Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7311
Peer-review started: April 14, 2021
First decision: May 11, 2021
Revised: May 16, 2021
Accepted: August 4, 2021
Article in press: August 4, 2021
Published online: September 6, 2021
Processing time: 138 Days and 23.9 Hours
Extracellular vesicles (EVs) are cystic vesicles naturally released by most mammalian cells and bacteria. EV contents include proteins, lipids, and nucleic acids. EVs can act as messengers to transmit a variety of molecules to recipient cells and thus play important regulatory roles in intercellular signal transduction. EVs, released by either a host cell or a pathogen, can carry pathogen-associated antigens and thus act as modulators of immune responses. EVs derived from Mycobacterium tuberculosis (Mtb)-infected cells can regulate the innate immune response through various pathways, such as regulating the release of inflammatory cytokines. In addition, EVs can mediate antigen presentation and regulate the adaptive immune response by transmitting immunoregulatory molecules to T helper cells. In this review, we summarize the regulatory roles of EVs in the immune response against Mtb.
Core Tip: Extracellular vesicles (EVs) are nanoscale membrane-bound structures released by mammalian cells and bacteria and play essential regulatory roles in intercellular signal transduction and the immune response. In this review, we discuss the regulatory role of EVs released by Mycobacterium tuberculosis (Mtb)-infected cells in the anti-Mtb immune response. Specifically, we focus on providing the most cutting-edge information on EVs released by Mtb-infected cells regulating the body’s immune response, including the regulatory roles in innate and acquired immune responses. In addition, we describe the basis for EV-mediated regulation of the immune response in detail, i.e., the EVs released by Mtb-infected host cells contain Mtb-associated anti
- Citation: Yan Z, Wang H, Mu L, Hu ZD, Zheng WQ. Regulatory roles of extracellular vesicles in immune responses against Mycobacterium tuberculosis infection. World J Clin Cases 2021; 9(25): 7311-7318
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7311.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7311
Tuberculosis (TB) is one of the major lethal infectious diseases caused by Mycobacterium tuberculosis (Mtb). According to the World Health Organization’s global TB report of 2020, approximately 10 million people were infected with Mtb in 2019, causing approximately 1.4 million deaths[1]. Although several public health measures have been taken to prevent the spread of Mtb, the situation is concerning[2]. Drug-resistant Mtb, especially rifampicin-resistant Mtb, has become one of the deadliest pathogens in the world[1,2]. Therefore, the development of novel anti-Mtb reagents or vaccines is urgent and essential. Investigating the molecular mechanism of the immune response against Mtb is of great value because this information is the basis for preventive and therapeutic approach developments.
Extracellular vesicles (EVs) are nanoscale membrane-bound structures released by mammalian cells and bacteria. They contain proteins, lipids, and nucleic acids. EVs can be categorized into four types according to their biological origin, release pathway, size, and content: Exosomes, microparticles, microvesicles, and apoptotic bodies. Exosomes are mainly formed through the fusion of multivesicular bodies with the plasma membrane and the extracellular release of intracavitary vesicles, with a diameter of 30–100 nm and a buoyancy density of 1.13-1.19 g/mL. Exosomes are cup-shaped under a transmission electron microscope and characterized by the expression of CD63 and CD61[3]. The term “exosome” was first proposed by Trams et al[4] in 1981. Johnstone and Harding first isolated exosomes while studying the transferrin cycle[5,6]. Exosomes can be secreted by various eukaryotic cells, including macro
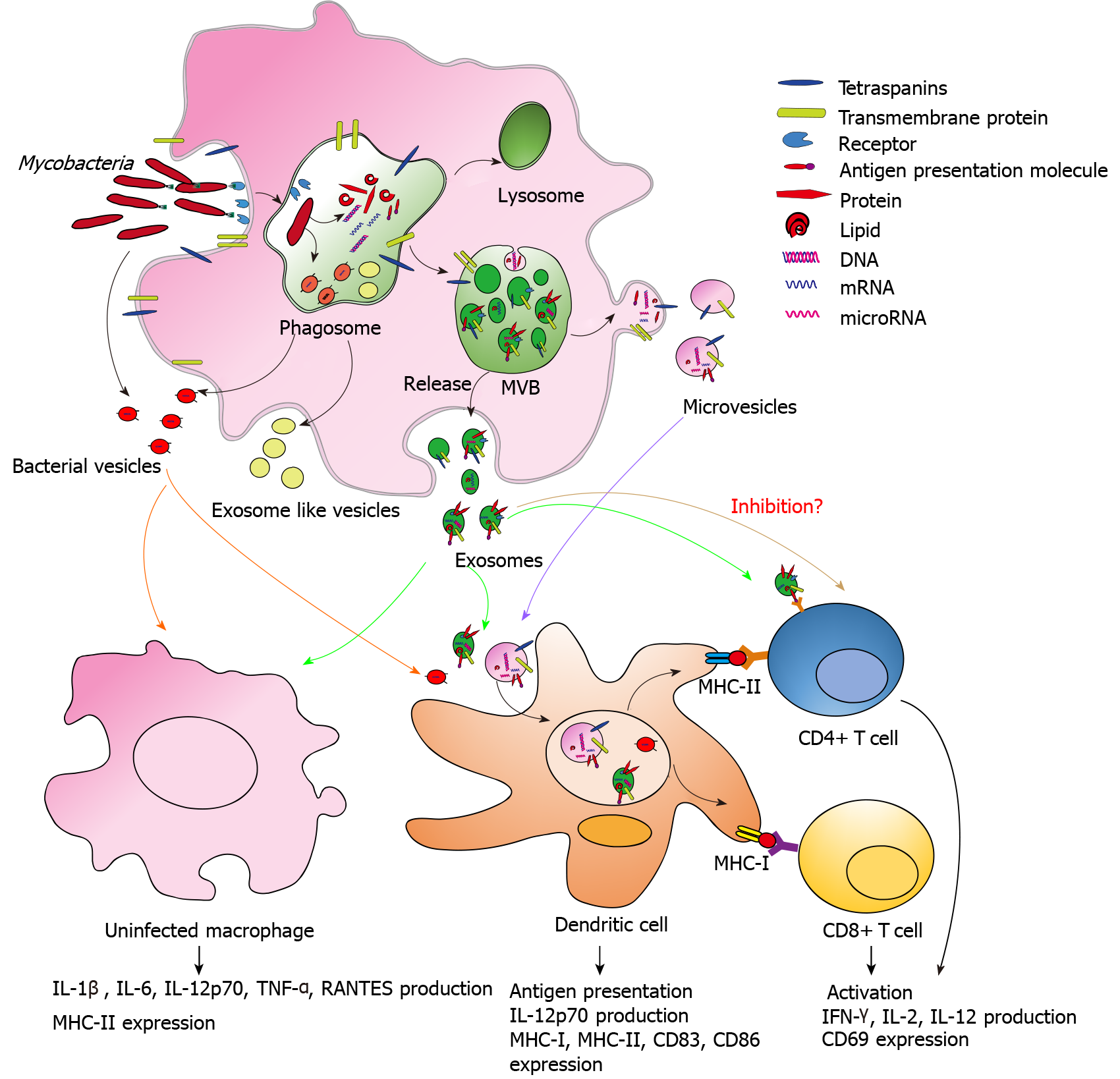
After being inhaled into the respiratory tract, Mtb is first recognized by antigen-presenting cells (APCs) resident in the lungs, including alveolar macrophages, pulmonary macrophages, and DCs[11]. The pattern-recognition receptors expressed on the APC surface sense pathogen-associated molecular patterns and endocytose Mtb to form a phagolysosome. Simultaneously, the innate immune response is initiated, and several inflammatory cytokines are released to promote the clearance of Mtb in APCs. Subsequently, the APCs migrate to the lymph nodes and initiate an adaptive immune response through antigen presentation and cytokine secretion[12-14].
However, Mtb has a variety of immune evasion strategies[15]. For example, it can inhibit the acidification and maturation of phagolysosomes via multiple virulence factors, such as protein tyrosine kinase[16], protein tyrosine phosphatase[17], and lipoarabinomannan (LAM)[18]. Mtb can also affect the adaptive immune response by regulating antigen presentation. For example, Mtb lipoarabinomannan mannose can bind to CD209 on DCs and inhibit DC maturation[19], promote the release of interleukin (IL)-10, reduce the synthesis of IL-12, and ultimately inhibit the release of interferon-γ (IFN-γ) by T helper (Th) cells[20]. The immune evasion mechanisms of Mtb are beyond the scope of this review; for more details, please refer to the review by Lerner et al[11].
Some in vitro experiments have indicated that Mtb infection can increase the exosome yield and alter the protein composition of EVs released by host cells. For example, the exosome yield of mouse macrophages increases approximately two-fold after Mtb infection[21]. However, not all types of cells produce increased amounts of exosomes after Mtb infection. For example, Diaz et al[22] found that the exosome yields of THP-1-derived macrophages infected with Mtb or left uninfected were comparable.
Mtb infection can alter the protein profile of exosomes released by a host cell. A study applying liquid chromatography-tandem mass spectrometry revealed that there were 355 host proteins in the exosomes released by Mtb-infected macrophages. Most of the proteins were membrane proteins, and 41 of the proteins, including Hsp90, vimentin, Coronin 1 C, and moesin, were increased after Mtb infection[22]. In addition, Mtb itself can also release vesicles, which are termed bacterial vesicles (BVs)[23,24]. BVs are rich in Mtb antigens, such as SodB, EsxN, and Ag85b[23,24]. The protein composition of BVs is different from that of Mtb itself[23]. Furthermore, there is great overlap between the protein profile of vesicles released by Mtb-infected cells and BVs in aseptic culture[22,23,25-27]. Several studies have confirmed that exosomes released by Mtb- or Mycobacterium bovis (M. bovis) BCG-infected cells contain Mtb-related anti
Notably, no Mtb antigens have been observed in exosomes released by mouse macrophages infected with heat-inactivated or γ-ray-inactivated Mtb[27,30], while the exosomes produced by J774 cells treated with Mtb culture filtrate protein 10 were shown to contain Mtb antigens[30]. In addition, heat-inactivated Mtb cannot release BVs when cultured alone[31]. Taken together, these findings imply that the Mtb antigens in Mtb-infected cell-released exosomes are induced by live Mtb or Mtb secreted proteins rather than mycobacterial lysis within the infected cell.
A previous study revealed that the Rab27a gene is essential for the synthesis of mammalian exosomes[32]. In vitro experiments indicated that Rab27a knockout could decrease the exosome yield and the content of Mtb proteins in exosomes[21]. Furthermore, compared with wild-type mice, Rab27a knockout mice had decreased serum exosome levels after Mtb infection[21]. The bacterial load was also shown to be increased in Rab27a knockout mice, suggesting that exosomes participate in the immune response against Mtb[21]. In another study, Bhatnagar et al[25] infected mice with M. bovis BCG and found that the exosomes in the bronchial lavage fluid contained both human components (Hsp70) and Mtb proteins, such as LAM and 19 kDa lipoproteins.
Mtb antigens have also been found in the serum exosomes of TB patients. Kruh-Garcia et al[33] used the multiple reaction monitoring technique to analyze the protein profiles of serum exosomes from patients with active TB. They found that there were 76 peptides (33 proteins) in the serum of TB patients, and 20 of them were increased when compared to the levels in TB-negative patients. These proteins were derived from Mtb and are critical to the survival of Mtb.
In conclusion, in vitro, animal, and clinical studies have revealed that Mtb can induce the release of exosomes that contain Mtb proteins. These exosomes may play crucial roles in the anti-Mtb immune response.
As mentioned above, EVs released by cells infected with Mtb or M. bovis BCG carry Mtb proteins, such as LAM[24,25,27], the Ag85 complex[26,30], lipoproteins (LpqH and LprG)[21,27], and 19 kDa lipoproteins[25,30]. Therefore, these EVs can trigger an inflammatory response after being taken up by APCs. In addition, these EVs have been confirmed to promote the migration of macrophages, neutrophils, and lymphocytes to the lungs both in vivo and in vitro[34].
Exosomes released from Mtb- or M. bovis BCG-infected J774 cells, THP-1 cells, and RAW264.7 cells can trigger mouse macrophages to release inflammatory cytokines, such as IL-1β, IL-6, IL-12p70, tumor necrosis factor-α (TNF-α), and regulated upon activation normal T cell expressed and secreted factor, and upregulate the expression of iNOS[25,30,31,34]. Exosomes from the serum of mice infected with M. bovis BCG can also promote the expression of inflammatory factors in mouse macrophages[34]. The induction of IL-1β and IL-6 is mediated by Toll-like receptor (TLR) 2, while the release of IL-10 and CCL3 is independent of TLR2[31]. The induction of TNF-α is also MyD88 and TLR4 dependent[25]. In addition, exosomes can promote the phosphorylation of p38 and IκB in mouse bone marrow-derived macrophages[25], suggesting that p38 and IκB are also involved in the production of inflammatory cytokines.
IFN-γ can enhance the clearance of Mtb in three ways: (1) Promoting the clearance of intracellular pathogens by supporting macrophages to enhance the response to reactive oxygen species or reactive nitrogen[35]; (2) Promoting the adaptive immune response by enhancing the expression of major histocompatibility complex II (MHC II)[36]; and (3) Promoting an autophagic response against pathogens[37]. The exosomes released by Mtb-infected RAW264.3 cells can inhibit the upregulation of MHC II and CD64 induced by IFN-γ in uninfected mouse macrophages through TLR2 and MyD88[24,38]. This inhibitory effect of exosomes is associated with the cargo Mtb lipoprotein, as exosomes produced by RAW264.7 cells infected with lspA knockout (unable to synthesize lipoprotein) Mtb fail to inhibit CD64 expression induced by IFN-γ[38].
The expression of miR-18a is increased in Mtb-infected RAW264.3 cells[39], while the expression of miR-20b-5p is decreased[40]. These two microRNAs can regulate the survival, apoptosis, and proliferation of macrophages[39,40]. Both of these microRNAs were also found to be elevated in exosomes released by Mtb-infected RAW 264.3 cells, but it remains unknown whether these exosomes can be taken up by uninfected macrophages. Two studies compared the microRNA profiles of exosomes released by cells infected with Mtb or M. bovis BCG or left uninfected and verified many differentially expressed microRNAs[29,41]. Bioinformatic analysis showed that these differentially expressed microRNAs are involved in the regulation of multiple signaling pathways, including central carbon, fatty acid, and sugar metabolism[42], but whether these microRNAs can regulate the immune response remains unclear.
As mentioned earlier, Rab27a is a key regulator of the fusion of exosomes and the plasma membrane[32]. Smith et al[21] found that Mtb-infected Rab27a gene-deficient mice released decreased amounts of exosomes and consequently had an increased bacterial load and a significantly reduced activated CD4+ T cell population in the spleen, indicating that exosomes promote the adaptive immune response against Mtb in vivo. Furthermore, exosomes promote the T cell response during Mtb mouse infection. Since IFN-γ is mainly produced by Th1 cells during Mtb infection[43], these studies suggest that exosomes are involved in the adaptive immune response against Mtb and can promote an antigen-specific T cell response[21].
The exosomes secreted by M. bovis BCG-infected J774 macrophages can enhance the expression of CD83, CD86, IL-12p40, and MHC II in mouse bone marrow-derived dendritic cells (BMDCs) and thus promote BMDCs mutation[26]. The release of IL-12p40 by DCs can promote the Th1 response[44,45]. Therefore, exosomes containing Mtb antigens can promote a subsequent Th1 response via DCs. In addition, Mtb itself can also release EVs (termed BVs) in culture, and these vesicles can upregulate the expression of CD86, MHC I, and MHC II in mouse BMDCs, which thereby enhances the release of IL-2 by Ag85-specific T cells[24]. Ramachandra et al[46] found that macrophages infected with Mtb could release EVs containing MHC II, including microvesicles and exosomes, in which ATP greatly enhanced the release of vesicles. Microvesicles and exosomes have the ability to present Mtb peptide–MHC II complexes to T cells[46]. These results suggest that innate immune cells can deliver Mtb antigens to T cells outside the infected site by releasing microvesicles and exosomes.
The exosomes released from M. bovis BCG-infected J774 cells can promote the proliferation of T cells and upregulate the expression of CD69[26], which is a marker of T cell activation[47]. In addition, these exosomes can directly enhance IFN-γ release from CD4+ and CD8+ T cells in M. bovis BCG-immunized mice[26]. These biological functions can be further enhanced in the presence of DCs[26]. In vivo studies have indicated that treating mice with exosomes can increase the proportion of spleen effector T cells (CD62L-low, CD44-high)[26]. These findings were further validated by subsequent studies. Exosomes from Mtb antigen-treated cells (Mtb CFP-treated J744 cells[30] or Mtb CFP-treated RAW264.7 cells[48]) can also activate T cells from Mtb antigen-immunized mice and enhance T cell production of IFN-γ[26,30] and IL-12[48] in vitro. Interestingly, adjuvants have little effect on the production of IFN-γ and IL-12[26,30], indicating that these exosomes may contain some types of substances similar to adjuvants. Furthermore, these exosomes can induce the production of memory T cells in mice[26,30] and reduce the susceptibility of mice to Mtb[48].
Athman et al[49] found that EVs released by macrophages from mice infected with Mtb and Mtb BVs could directly inhibit the anti-CD3 and anti-CD28 antibody-induced activation of naive T cells and effector T cells. This inhibitory effect was mainly attributed to Mtb antigens in the EVs, including LAM, LM, PIM1/2, and PIM6. Previous studies have shown that these Mtb antigens can inhibit the activation of T cells, which represents one of the immune evasion mechanisms of Mtb[50]. EVs can transmit Mtb antigens to T cells and promote the expression of GRAIL[49], a negative regulator of T cell activation[51,52]. Therefore, EVs can regulate the adaptive immune response against Mtb in at least two ways: Modulating the antigen presentation process and directly regulating T cells.
In recent years, several studies have been performed to explore the characteristics and potential biological functions of EVs in the immune response against Mtb. However, our understanding of the immunomodulatory role of EVs in Mtb infection is still in its early stages. The regulatory roles of EVs in the immune response against Mtb are summarized in Figure 1. The EVs released by Mtb-infected host cells contain Mtb-related proteins and nucleic acids, which establishes the foundation for a regulatory role in the immune response against Mtb. EVs regulate both the innate and adaptive immune responses against Mtb through various pathways. Therefore, EVs may represent a key factor in the development of an Mtb vaccine.
Manuscript source: Invited manuscript
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sahin TT S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Organisation WWH. Global Tuberculosis Report 2020. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports. |
| 2. | Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393:1642-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 546] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 3. | Wang J, Wang Y, Tang L, Garcia RC. Extracellular Vesicles in Mycobacterial Infections: Their Potential as Molecule Transfer Vectors. Front Immunol. 2019;10:1929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 792] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 5. | Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1361] [Cited by in RCA: 1300] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 6. | Johnstone RM, Adam M, Pan BT. The fate of the transferrin receptor during maturation of sheep reticulocytes in vitro. Can J Biochem Cell Biol. 1984;62:1246-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1410] [Cited by in RCA: 1722] [Article Influence: 156.5] [Reference Citation Analysis (0)] |
| 8. | Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1019] [Article Influence: 169.8] [Reference Citation Analysis (0)] |
| 9. | Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 2597] [Article Influence: 432.8] [Reference Citation Analysis (0)] |
| 10. | Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1828] [Cited by in RCA: 2556] [Article Influence: 284.0] [Reference Citation Analysis (0)] |
| 11. | Lerner TR, Borel S, Gutierrez MG. The innate immune response in human tuberculosis. Cell Microbiol. 2015;17:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 476] [Cited by in RCA: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 13. | de Martino M, Lodi L, Galli L, Chiappini E. Immune Response to Mycobacterium tuberculosis: A Narrative Review. Front Pediatr. 2019;7:350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 14. | Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol. 2010;8:296-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 314] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 15. | Zhai W, Wu F, Zhang Y, Fu Y, Liu Z. The Immune Escape Mechanisms of Mycobacterium Tuberculosis. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 16. | Wong D, Li W, Chao JD, Zhou P, Narula G, Tsui C, Ko M, Xie J, Martinez-Frailes C, Av-Gay Y. Protein tyrosine kinase, PtkA, is required for Mycobacterium tuberculosis growth in macrophages. Sci Rep. 2018;8:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Wong D, Bach H, Sun J, Hmama Z, Av-Gay Y. Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. Proc Natl Acad Sci U S A. 2011;108:19371-19376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 295] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 18. | Welin A, Winberg ME, Abdalla H, Särndahl E, Rasmusson B, Stendahl O, Lerm M. Incorporation of Mycobacterium tuberculosis lipoarabinomannan into macrophage membrane rafts is a prerequisite for the phagosomal maturation block. Infect Immun. 2008;76:2882-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 795] [Cited by in RCA: 793] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 20. | Wu T, Guo S, Wang J, Li L, Xu L, Liu P, Ma S, Zhang J, Luo Y. Interaction between mannosylated lipoarabinomannan and dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin influences dendritic cells maturation and T cell immunity. Cell Immunol. 2011;272:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Smith VL, Cheng Y, Bryant BR, Schorey JS. Exosomes function in antigen presentation during an in vivo Mycobacterium tuberculosis infection. Sci Rep. 2017;7:43578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Diaz G, Wolfe LM, Kruh-Garcia NA, Dobos KM. Changes in the Membrane-Associated Proteins of Exosomes Released from Human Macrophages after Mycobacterium tuberculosis Infection. Sci Rep. 2016;6:37975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Lee J, Kim SH, Choi DS, Lee JS, Kim DK, Go G, Park SM, Shin JH, Chang CL, Gho YS. Proteomic analysis of extracellular vesicles derived from Mycobacterium tuberculosis. Proteomics. 2015;15:3331-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Jurkoshek KS, Wang Y, Athman JJ, Barton MR, Wearsch PA. Interspecies Communication between Pathogens and Immune Cells via Bacterial Membrane Vesicles. Front Cell Dev Biol. 2016;4:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234-3244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 503] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 26. | Giri PK, Schorey JS. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One. 2008;3:e2461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 27. | Athman JJ, Wang Y, McDonald DJ, Boom WH, Harding CV, Wearsch PA. Bacterial Membrane Vesicles Mediate the Release of Mycobacterium tuberculosis Lipoglycans and Lipoproteins from Infected Macrophages. J Immunol. 2015;195:1044-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Karbalaei Zadeh Babaki M, Soleimanpour S, Rezaee SA. Antigen 85 complex as a powerful Mycobacterium tuberculosis immunogene: Biology, immune-pathogenicity, applications in diagnosis, and vaccine design. Microb Pathog. 2017;112:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Singh PP, Li L, Schorey JS. Exosomal RNA from Mycobacterium tuberculosis-Infected Cells Is Functional in Recipient Macrophages. Traffic. 2015;16:555-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Giri PK, Kruh NA, Dobos KM, Schorey JS. Proteomic analysis identifies highly antigenic proteins in exosomes from M. tuberculosis-infected and culture filtrate protein-treated macrophages. Proteomics. 2010;10:3190-3202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 31. | Prados-Rosales R, Baena A, Martinez LR, Luque-Garcia J, Kalscheuer R, Veeraraghavan U, Camara C, Nosanchuk JD, Besra GS, Chen B, Jimenez J, Glatman-Freedman A, Jacobs WR Jr, Porcelli SA, Casadevall A. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J Clin Invest. 2011;121:1471-1483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 32. | Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19-30; sup pp 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1525] [Cited by in RCA: 1953] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 33. | Kruh-Garcia NA, Wolfe LM, Chaisson LH, Worodria WO, Nahid P, Schorey JS, Davis JL, Dobos KM. Detection of Mycobacterium tuberculosis peptides in the exosomes of patients with active and latent M. tuberculosis infection using MRM-MS. PLoS One. 2014;9:e103811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 34. | Singh PP, Smith VL, Karakousis PC, Schorey JS. Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo. J Immunol. 2012;189:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 35. | Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991;132:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 258] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 36. | Sicher SC, Chung GW, Vazquez MA, Lu CY. Augmentation or inhibition of IFN-gamma-induced MHC class II expression by lipopolysaccharides. The roles of TNF-alpha and nitric oxide, and the importance of the sequence of signaling. J Immunol. 1995;155:5826-5834. [PubMed] |
| 37. | Rovetta AI, Peña D, Hernández Del Pino RE, Recalde GM, Pellegrini J, Bigi F, Musella RM, Palmero DJ, Gutierrez M, Colombo MI, García VE. IFNG-mediated immune responses enhance autophagy against Mycobacterium tuberculosis antigens in patients with active tuberculosis. Autophagy. 2014;10:2109-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Singh PP, LeMaire C, Tan JC, Zeng E, Schorey JS. Exosomes released from M. tuberculosis infected cells can suppress IFN-γ mediated activation of naïve macrophages. PLoS One. 2011;6:e18564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | Yuan Q, Chen H, Yang Y, Fu Y, Yi Z. miR-18a promotes Mycobacterial survival in macrophages via inhibiting autophagy by down-regulation of ATM. J Cell Mol Med. 2020;24:2004-2012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Zhang D, Yi Z, Fu Y. Downregulation of miR-20b-5p facilitates Mycobacterium tuberculosis survival in RAW 264.7 macrophages via attenuating the cell apoptosis by Mcl-1 upregulation. J Cell Biochem. 2019;120:5889-5896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Alipoor SD, Mortaz E, Tabarsi P, Farnia P, Mirsaeidi M, Garssen J, Movassaghi M, Adcock IM. Bovis Bacillus Calmette-Guerin (BCG) infection induces exosomal miRNA release by human macrophages. J Transl Med. 2017;15:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Alipoor SD, Adcock IM, Folkerts G, Garssen J, Mortaz E. A bioinformatics analysis of exosomal microRNAs released following mycobacterial infection. Int J Mycobacteriol. 2019;8:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249-2254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1855] [Cited by in RCA: 1917] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 44. | Prendergast KA, Kirman JR. Dendritic cell subsets in mycobacterial infection: control of bacterial growth and T cell responses. Tuberculosis (Edinb). 2013;93:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 835] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 46. | Ramachandra L, Qu Y, Wang Y, Lewis CJ, Cobb BA, Takatsu K, Boom WH, Dubyak GR, Harding CV. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing major histocompatibility complex class II molecules capable of antigen presentation. Infect Immun. 2010;78:5116-5125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | Testi R, Phillips JH, Lanier LL. T cell activation via Leu-23 (CD69). J Immunol. 1989;143:1123-1128. [PubMed] |
| 48. | Cheng Y, Schorey JS. Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur J Immunol. 2013;43:3279-3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 49. | Athman JJ, Sande OJ, Groft SG, Reba SM, Nagy N, Wearsch PA, Richardson ET, Rojas R, Boom WH, Shukla S, Harding CV. Mycobacterium tuberculosis Membrane Vesicles Inhibit T Cell Activation. J Immunol. 2017;198:2028-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 50. | Mahon RN, Sande OJ, Rojas RE, Levine AD, Harding CV, Boom WH. Mycobacterium tuberculosis ManLAM inhibits T-cell-receptor signaling by interference with ZAP-70, Lck and LAT phosphorylation. Cell Immunol. 2012;275:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Kriegel MA, Rathinam C, Flavell RA. E3 ubiquitin ligase GRAIL controls primary T cell activation and oral tolerance. Proc Natl Acad Sci U S A. 2009;106:16770-16775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Whiting CC, Su LL, Lin JT, Fathman CG. GRAIL: a unique mediator of CD4 T-lymphocyte unresponsiveness. FEBS J. 2011;278:47-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |