Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7297
Peer-review started: March 24, 2021
First decision: May 1, 2021
Revised: May 14, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: September 6, 2021
Processing time: 167 Days and 16.1 Hours
Since the 19th century, appropriate lymphadenectomy has been considered a cornerstone of oncologic surgery and one of the most important prognostic factors. This approach can be applied to any surgery for gastrointestinal cancer. During surgery for colon and rectal cancer, an adequate portion of the mesentery is removed together with the segment of bowel affected by the disease. The adequate number of lymph nodes to be removed is standardized and reported by several guidelines. It is mandatory to determine the appropriate extent of lymphadenectomy and to balance its oncological benefits with the increased morbidity associated with its execution in cancer patients. Our review focuses on the concept of “complete mesenteric excision (CME) with central vascular ligation (CVL),” a radical lymphadenectomy for colorectal cancer that has gained increasing interest in recent years. The aim of this study was to evaluate the evolution of this approach over the years, its potential oncologic benefits and potential risks, and the improvements offered by laparoscopic techniques. Theoretical advantages of CME are improved local-relapse rates due to complete removal of the intact mesocolic fascia and improved distance recurrence rates due to ligation of vessels at their origin (CVL) which guarantees removal of a larger number of lymph nodes. The development and worldwide diffusion of laparoscopic techniques minimized postoperative trauma in oncologic surgery, providing the same oncologic results as open surgery. This has been widely applied to colorectal cancer surgery; however, CME entails a technical complexity that can limit its wide minimally-invasive application. This review analyzes results of these procedures in terms of oncological outcomes, technical feasibility and complexity, especially within the context of minimally invasive surgery.
Core Tip: An optimal lymphadenectomy is the cornerstone of oncologic surgery. The concept of “optimal” or “adequate” relies on the balance between oncologic advantages and increased morbidity. The extent of lymphadenectomy in colorectal cancer surgery is a highly debated issue. The concept of “central vascular ligation” and “complete mesocolic excision” for radical lymphadenectomy in the era of minimally invasive surgery for colorectal cancer have been investigated.
- Citation: Franceschilli M, Di Carlo S, Vinci D, Sensi B, Siragusa L, Bellato V, Caronna R, Rossi P, Cavallaro G, Guida A, Sibio S. Complete mesocolic excision and central vascular ligation in colorectal cancer in the era of minimally invasive surgery. World J Clin Cases 2021; 9(25): 7297-7305
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7297.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7297
Cancer represents a social disease related to lifestyle habits, environmental pollution, and aging societies, and its incidence has progressively increased during the last several decades, since 20% of men and 15% of women will be diagnosed with cancer in their lifetime and 12% and 10%, respectively, will die of the disease, namely from metastatic progression[1]. For this reason, preventing metastatic spread is of key importance in cancer treatments. Currently, these treatments are highly integrated, combining neoadjuvant and adjuvant chemotherapy with radiotherapy and early or subsequent surgery in several different settings[2].
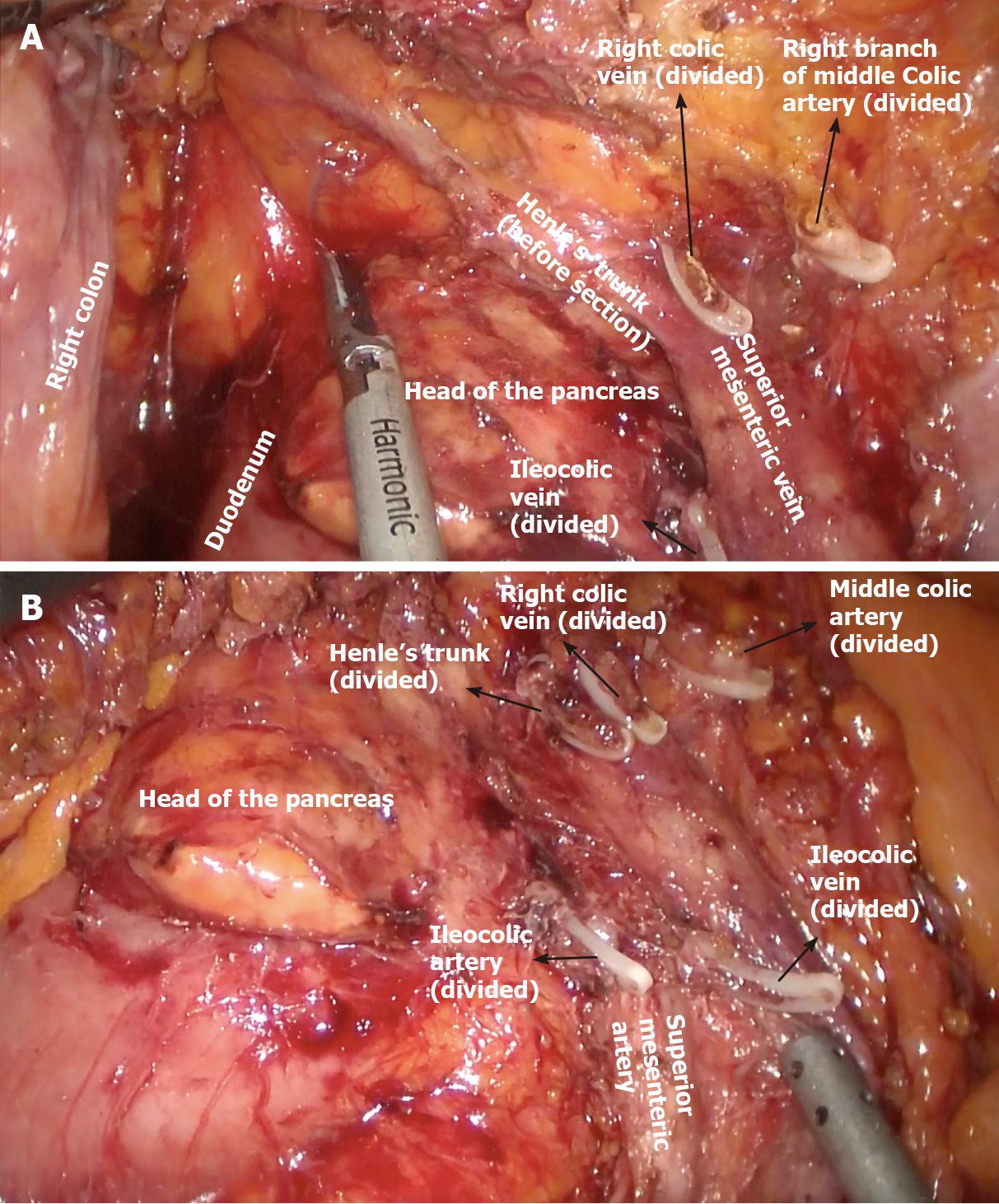
The keystone of surgery is removal of an adequate number of lymph nodes that have both staging and prognostic value. Biological bases of lymphatic spread are theorized by the Halsted and Fisher models. The former describes a highly organized and progressive proximal to distal spread of metastases, whereas the latter suggests an early and completely random spread of metastases[2,3]. Regardless, the adequate extent of lymphadenectomy is always advocated in cancer surgery and worldwide consensus guidelines state the minimal number of lymph nodes to be removed for each type of cancer. In this landscape, complete mesocolic excision with central vascular ligation procedures have been developed to optimize lymph node removal and improve the radicality of surgery. Complete mesocolic excision (CME) is based on a dissection conducted on the embryological plane separating the right mesocolon and the retroperitoneum and a high tide of ileocolic, right colic, and right branch of the middle colic vessels[4,5]. A key point of the CME technique is the retrieval of an unbreached mesocolon package as the result of careful dissection between mesocolon and retroperitoneum along the Toldt’s layer together with central vascular ligation to remove the largest amount of lymph nodes.
To be more specific, D1 lymph node resection represents transection of the feeding vessel just proximal to the marginal vessels; D2 resection is a more traditional resection of the main feeding vessels to a given colonic segment and lymphadenectomy that includes the origin of the feeding vessels[6]; D3 represents an extended lymphadenectomy that includes dissection of the lymphoadipose tissue covering the medial side of the superior mesenteric vein (SMV) and dissection of the lympho
CME and D3 lymphadenectomy share common oncologic results, and as first described by Hohenberger et al[7], CME and CVL offer better results if performed together. The authors proposed a nodal dissection even more extended than the standard D3 proposed by Japanese surgical societies, known as CVL[7].
Furthermore, in recent years, the wide spread of minimally invasive techniques in colorectal surgery has introduced new issues regarding technical complexity and increased morbidity of these procedures[8]. Concerns have been raised, especially about the proper extent of laparoscopic lymphadenectomy and its feasibility.
Curative treatment of colorectal cancer (CRC) is focused on surgery. The development of cancer is supposed to be a result of interactions among environmental factors, genetic alterations, and immune response that can promote or inhibit tumor cell growth[11-16].
Once developed, CRC cells can diffuse away from the primary tumor by means of the embryological envelope constituted by the primitive dorsal mesenterium, a double layered fibrofatty mesenchymal tissue. The concept of radicality in CRC must include complete excision of this “meso-structure,” which represents the main procedure able to prevent local recurrence. On the other hand, distant metastases spread has to be prevented by means of an extended local lymph nodes removal. From this point of view, CVL is able to provide extensive lymph node dissection, limiting regional recurrence and systemic dissemination rates, thus providing improved survival in stage I-III colonic cancer[17].
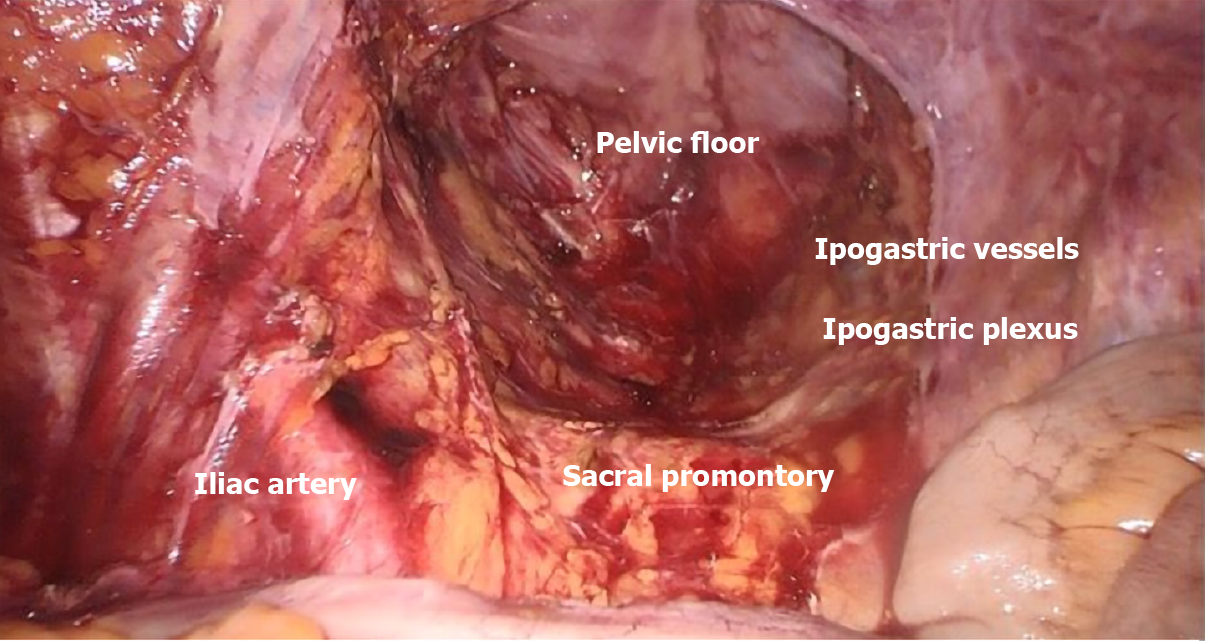
Regarding rectal cancer, the concept of total mesorectal excision (TME) introduced by Heald[4] demonstrated as a complete excision of mesorectal fascia yields better outcomes and it has become the gold standard for rectal surgery (Figure 2). Again, the underlying concept is that complete removal of lymphatic drainage together with the primary tumor, while preserving the integrity of enveloping fascial layers is able to provide improved local control of disease and lower distant diffusion rates[18]. Definitely, the integrity of the dissection plane described by Heald, remains the principal predictive factor for local recurrence as clearly stated in recent meta-analyses and reviews comparing TME plus lateral lymph node dissection (LLND) vs TME alone[19-22]: to perform LLND doesn’t provide significant reduction of recurrence rates or improvement in survival; indeed, LLND is reported to require longer operation time (360 min vs 294.7 min; P = 0.02) and increased complication rates (odds ratio [OR] = 1.48, 95% confidence interval [CI]: 1.18-1.87; P < 0.001) such as urinary dysfunction[19].
In recent years, the same concept of extensive dissection adhering to embryological planes (CME) and central vascular ligation (CVL) have also been introduced for colonic resections[23]. CME is a well standardized procedure providing increased DFS in right colectomy[24], while little is known about perioperative morbidity and mortality when it is associated with CVL[25].
Kanemitsu et al[26] examined 370 consecutive patients who had right colectomy with D3 lymphadenectomy for right colon cancer; 3% of patients had N3 nodal involvement (patients with T3-T4 tumors) and 13.2% had N2 nodal involvement. The 5-year DFS was 36.4% for the patient with N3 nodal involvement vs 83.5% for N2 nodal involvement, suggesting that patients with proximal nodal metastasis exhibit a different tumor biology than patients with more intermediate-level nodal metastasis. Nagasaki et al[27] suggested that lymph nodes are a key element of the tumor-node-metastasis staging system and are considered a significant factor for predicting disease-free survival (DFS) and overall survival (OS) in patients with CRC without distant metastasis. Integrity of the surgical field provided by dissection conducted along the embryological planes is also very important to limit the amount of cancer cells exfoliating from traumatized tissues. In fact, in CRC surgery, intraperitoneal-free cancer cells presence is not routinely investigated but data exist on worse survival for patients who show a positive peritoneal washing[28-30] .
The wide application of CME and CVL techniques seem to be also limited by the large number of CRC patients who present in emergency: a recent study demonstrated that disease free survival for patients with pT3 mucinous and signet ring cell tumor is related to emergency presentation and that they have poorest outcomes and survival. Although the debate whether emergency colon surgery is associated with worse oncological outcome is still ongoing, the finding of a “bridge to surgery” strategy (if possible) might provide better oncologic outcomes in T3 patients[31] and might allow application of CME and CVL also in these patients, if successfully shifted to elective surgery.
However, the correct extent of lymphadenectomy is still debated: 2019 guidelines of the Japanese Society for Cancer of the Colon and Rectum (JSCCR) recommend D3 lymph node dissection for clinical stage II/III CRC[32]. However, when performing a left hemicolectomy, it is still unclear whether D3 lymph node dissection with preservation of the left colic artery (LCA) is different in terms of clinical outcomes, compared to D3 without LCA preservation. The advantages in D3 without LCA preservation have been identified in the prevention of the micrometastatic cell spillage through the en bloc lymph node dissection of the root of the inferior mesenteric artery (IMA); disadvantages include a higher possibility of anastomotic leakage and the sacrifice of the autonomic nerves around the IMA; no significant differences in terms of operation time and blood loss have been found. Despite a higher incidence of complications, D3 with LCA preservation was associated with a higher OS[33-37]
Moreover, while Kotake et al[38] demonstrated no difference in the OS of patients who had T2 colon cancers treated with D2 or D3 resection, Slanetz et al[39] showed that the level of mesenteric resection influenced outcomes only for patients who had moderate or well-differentiated cancer with intermediate-level nodal involvement. Patients with more than four positive lymph nodes or poorly differentiated tumors had poor survival regardless of the extension of lymphadenectomy. These studies had limitations such as outdated staging methods, lack of modern chemotherapy, and no audit of the pathology specimen.
Minimal invasive surgery, such as robotic and laparoscopic techniques, has revolutionized the approach to gastrointestinal surgery, especially in colorectal surgery, notably lowering surgical and post-operative trauma and shortening post-operative course[40,41]. The concept of extended lymphadenectomy might appear in contrast with this leading point of view. Technical complexity as well as increased morbidity are important issues to be solved in order to consider these procedures in the scope of minimal invasive surgery. Regarding safety of laparoscopy from a general point of view, several trials reported promising results when comparing it to open surgery: the COST trial[42], COLOR I and II trials[43,44], CLASICC trial[45,46], and COREAN[47] demonstrated non-inferior outcomes to open surgery. A Cochrane Review clearly showed the laparoscopic approach features advantages such as decreased blood loss, quicker oral intake, decreased narcotic use, and lower rates of surgical site infections[48]. Furthermore, Arezzo et al[49] in a meta-analysis including 4539 patients found decreased mortality (2.4% vs 1.0%; P = 0.048) and morbidity (35.4% vs 31.8%; P < 0.001) in the laparoscopic group.
Regarding the correct performance of CME and CVL, a recent systematic review reported no differences in the local and distant recurrence rate, the 3- and 5-year OS rates and the DFS rates between the laparoscopic and open CME groups[48]. Furthermore, the quality of the surgical specimen from laparoscopic CME/CVL seems to be similar to that obtained with the open technique[50-53]. In one of the few randomized controlled trials, Yamamoto et al[54] compared laparoscopic and open D3 colonic resections demonstrating lower morbidity rates in the laparoscopic group with the usual benefits of minimally invasive surgery.
Central vascular ligation can be considered a widely accepted reality in colorectal surgical oncology with clear benefits in terms of oncologic outcomes. Concerns remain regarding increased rate of postoperative complications[7,24,55]. Different awareness on the benefits and feasibility of these extended dissections is reported for rectal and colonic cancer. Nowadays, TME is considered the gold standard in rectal cancer and it provides an optimal local disease control confining tumor deposits as well as nodal involvement within the mesorectal fascia. Complete excision of the mesorectum should be performed en bloc with the rectum by dissecting along the rectal fascia in the plane that separates this from the parietal pelvic fascia (the so called “holy plane”), thereby preserving integrity of the rectal fascia and mesorectal contents, and sparing the autonomic pelvic nerves and plexuses[5].
In agreement with this principle, the same concept of preservation of the embryological envelope has been applied to colonic resections[24]. For what concerns right colectomy, it is the author’s opinion that a true CME does not exist without CVL and extended dissection along the vascular plane offered by the anterior surface of the SMV and SMA.
The adherence to the 2019 guidelines of the JSCCR for the treatment of CRC recommending D3 lymph node dissection for clinical stage II/III CRC is suggested and shared by our institution[34]. For what concerns arterial ligation in sigmoid and rectosigmoid colon cancer there is still no consensus, but we agree that LCA preservation should be attempted whenever possible as fewer postoperative complications might contribute to a better prognosis.
The aim of this review is to create an increasing awareness that the idea of an extended lymphadenectomy with CVL must be in the contest of a multimodal approach where neoadjuvant chemotherapy is increasing its role in the treatment of advanced stage cancers, allowing more conservative surgery. This idea appears even more important in a context where minimally invasive techniques and the idea of “less is more” is becoming the standard surgical approach. Further trials are needed to better investigate the correct role of these techniques in the era of multimodal approach to cancer treatment.
The achievement of reliable of laparoscopic lymphadenectomy in terms of oncological appropriateness has allowed the transfer of the many advantages of mini-invasiveness to the treatment of gastro-intestinal cancer. Improvement of surgical expertise in minimally-invasive surgery, technical/instrumental innovations and the development of newly designed operative techniques will make it possible to consider laparoscopic CME/CVL as a common surgical technique[9,10].
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koukoulis GD S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | The Lancet. GLOBOCAN 2018: counting the toll of cancer. Lancet. 2018;392:985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 2. | Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 843] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 3. | Torino F, Bonmassar E, Bonmassar L, De Vecchis L, Barnabei A, Zuppi C, Capoluongo E, Aquino A. Circulating tumor cells in colorectal cancer patients. Cancer Treat Rev. 2013;39:759-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Garcia-Granero A, Pellino G, Frasson M, Fletcher-Sanfeliu D, Bonilla F, Sánchez-Guillén L, Domenech Dolz A, Primo Romaguera V, Sabater Ortí L, Martinez-Soriano F, Garcia-Granero E, Valverde-Navarro AA. The fusion fascia of Fredet: an important embryological landmark for complete mesocolic excision and D3-lymphadenectomy in right colon cancer. Surg Endosc. 2019;33:3842-3850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med. 1988;81:503-508. [PubMed] |
| 6. | Paquette IM, Madoff RD, Sigurdson ER, Chang GJ. Impact of Proximal Vascular Ligation on Survival of Patients with Colon Cancer. Ann Surg Oncol. 2018;25:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354-364; discussion 364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1102] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 8. | Kim NK, Kim YW, Han YD, Cho MS, Hur H, Min BS, Lee KY. Complete mesocolic excision and central vascular ligation for colon cancer: Principle, anatomy, surgical technique, and outcomes. Surg Oncol. 2016;25:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Feldman LS, Delaney CP. Laparoscopy plus enhanced recovery: optimizing the benefits of MIS through SAGES 'SMART' program. Surg Endosc. 2014;28:1403-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Amelio I, Bertolo R, Bove P, Candi E, Chiocchi M, Cipriani C, Di Daniele N, Ganini C, Juhl H, Mauriello A, Marani C, Marshall J, Montanaro M, Palmieri G, Piacentini M, Sica G, Tesauro M, Rovella V, Tisone G, Shi Y, Wang Y, Melino G. Cancer predictive studies. Biol Direct. 2020;15:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | EuroSurg Collaborative. EuroSurg: a new European student-driven research network in surgery. Colorectal Dis. 2016;18:214-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Franzè E, Laudisi F, Di Grazia A, Marônek M, Bellato V, Sica G, Monteleone G. Macrophages produce and functionally respond to interleukin-34 in colon cancer. Cell Death Discov. 2020;6:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Franzè E, Di Grazia A, Sica GS, Biancone L, Laudisi F, Monteleone G. Interleukin-34 Enhances the Tumor Promoting Function of Colorectal Cancer-Associated Fibroblasts. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Franzè E, Marafini I, De Simone V, Monteleone I, Caprioli F, Colantoni A, Ortenzi A, Crescenzi F, Izzo R, Sica G, Sileri P, Rossi P, Pallone F, Monteleone G. Interleukin-34 Induces Cc-chemokine Ligand 20 in Gut Epithelial Cells. J Crohns Colitis. 2016;10:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Biancone L, Onali S, Calabrese E, Petruzziello C, Zorzi F, Condino G, Sica GS, Pallone F. Non-invasive techniques for assessing postoperative recurrence in Crohn's disease. Dig Liver Dis. 2008;40 Suppl 2:S265-S270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Sileri P, Sica G, Gentileschi P, Venza M, Manzelli A, Palmieri G, Spagnoli LG, Testa G, Benedetti E, Gaspari AL. Ischemic preconditioning protects intestine from prolonged ischemia. Transplant Proc. 2004;36:283-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | De Simone V, Ronchetti G, Franzè E, Colantoni A, Ortenzi A, Fantini MC, Rizzo A, Sica GS, Sileri P, Rossi P, MacDonald TT, Pallone F, Monteleone G, Stolfi C. Interleukin-21 sustains inflammatory signals that contribute to sporadic colon tumorigenesis. Oncotarget. 2015;6:9908-9923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Siani LM, Lucchi A, Berti P, Garulli G. Laparoscopic complete mesocolic excision with central vascular ligation in 600 right total mesocolectomies: Safety, prognostic factors and oncologic outcome. Am J Surg. 2017;214:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Knol J, Keller DS. Total Mesorectal Excision Technique-Past, Present, and Future. Clin Colon Rectal Surg. 2020;33:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 20. | Emile SH, Elfeki H, Shalaby M, Sakr A, Kim NK. Outcome of lateral pelvic lymph node dissection with total mesorectal excision in treatment of rectal cancer: A systematic review and meta-analysis. Surgery. 2021;169:1005-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Longchamp G, Meyer J, Christou N, Popeskou S, Roos E, Toso C, Buchs NC, Ris F. Total mesorectal excision with and without lateral lymph node dissection: a systematic review of the literature. Int J Colorectal Dis. 2020;35:1183-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Wang X, Qiu A, Liu X, Shi Y. Total mesorectal excision plus lateral lymph node dissection vs TME on rectal cancer patients: a meta-analysis. Int J Colorectal Dis. 2020;35:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Hajibandeh S, Hajibandeh S, Matthews J, Palmer L, Maw A. Meta-analysis of survival and functional outcomes after total mesorectal excision with or without lateral pelvic lymph node dissection in rectal cancer surgery. Surgery. 2020;168:486-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Søndenaa K, Quirke P, Hohenberger W, Sugihara K, Kobayashi H, Kessler H, Brown G, Tudyka V, D'Hoore A, Kennedy RH, West NP, Kim SH, Heald R, Storli KE, Nesbakken A, Moran B. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery : proceedings of a consensus conference. Int J Colorectal Dis. 2014;29:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 25. | Gouvas N, Agalianos C, Papaparaskeva K, Perrakis A, Hohenberger W, Xynos E. Surgery along the embryological planes for colon cancer: a systematic review of complete mesocolic excision. Int J Colorectal Dis. 2016;31:1577-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Kanemitsu Y, Komori K, Kimura K, Kato T. D3 Lymph Node Dissection in Right Hemicolectomy with a No-touch Isolation Technique in Patients With Colon Cancer. Dis Colon Rectum. 2013;56:815-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Nagasaki T, Akiyoshi T, Fujimoto Y, Konishi T, Nagayama S, Fukunaga Y, Arai M, Ueno M. Prognostic Impact of Distribution of Lymph Node Metastases in Stage III Colon Cancer. World J Surg. 2015;39:3008-3015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Sibio S, Fiorani C, Stolfi C, Divizia A, Pezzuto R, Montagnese F, Bagaglini G, Sammartino P, Sica GS. Detection methods and clinical significance of free peritoneal tumor cells found during colorectal cancer surgery. World J Gastrointest Surg. 2015;7:178-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Sica GS, Fiorani C, Stolfi C, Monteleone G, Candi E, Amelio I, Catani V, Sibio S, Divizia A, Tema G, Iaculli E, Gaspari AL. Peritoneal expression of Matrilysin helps identify early post-operative recurrence of colorectal cancer. Oncotarget. 2015;6:13402-13415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Amelio I, Bertolo R, Bove P, Buonomo OC, Candi E, Chiocchi M, Cipriani C, Di Daniele N, Ganini C, Juhl H, Mauriello A, Marani C, Marshall J, Montanaro M, Palmieri G, Piacentini M, Sica G, Tesauro M, Rovella V, Tisone G, Shi Y, Wang Y, Melino G. Liquid biopsies and cancer omics. Cell Death Discov. 2020;6:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Sibio S, Di Giorgio A, D'Ugo S, Palmieri G, Cinelli L, Formica V, Sensi B, Bagaglini G, Di Carlo S, Bellato V, Sica GS. Histotype influences emergency presentation and prognosis in colon cancer surgery. Langenbecks Arch Surg. 2019;404:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 1301] [Article Influence: 260.2] [Reference Citation Analysis (1)] |
| 33. | Akagi T, Inomata M, Hara T, Mizusawa J, Katayama H, Shida D, Ohue M, Ito M, Kinugasa Y, Saida Y, Masaki T, Yamamoto S, Hanai T, Yamaguchi S, Watanabe M, Sugihara K, Fukuda H, Kanemitsu Y, Kitano S. Clinical impact of D3 Lymph node dissection with left colic artery (LCA) preservation compared to D3 without LCA preservation: Exploratory subgroup analysis of data from JCOG0404. Ann Gastroenterol Surg. 2020;4:163-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Yasuda K, Kawai K, Ishihara S, Murono K, Otani K, Nishikawa T, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, Yamaguchi H, Aoki S, Mishima H, Maruyama T, Sako A, Watanabe T. Level of arterial ligation in sigmoid colon and rectal cancer surgery. World J Surg Oncol. 2016;14:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Sekimoto M, Takemasa I, Mizushima T, Ikeda M, Yamamoto H, Doki Y, Mori M. Laparoscopic lymph node dissection around the inferior mesenteric artery with preservation of the left colic artery. Surg Endosc. 2011;25:861-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Titu LV, Tweedle E, Rooney PS. High tie of the inferior mesenteric artery in curative surgery for left colonic and rectal cancers: a systematic review. Dig Surg. 2008;25:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Kanemitsu Y, Hirai T, Komori K, Kato T. Survival benefit of high ligation of the inferior mesenteric artery in sigmoid colon or rectal cancer surgery. Br J Surg. 2006;93:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Kotake K, Kobayashi H, Asano M, Ozawa H, Sugihara K. Influence of extent of lymph node dissection on survival for patients with pT2 colon cancer. Int J Colorectal Dis. 2015;30:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Slanetz CA Jr, Grimson R. Effect of high and intermediate ligation on survival and recurrence rates following curative resection of colorectal cancer. Dis Colon Rectum. 1997;40:1205-18; discussion 1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Sica GS, Iaculli E, Biancone L, Di Carlo S, Scaramuzzo R, Fiorani C, Gentileschi P, Gaspari AL. Comparative study of laparoscopic vs open gastrectomy in gastric cancer management. World J Gastroenterol. 2011;17:4602-4606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Sica GS, Campanelli M, Bellato V, Monteleone G. Gastrointestinal cancer surgery and enhanced recovery after surgery (ERAS) during COVID-19 outbreak. Langenbecks Arch Surg. 2020;405:357-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Clinical Outcomes of Surgical Therapy Study Group; Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Ota D. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2514] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 43. | Colon Cancer Laparoscopic or Open Resection Study Group; Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ. Survival after laparoscopic surgery vs open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 1054] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 44. | Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E; COLOR II Study Group. A randomized trial of laparoscopic vs open surgery for rectal cancer. N Engl J Med. 2015;372:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 927] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 45. | Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM; UK MRC CLASICC Trial Group. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1104] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 46. | Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted vs open surgery for colorectal cancer. Br J Surg. 2010;97:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 737] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 47. | Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, Jung KH, Kim TY, Kang GH, Chie EK, Kim SY, Sohn DK, Kim DH, Kim JS, Lee HS, Kim JH, Oh JH. Open vs laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 637] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 48. | Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. 2008;CD003432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 250] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 49. | Arezzo A, Passera R, Scozzari G, Verra M, Morino M. Laparoscopy for rectal cancer reduces short-term mortality and morbidity: results of a systematic review and meta-analysis. Surg Endosc. 2013;27:1485-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 50. | Negoi I, Hostiuc S, Negoi RI, Beuran M. Laparoscopic vs open complete mesocolic excision with central vascular ligation for colon cancer: A systematic review and meta-analysis. World J Gastrointest Oncol. 2017;9:475-491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Subbiah R, Bansal S, Jain M, Ramakrishnan P, Palanisamy S, Palanivelu PR, Chinusamy P. Initial retrocolic endoscopic tunnel approach (IRETA) for complete mesocolic excision (CME) with central vascular ligation (CVL) for right colonic cancers: technique and pathological radicality. Int J Colorectal Dis. 2016;31:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | West NP, Kennedy RH, Magro T, Luglio G, Sala S, Jenkins JT, Quirke P. Morphometric analysis and lymph node yield in laparoscopic complete mesocolic excision performed by supervised trainees. Br J Surg. 2014;101:1460-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Melich G, Jeong DH, Hur H, Baik SH, Faria J, Kim NK, Min BS. Laparoscopic right hemicolectomy with complete mesocolic excision provides acceptable perioperative outcomes but is lengthy--analysis of learning curves for a novice minimally invasive surgeon. Can J Surg. 2014;57:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Yamamoto S, Inomata M, Katayama H, Mizusawa J, Etoh T, Konishi F, Sugihara K, Watanabe M, Moriya Y, Kitano S; Japan Clinical Oncology Group Colorectal Cancer Study Group. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg. 2014;260:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 55. | Strey CW, Wullstein C, Adamina M, Agha A, Aselmann H, Becker T, Grützmann R, Kneist W, Maak M, Mann B, Moesta KT, Runkel N, Schafmayer C, Türler A, Wedel T, Benz S. Laparoscopic right hemicolectomy with CME: standardization using the "critical view" concept. Surg Endosc. 2018;32:5021-5030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |