Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7205
Peer-review started: March 5, 2021
First decision: April 24, 2021
Revised: April 27, 2021
Accepted: July 15, 2021
Article in press: July 15, 2021
Published online: August 26, 2021
Processing time: 171 Days and 17.5 Hours
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are tolerable drugs used for patients with EGFR-mutant advanced non-small cell lung cancer (NSCLC). Serious adverse reactions are uncommon compared with cytotoxic drugs.
A 52-year-old man presented with general weakness and cytopenia. He had been taking erlotinib for 11 mo to treat NSCLC. The pathological diagnosis from the right upper lobe mass was adenocarcinoma with an EGFR mutation in exon 21 (L858R). He had previously received paclitaxel/carboplatin, gemcitabin/ vinorelbine chemotherapy, stereotactic radiosurgery for brain metastasis, and whole-brain radiotherapy as treatment for NSCLC. We diagnosed the patient with acute myeloid leukemia (AML). During the induction and consolidation chemotherapy for AML, the erlotinib was discontinued. When complete remission of the AML was achieved, since the lung masses were increased, pemetrexed/ cisplatin for the NSCLC was initiated. After two cycles of chemotherapy, the cytopenia was prolonged. AML relapse occurred with the same karyotype.
Therapy-related acute myeloid neoplasm (t-MN) is a rare but fatal late complication. Although a patient may be taking EGFR-TKIs, the possibility of t-MN should be considered. Further studies are needed to determine whether EGFR-TKI usage is a predisposing factor for t-MN.
Core Tip: Therapy-related acute myeloid leukemia (t-AML) developed during erlotinib treatment in a patient with epidermal growth factor receptor (EGFR)–mutant advanced non-small cell lung cancer (NSCLC). Alkylating cytotoxic drugs and radiotherapy are common treatments for patients with NSCLC. Cases of t-AML related to alkylating agents typically have a long latency period. Since it was 20 mo in this case, EGFR–tyrosine kinase inhibitor (EGFR-TKI) usage may be related to or hasten AML development in patients who previously received cytotoxic chemotherapy. Although the mechanism remains unclear, when a patient takes an EGFR-TKI, t-AML development should be considered, especially if cytopenia persists.
- Citation: Koo SM, Kim KU, Kim YK, Uh ST. Therapy-related myeloid leukemia during erlotinib treatment in a non-small cell lung cancer patient: A case report. World J Clin Cases 2021; 9(24): 7205-7211
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7205.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7205
Therapy-related acute myeloid leukemia (t-AML) is a rare but fatal late complication of cytotoxic chemotherapy. According to Surveillance, Epidemiology, and End Results data in United States cancer registries, 18 patients with t-AML were identified in 2001–2008 among 37008 non-small cell lung cancer (NSCLC) patients who received initial chemotherapy in adulthood[1]. Since patients with NSCLC receive multiple lines of treatment, it is difficult to determine which of the cytotoxic drugs are related to the AML that occurs several years later.
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are the target therapy for patients with EGFR-mutant NSCLC. EGFR-TKIs are known to be relatively tolerable drugs for patients and less toxic than cytotoxic agents. Moreover, serious adverse reactions to them are uncommon[2].
We identified a case of AML that developed during erlotinib treatment and reviewed cases of myeloid neoplasms that occurred during EGFR-TKI treatment in the literature.
A 52-year-old man presented with general weakness. His symptoms had waxed and waned during the past several months.
He was under lung cancer treatment consisting of erlotinib 100 mg/d, levetiracetam 1000 mg/d, and prednisolone 10 mg/d to control his symptoms and prevent seizure that related with brain metastasis.
He had a medical history of chemotherapy and radiotherapy for NSCLC diagnosed as adenocarcinoma, T2bN2M1a by the International Association for the Study of Lung Cancer seventh edition guidelines 20 mo prior. The pathological diagnosis of the right upper lobe mass was adenocarcinoma with an EGFR mutation in exon 21(L858R). As first-line chemotherapy, he received six cycles of combination paclitaxel (175 mg/m2) and carboplatin (AUC 4). At that time, EGFR-TKI was not covered by medical insurance benefits as the first-line therapy, so cytotoxic chemotherapy was admi
Our patient, who ran a flower shop, was a former smoker with a 26 pack-years history of smoking. He had quit smoking a year prior.
His vital signs and physical examination findings were unremarkable except for a chronic ill-looking appearance.
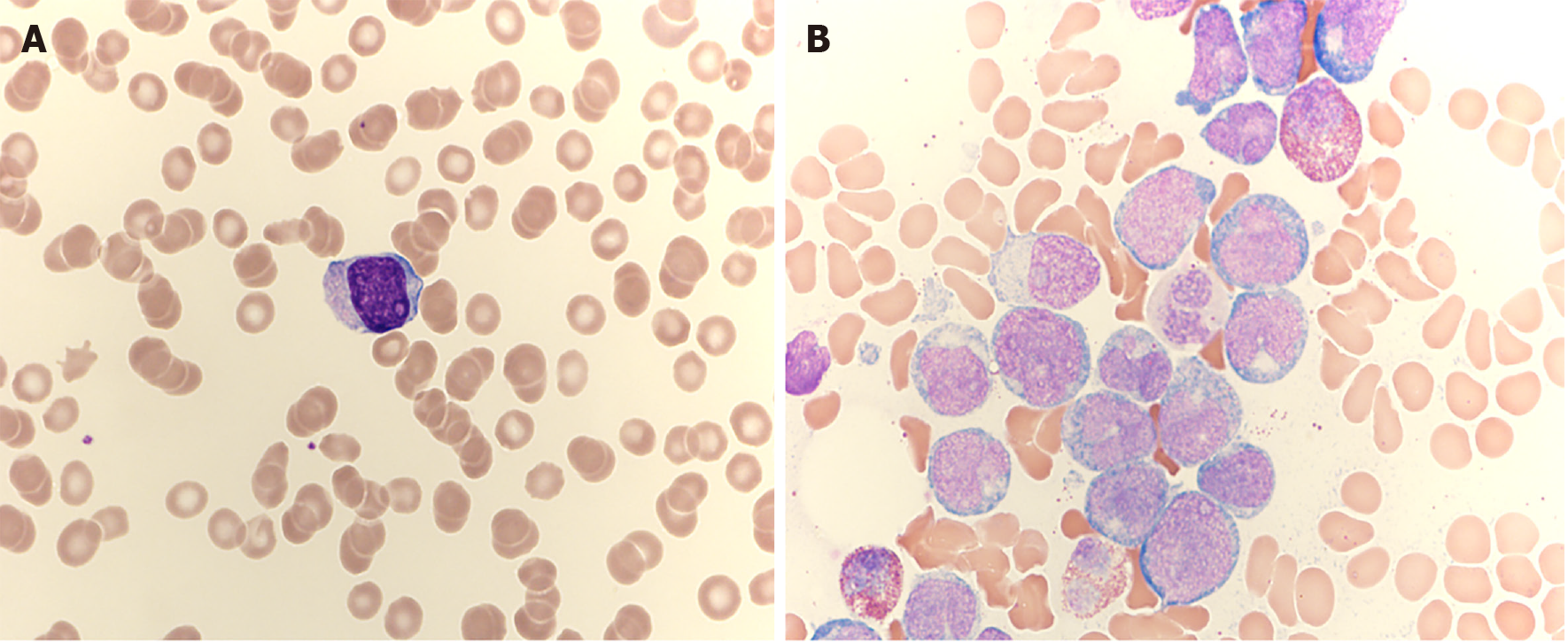
A complete blood count revealed a white blood cell count of 7600 (absolute neutrophil count, 500), hemoglobin level of 12.4 g/dL, and platelet count of 83000/μL. A leukocyte differential count revealed an absolute neutrophil count of 500/μL, lymphocyte count of 2400/μL, and monocyte count of 1000/μL. Immature cells were observed in the peripheral blood (Figure 1A). The chemistry results were normal except for a lactate dehydrogenase level of 293 U/L (reference interval, 106–211 U/L).
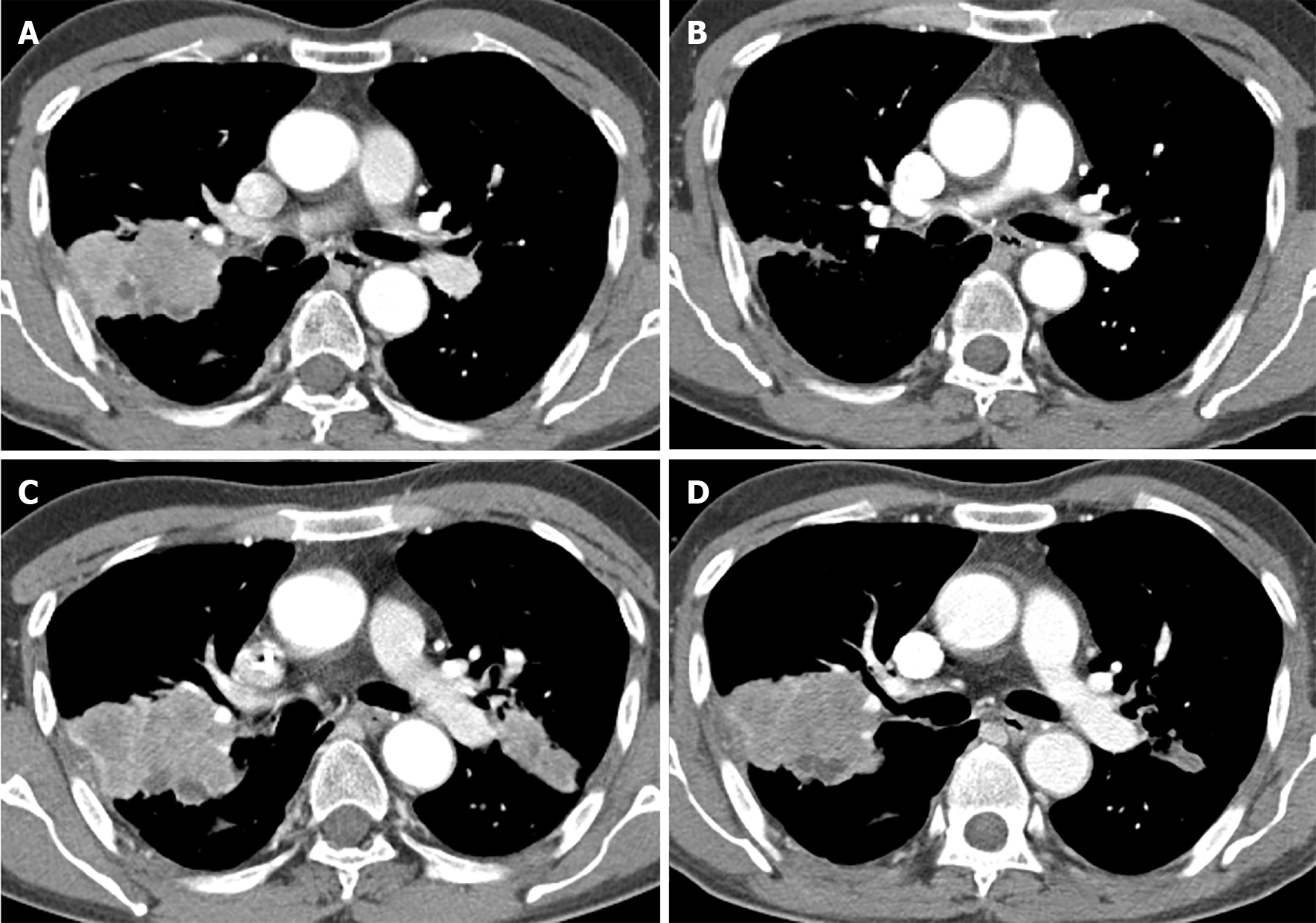
In the initial diagnosis, chest computed tomography revealed a 61 mm × 44 mm lobulated mass in the right upper lobe and another 31 mm × 23 mm spiculated mass in the left upper lobe. As the third-line therapy, we started erlotinib, an EGFR-TKI, at a dose of 150 mg/d 11 mo prior. During erlotinib treatment, the mass in the right upper lung became markedly improved (Figure 2A and B).
Bone marrow aspirate smears revealed a monocytic blast rate of 77.3% (Figure 1B). Eosinophil levels in the bone marrow were increased (14.4%). A bone marrow biopsy revealed 80% cellularity. While megakaryocytes were decreased, immature cells diffusely infiltrated the bone marrow. CBFB/MYH11 rearrangement was observed by fluorescence in situ hybridization analysis. On a chromosome analysis, inv(1) and inv(16) were detected. The patient’s karyotype was 46, XY, inv(1) (p22q32), inv(16) (p13.1q22).
After receiving the diagnosis of AML, the patient started induction chemotherapy with daunorubicin/cytarabine and consolidation chemotherapy with high-dose cytarabine. During AML treatment, the erlotinib treatment was stopped.
At the time that complete remission of the AML was achieved, both upper lobe masses had increased in size (Figure 2C and D). Fourth-line chemotherapy with pemetrexed/cisplatin for NSCLC was initiated. After two cycles of chemotherapy, the cytopenia persisted. The complete blood count revealed a white blood cell count of 1800 (absolute neutrophil count, 972), hemoglobin level of 9.6 g/dL, and platelet count of 88000/μL. Immature cells were observed in the peripheral blood. The result of the bone marrow study was AML relapse with the same karyotype. Re-induction chemotherapy with mitoxantrone/etoposide did not achieve remission. We administered low-dose cytarabine chemotherapy; the patient was exhausted with poor performance status and died of respiratory failure 10 mo after receiving the AML diagnosis.
This case involved t-AML that developed during erlotinib treatment in a patient with NSCLC. The case was considered a therapy-related myeloid neoplasm rather than de novo AML because the patient had a clinical history of antecedent cytotoxic therapy and radiotherapy. Carboplatin and radiotherapy in this patient were risk factors for t-AML[3]. Patients with t-AML related to alkylating agents generally experience a longer latency period (5–7 years) than those with t-AML related to topoisomerase-2 inhibitors (2–3 years) between therapy and myeloid neoplasm development[4]. This can predict the long interval between alkylating chemotherapy and an AML diagnosis. However, in the present case, the interval between the initiation of carboplatin treatment and AML development was 20 mo, which was relatively short.
Differentiating therapy-related acute myeloid neoplasm (t-MN) from de novo myelodysplastic syndrome (MDS)/AML is difficult because mutation profiling has demonstrated similar abnormalities for both conditions. Common patterns in recurrent mutations and chromosomal abnormalities are chromosome 5 and 7 loss with alkylating agent exposure and MLL translocations at 11q23 or RUNX1/AML1 at 21q22 with topoisomerase-2 inhibitor exposure[4,5]. However, de novo and therapy-related MDS/AML can share genetic features (especially the 11q23 anomaly). There are no pathognomonic morphologic or genetic features of t-MN[5].
EGFR-TKIs are the current first-line treatment for patients with EGFR mutation-positive advanced NSCLC. There have been a few reports related to leukemia and EGFR-TKIs[6-9]. In the literature review (Table 1), we identified eight patients with t-MN after EGFR-TKI treatment for antecedent NSCLC. The mean interval from the first-line NSCLC treatment to AML development was 40.7 (range, 11–120) months. None of the patients who were diagnosed with t-MN after EGFR-TKI treatment for NSCLC had taken topoisomerase II inhibitors. Since the latency period for these patients was not longer than expected, we considered the possibility that EGFR-TKI might be associated with the development of myeloid neoplasms.
| Patients number | Ref. | Age/sex | Pathological type of NSCLC | Initial stage | EGFR mutation | Prior chemotherapy | Interval from first line treatment | Prior radiotherapy | Duration of EGFR-TKI treatment | Interval from EGFR-TKI treatment until leukemia | CBC profiles at leukemia presentation: WBC (ANC) (μL)-hemoglobin (g/dL)/hematocrit (%)-platelets (mL) | Characteristics (Karyotype) of leukemia/MDS | Survival after diagnosis of leukemia |
| 1 | Uchida et al[8], 2005 | 49/M | Adenocarinoma | IV (T1N0M1) | NA | Cisplatin/docetaxel/ irinotecan 2 cycles | 36 mo | Cyberknife for brain metastasis (22.993Gy) | Gefitinib, 15 mo | 15 mo | NA | APL, normal karyotype, PML RARα positive | NA |
| 2 | Uchida et al[8], 2005 | 65/M | Squamous cell carcinoma | IIIB (T4N3M0) | NA | Cisplatin/mitomycin/ vinorelbine 2 cycles, Uracil/tegafur for 2 mo in adjuvant setting | 48 mo | Fractionated radiotherapy (2Gy* 25) | Gefitinib, 25 mo | 25 mo | NA | APL, normal karyotype, PML RARα positive | NA |
| 3 | Uchida et al[8], 2005 | 72/M | Adenocarinoma | IA (T1N0M0) | NA | Carboplatin/paclitaxel 1 cycle, Cisplatin, carboplatin, irinotecan, docetaxel, gemcitabine, vinorelbine, paclitaxel, amrubicin | 69 mo | None | Gefitinib, 5 mo + 4 mo (discontinued and restarted) | 26 mo | NA | APL, normal karyotype, PML RARα positive | NA |
| 4 | Ennishi et al[9], 2006 | 51/F | Adenocarcinoma | Recurrence after LLL lobectomy, mediastinal LN metastasis | NA | Carboplatin/paclitaxel 6 cycles | NA | Radiotherapy at a total dosage of 60 Gy | Gefitinib, 14 mo | 14 mo | 2300-13.2/-15200 | APL, t(15;17)(q22;q21), PML/RARα positive | NA |
| 5 | Stathopoulos et al[6], 2010 | 67/M | Adenocarinoma | IIIB | NA | Cisplatin/gemcitabine 6 cycles | 11 mo | None | Erlotinib, 4 mo | 8 mo | Grade 4 thrombocytopenia | MDS, 46, XY, del(20)(q11)[15]/47, idem, + 21(7)/48,idem, 21, + 21[-3] | NA |
| 6 | Stathopoulos et al[6], 2010 | 70/M | Adenocarinoma | IIIB | NA- | Cisplatin/paclitaxel 6 cycles | 12 mo | None | Erlotinib, 8 mo | 8 mo | WBC 92000 | CML, BCR-ABL+ | NA |
| 7 | Stathopoulos et al[6], 2010 | 60/F | Adenocarinoma | IIIB | NA | Cisplatin/vinorelbine 6 cycles. Carboplatin/etoposide 3 cycles | 36 mo | Radiotherapy (RT) of the primary lung lesions and of themediastinum | Erlotinib, 8.5 mo | 8.5 mo | 2500-/28.8-72000 | MDS, RAEB-T/t-AML, 46, XX, del(7)(q22), add(21)(q22) | Died 3.5 mo later |
| 8 | Stathopoulos et al[6], 2010 | 59/F | Squamous cell carcinoma | IV | NA | Carboplatin/etoposide 6 cycles | 14 mo | Erlotinib, 5.5 mo | 5.5 mo | 3200-/25.7 | MDS, RAEB 47, XX, +8, t(5;9)(q13;q34) | Died 8 mo later | |
| 9 | Moon et al[7], 2014 | 72/M | Squamous cell carcinoma | II (T2N1M0) | NA | A combination of radiotherapy and a repeated chemotherapy regimen (4 trials, 13 cycles) consisting of docetaxel, cisplatin, gemcitabine, vinorelbine, gefitinib, irinotecan, and carboplatin | 10 yr | Combination of radiotherapy and a repeated chemotherapy regimen | Gefitinib, Duration:NA | NA | 1700 (780)-5.2/-34000 | T-AML (Acute megakaryoblastic leukemia)-5,-7,+2mar | Being followed up |
| 10 | Present case | 52/M | Adenocarcinoma | IV (T2bN2M1a) | L858R | Paclitaxel/carboplatin 6 cycles, gemcitabin/vinorelbine 3 cycles | 20 mo | Cyberknife for brain metastasis (23Gy), WBRT (30Gy) | Erlotinib, 11 mo | 11 mo | 7600(500)-12.4/-83000 | AML, 46, XY, inv(1) (p22q32), inv(16)(p13.1q22) | Died 10 mo later |
The duration of EGFR-TKI therapy before AML was relatively short (mean, 13.4 mo; range, 5.5–26 mo). However, since cytotoxic chemotherapy drugs were administered prior to EGFR-TKI in all of these patients, the development of myeloid neoplasm may be related to both classes of drugs. It was not known whether the occurrence of t-MN was different depending on the type of EGFR mutation. This is because out of the 8 reported cases, only our case (L858R) provided the type of EGFR mutation.
Several case reports have shown that EGFR-TKIs might have leukemogenic effects in patients with t-MN after the administration of EGFR-TKIs[6,8,10]. However, after EGFR-TKIs were commercially approved, to the best of our knowledge, no cases of myeloid neoplasms after the administration of EGFR-TKI alone for NSCLC treatment have been reported in the literature. All patients of case reports with t-MN received chemotherapy or radiotherapy for NSCLC treatment before receiving EGFR-TKIs. We suggest that EGFR-TKI may be related to or shorten the interval to AML development when patients previously received cytotoxic chemotherapy. According to the historical concept, cytotoxic drugs such as topoisomerase II inhibitors or radiotherapy induce DNA damage that leads to translocation. In the model for the role of clonal selection[4], clonal hematopoiesis of indeterminate potential (CHIP) is characterized by the absence of morphological evidence of disease and the presence of a clonal population of hematopoietic cells with somatic mutations in the genes associated with hematologic malignancies[11].
Toxins, drugs, oligoclonality with aging, chemoradiation, chemical exposure, immune destruction, or dysfunctional hematopoiesis may be selected for mutant clones and induce CHIP. Additional somatic alterations in CHIP can promote high-risk myeloid neoplasms. We considered the possibility that certain effects of EGFR-TKI on the tyrosine kinase pathway could play a role in CHIP.
Long-term epidemiological research is needed to clarify whether there is a relationship between EGFR-TKI treatment and the rare but serious events noted here. If the incidence of this complication in the EGFR-TKI-treated cohort is beyond that expected on the basis of patients with NSCLC before EGFR-TKIs are commercially available, we must consider EGFR-TKIs as the predisposing factor of t-MN.
In summary, if cytopenia persists in patients treated with EGFR-TKIs for NSCLC, the possibility of t-MN should be considered. Further studies are needed to determine whether the administration of EGFR-TKIs is a predisposing factor for t-MN.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Neninger E, Yang TY S-Editor: Fan JR L-Editor: A P-Editor: Li X
| 1. | Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES, Fraumeni JF Jr, Curtis RE. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121:2996-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 2. | Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L; National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 4215] [Article Influence: 210.8] [Reference Citation Analysis (0)] |
| 3. | Beaumont M, Sanz M, Carli PM, Maloisel F, Thomas X, Detourmignies L, Guerci A, Gratecos N, Rayon C, San Miguel J, Odriozola J, Cahn JY, Huguet F, Vekhof A, Stamatoulas A, Dombret H, Capote F, Esteve J, Stoppa AM, Fenaux P. Therapy-related acute promyelocytic leukemia. J Clin Oncol. 2003;21:2123-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 4. | McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17:513-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 282] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 5. | Klimek VM, Tray NJ. Therapy-related myeloid neoplasms: what's in a name? Curr Opin Hematol. 2016;23:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Stathopoulos GP, Trafalis D, Athanasiou A, Bardi G, Chandrinou H. Serious hematologic complications following erlotinib treatment. Anticancer Res. 2010;30:973-976. [PubMed] |
| 7. | Moon JJ, Nam MH, Lim CS, Lee CK, Cho Y, Yoon SY. Therapy-related acute megakaryoblastic leukemia in a lung cancer patient. Ann Lab Med. 2014;34:155-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Uchida A, Matsuo K, Tanimoto M. APL during gefitinib treatment for non-small-cell lung cancer. N Engl J Med. 2005;352:843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Ennishi D, Sezaki N, Senoo T, Terui Y, Hatake K, Hino N. A case of acute promyelocytic leukemia during gefitinib treatment. Int J Hematol. 2006;84:284-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Hotta K, Kiura K, Takigawa N, Matsuo K, Tabata M, Fujiwara Y, Tanimoto M. Paradoxical clinical effects of epidermal growth factor receptor-tyrosine kinase inhibitors for acute myelogenous leukemia. J Clin Oncol. 2008;26:5826-5827; author reply 5827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, Ebert BL. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1086] [Cited by in RCA: 1422] [Article Influence: 142.2] [Reference Citation Analysis (0)] |