosis: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2017;69:1313-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 387] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 60. | Gargiulo G, Collet JP, Valgimigli M. Antithrombotic therapy in TAVI patients: changing concepts. EuroIntervention. 2015;11 Suppl W:W92-W95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Collet JP, Montalescot G. Antithrombotic and antiplatelet therapy in TAVI patients: a fallow field? EuroIntervention. 2013;9 Suppl:S43-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Capodanno D, Angiolillo DJ. Antithrombotic Therapy for Prevention of Cerebral Thromboembolic Events After Transcatheter Aortic Valve Replacement: Evolving Paradigms and Ongoing Directions. JACC Cardiovasc Interv. 2017;10:1366-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Brouwer J, Nijenhuis VJ, Delewi R, Hermanides RS, Holvoet W, Dubois CLF, Frambach P, De Bruyne B, van Houwelingen GK, Van Der Heyden JAS, Toušek P, van der Kley F, Buysschaert I, Schotborgh CE, Ferdinande B, van der Harst P, Roosen J, Peper J, Thielen FWF, Veenstra L, Chan Pin Yin DRPP, Swaans MJ, Rensing BJWM, van 't Hof AWJ, Timmers L, Kelder JC, Stella PR, Baan J, Ten Berg JM. Aspirin with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N Engl J Med. 2020;383:1447-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 64. | Rogers T, Shults C, Torguson R, Shea C, Parikh P, Bilfinger T, Cocke T, Brizzio ME, Levitt R, Hahn C, Hanna N, Comas G, Mahoney P, Newton J, Buchbinder M, Moreno R, Zhang C, Craig P, Asch FM, Weissman G, Garcia-Garcia HM, Ben-Dor I, Satler LF, Waksman R. Randomized Trial of Aspirin Versus Warfarin After Transcatheter Aortic Valve Replacement in Low-Risk Patients. Circ Cardiovasc Interv. 2021;14:e009983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 65. | Spiewak T, Eskandari A, Bang H, Tejaswi S. Gastrointestinal Bleeding During Hospitalization for Transcatheter Aortic Valve Replacement. Am J Gastroenterol. 2018;113:S316. [DOI] [Full Text] |
| 66. | Stanger DE, Abdulla AH, Wong FT, Alipour S, Bressler BL, Wood DA, Webb JG. Upper gastrointestinal bleeding following transcatheter aortic valve replacement: A retrospective analysis. Catheter Cardiovasc Interv. 2017;90:E53-E61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Iyengar A, Sanaiha Y, Aguayo E, Seo YJ, Dobaria V, Toppen W, Shemin RJ, Benharash P. Comparison of Frequency of Late Gastrointestinal Bleeding With Transcatheter Versus Surgical Aortic Valve Replacement. Am J Cardiol. 2018;122:1727-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 68. | Kibler M, Marchandot B, Messas N, Labreuche J, Vincent F, Grunebaum L, Hoang VA, Reydel A, Crimizade U, Kindo M, Hoang MT, Zeyons F, Trinh A, Petit-Eisenmann H, De Poli F, Leddet P, Duhamel A, Jesel L, Ohana M, Susen S, Ohlmann P, Van Belle E, Morel O. Primary Hemostatic Disorders and Late Major Bleeding After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2018;72:2139-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 69. | Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2971] [Cited by in RCA: 3354] [Article Influence: 223.6] [Reference Citation Analysis (0)] |