Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.6987
Peer-review started: January 1, 2021
First decision: January 24, 2021
Revised: March 1, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: August 26, 2021
Processing time: 235 Days and 0.4 Hours
The accuracy of discriminating pT3a from pT3b-c rectal cancer using high-resolution magnetic resonance imaging (MRI) remains unsatisfactory, although texture analysis (TA) could improve such discrimination.
To investigate the value of TA on apparent diffusion coefficient (ADC) maps in differentiating pT3a rectal adenocarcinomas from pT3b-c tumors.
This was a case-control study of 59 patients with pT3 rectal adenocarcinoma, who underwent diffusion-weighted imaging (DWI) between October 2016 and December 2018. The inclusion criteria were: (1) Proven pT3 rectal adenocarcinoma; (2) Primary MRI including high-resolution T2-weighted image (T2WI) and DWI; and (3) Availability of pathological reports for surgical specimens. The exclusion criteria were: (1) Poor image quality; (2) Preoperative chemoradiation therapy; and (3) A different pathological type. First-order (ADC values, skewness, kurtosis, and uniformity) and second-order (energy, entropy, inertia, and correlation) texture features were derived from whole-lesion ADC maps. Receiver operating characteristic curves were used to determine the diagnostic value for pT3b-c tumors.
The final study population consisted of 59 patients (34 men and 25 women), with a median age of 66 years (range, 41-85 years). Thirty patients had pT3a, 24 had pT3b, and five had pT3c. Among the ADC first-order textural differences between pT3a and pT3b-c rectal adenocarcinomas, only skewness was significantly lower in the pT3a tumors than in pT3b-c tumors. Among the ADC second-order textural differences, energy and entropy were significantly different between pT3a and pT3b-c rectal adenocarcinomas. For differentiating pT3a rectal adenocarcinomas from pT3b-c tumors, the areas under the curves (AUCs) of skewness, energy, and entropy were 0.686, 0.657, and 0.747, respectively. Logistic regression analysis of all three features yielded a greater AUC (0.775) in differentiating pT3a rectal adenocarcinomas from pT3b-c tumors (69.0% sensitivity and 83.3% specificity).
TA features derived from ADC maps might potentially differentiate pT3a rectal adenocarcinomas from pT3b-c tumors.
Core Tip: Texture features derived from apparent diffusion coefficient maps might potentially differentiate pT3a rectal adenocarcinomas from pT3b-c tumors.
- Citation: Lu ZH, Xia KJ, Jiang H, Jiang JL, Wu M. Textural differences based on apparent diffusion coefficient maps for discriminating pT3 subclasses of rectal adenocarcinoma. World J Clin Cases 2021; 9(24): 6987-6998
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/6987.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.6987
Colorectal cancer, the third commonest malignancy around the world, most commonly affects elderly individuals ≥ 60 years old, with a male predominance[1-3]. The incidence of rectal cancer was 5.2 per 100000 in 2014[4]. The 5-year relative survival is 37%-100% for local disease (varying widely according to perineural invasion and satellite nodules), 44% for locoregional disease, and 8%-15% for metastatic disease[5].
The majority (60%-80%) of rectal tumors are in the pT3 stage at diagnosis, constituting a heterogeneous group[6,7]. The extramural depth (EMD) of tumor invasion is one of the most important prognostic factors in rectal cancer, and strongly influences survival and local recurrence[6]. The 5-mm cut-off of EMD has been determined to be the most discriminating and simple to use in clinical practice regardless of differences in overall survival (OS) and local recurrence[6-9]. Locoregional recurrence and cancer-related 5-year survival rates are 10.4% and 85.4% for pT3a (EMD < 5 mm), respec
Magnetic resonance imaging (MRI) with a phased-array surface coil has an undeniable role in the preoperative staging of rectal cancer. In preoperative local T staging, the reported overall accuracy of MRI is 71%-90%[10-13]. In two studies on pT3 subclassification, the accuracies of MRI were 71.2% and 86%[10,12]. However, the accuracy of staging using high-resolution MRI remains unsatisfactory. In recent years, the potential of MRI has extended from morphological assessments to texture analysis (TA). TA refers to various mathematical-statistical methods used to extract texture features by evaluating the spatial variation of gray levels within given images[14]. These features can be used to characterize and measure tissue heterogeneity in medical images. Some texture features are useful tools for accurate diagnosis, preoperative risk stratification, and assessment of treatment response in several cancers[15-18]. Previous studies have demonstrated that MRI-based texture features are efficient in identifying preoperative KRAS mutation status in rectal cancer cases[19,20]. In addition, several MRI features and TA were shown to be valuable for predicting the therapeutic response to neoadjuvant chemoradiotherapy (nCRT) for rectal cancer and tumor recurrence[21]. However, another study reported that MRI T2-weighted sequence-based TA is not effective in predicting pathological complete response to nCRT in patients with locally advanced rectal cancer, suggesting that additional trials are required for comprehensively analyzing the potential of MRI-derived TA in this setting[22].
Diffusion-weighted imaging (DWI) is important in rectal cancer imaging because it provides superior contrast between cancerous and non-cancerous tissues. In a previous study[11], DWI was shown to be useful for evaluating the pT stage of rectal cancer and guiding EMD measurement. In addition, the apparent diffusion coefficient (ADC), a quantitative value of DWI, has been reported to correlate with the pT stage[11,23]. Nevertheless, the mean and median ADC values are not always significantly sensitive to small changes or precise status of the tumor owing to the intrinsic chaotic environment of tumors[24]. In addition, pT3 subclasses of rectal cancer have not been previously determined by ADC maps.
Given the increasing use of DWI in rectal cancer imaging and the quantitative value of ADC for accurate diagnosis, we hypothesized that texture features derived from ADC maps could discriminate pT3 subclasses. Therefore, the aim of this study was to investigate the value of TA on ADC maps in differentiating pT3a rectal adenocarcinomas from pT3b-c tumors. The results could provide interesting features for the tailoring of treatment strategies.
This case-control study was approved by the Institutional Review Board of the Changshu Hospital Affiliated to Soochow University (2018 Ethics Audit (Declaration) Batch No. 3). The requirement for individual informed consent was waived because of the retrospective design of the study. The patients who underwent imaging at the Changshu Hospital Affiliated to Soochow University between October 2016 and December 2018 were included (n = 120).
The inclusion criteria were: (1) Proven pT3 rectal adenocarcinoma; (2) Primary MRI including high-resolution T2-weighted image (T2WI) and DWI; and (3) Availability of pathological reports of surgical specimens. A total of 67 patients met these criteria. Eight patients were excluded for the following reasons: (1) Poor image quality apparent on DWI (n = 2); (2) Treatment with preoperative chemoradiation therapy (n = 4); and (3) A different pathological type, such as mucinous adenocarcinoma (n = 2).
Patient features (age and sex), pathological characteristics (grade and stage), and DWI TA features (ADC, skewness, kurtosis, uniformity, energy, entropy, inertia, and correlation) were collected from the medical charts.
All patients were imaged using a 3.0-T MRI system (Intera Achieva 3.0T TX, Philips Medical System, Best, The Netherlands) and a 16-channel phased-array surface coil. Thirty minutes before the MRI examination, all patients were asked to take clyster in order to reduce artifacts caused by gas within the rectum. The patients also received 10 mg of anisodamine (Hangzhou Mingsheng Pharmaceuticals Co., Ltd., Zhejiang, China) by intramuscular injection 10 min before the examination to reduce bowel peristalsis.
The standard rectal imaging protocol consisted of sagittal T2WI turbo spin-echo (TSE) [repetition time/echo time (TR/TE), 3577/70 ms; TSE factor, 20; slice thickness, 3 mm; interspace, 0 mm; field of view (FOV), 24 cm] and high-resolution axial and coronal T2W (TR/TE, 3000/75 ms; TSE factor, 18; slice thickness, 2 mm for coronal and 3 mm for axial; interspace, 0 mm; FOV: 18 cm). Axial T2WI was perpendicular to the tumor axis, as identified on sagittal T2WI. Coronal T2WI was angled parallel to the tumor axis. In addition, axial DWI was used (TR/TE, 2750/76 ms; slice thickness, 3 mm; interspace, 0 mm; FOV, 22-24 cm; b-values, 0, 1000 s/mm2). Dynamic contrast-enhanced imaging was not assessed in this study. ADC maps were automatically generated from DWI data using a mono-exponential decay model.
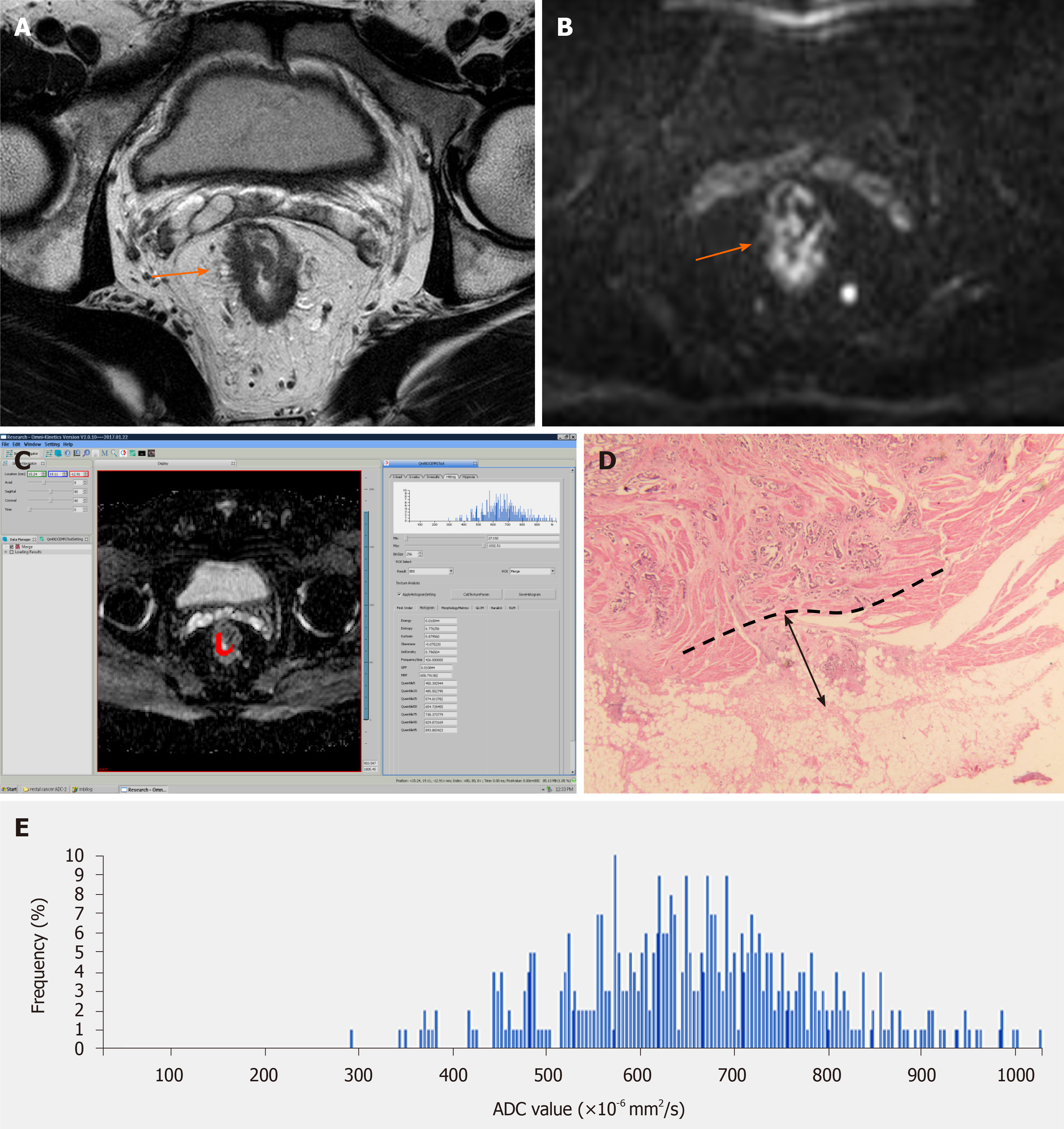
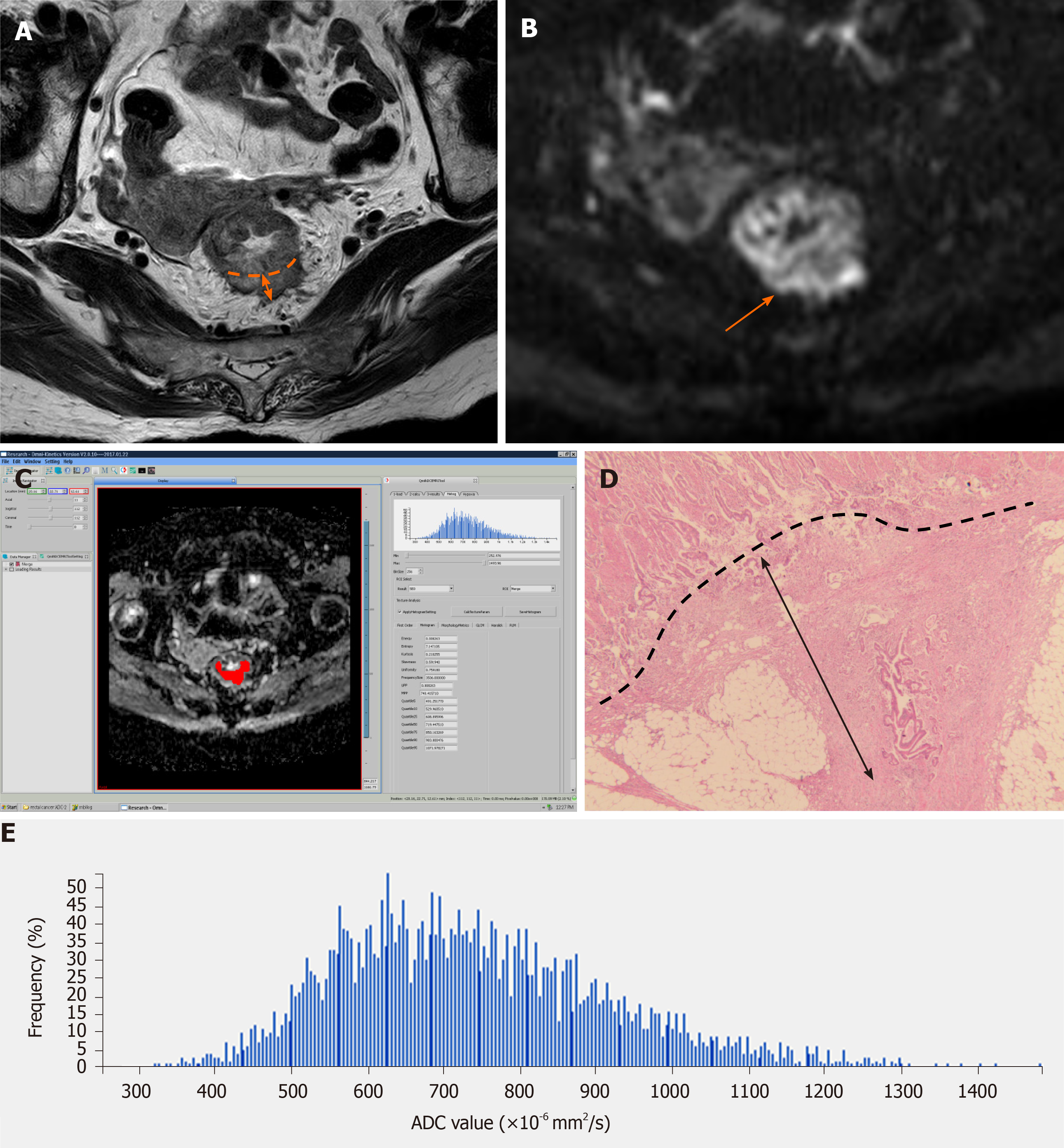
The primary tumor was distinguished on high-resolution T2WI and DWI based on final pathological outcomes. The primary tumor site was determined as a focal mass or abnormal wall thickening with intermediate signal intensity on T2WI, hyperintensity on DWI (b value = 1000 s/mm2), and the corresponding hypointensity on grey-scale ADC map. The entire tumor volumes were segmented independently by two experienced radiologists (Z.L. and H.J., with 9 and 4 years of experience in gastrointestinal MRI, respectively), using the Omni-Kinetics software (GE Healthcare, Waukesha, WI, United States). The resulting ADC maps were imported into Omni-Kinetics. The regions of interest (ROIs) were manually drawn slice by slice just inside the outer margin of the lesion to minimize partial volume error. The entire tumor area was covered as much as possible on ADC maps with reference to T2WI and DWI. Areas of necrosis, cysts, and gas were avoided to minimize bias. All ROIs were selected to derive the volume of interest (VOI). Then, first- and second-order texture features based on ADC maps were calculated automatically. ADC first-order texture features, as histogram features, included mean value, ADC percentiles (5th, 10th, 25th, and 90th percentiles), skewness, kurtosis, and uniformity. The ADC second-order texture features were derived from the gray-level co-occurrence matrix (GLCM), and included energy, entropy, inertia, and correlation.
The mean time between MRI and surgery was 3.6 ± 1.4 d (range, 2-7 d). One pathologist (M.W., 15 years of experience) measured the EMD of tumor invasion on histopathologic specimens (stained routinely with hematoxylin and eosin) according to the criteria of The Radiologic Society of North America (RSNA)[7]. EMD was defined as the maximum depth of the extramural tumor spreading outside the muscularis propria (pT3a, < 5 mm; pT3b, 5-10 mm; pT3c, > 10 mm)[7]. The pathologist measured the distance between the outer border of the identifiable muscularis propria layer and the outermost border of the tumor. If the outer border was not clear, then the pathologist checked the outer border at both ends of the tumor to determine clear areas, and drew a tentative line designating the outer border of the muscle layer.
Statistical analyses were performed using SPSS 16.0 (IBM, Armonk, NY, United States) and MedCalc 9.0 (MedCalc Software, Mariakierke, Belgium). P values < 0.05 were considered statistically significant. The KolmogorovSmirnov test was used to determine the distribution of all continuous data. Normally distributed data are presented as the mean ± SD, while those with a skewed distribution are presented as the median and interquartile range. ADC texture features were compared between the pT3a and pT3b-c stages by independent samples Student's t-test or the Mann-Whitney U-test. Receiver operating characteristic (ROC) analysis was used to assess the diagnostic value of each statistically significant feature for the detection of pT3a tumors as previously proposed[25]. The areas under the curves (AUCs) were compared using the method of Delong et al[26]. Interobserver variability of ADC texture features extraction was evaluated using intra-class correlation coefficients (ICCs) as follows: 0.00-0.20, poor agreement; 0.21-0.40, fair agreement; 0.41-0.60, moderate agreement; 0.61-0.80, good agreement; and 0.81-1.00, excellent agreement.
A summary of the baseline characteristics of the patients is shown in Table 1. The final study population consisted of 59 patients (34 men and 25 women), with a median age of 66 years (range, 41-85 years). According to the histological measurement of the maximal tumor invasion beyond the outer border of the muscularis propria, there were 30 patients with pT3a (Figure 1), 24 with pT3b (Figure 2), and five with pT3c. Twenty-seven patients were staged as N0, 22 as N1, and 10 as N2. Fifty-five patients were staged as M0, and four as M1. Forty-four patients had moderately differentiated tumors, and 15 had poorly differentiated ones.
| Characteristic | n = 59 |
| Age (yr), median (IQR) | 66 (58-74) |
| Sex | |
| Men | 34 (57.6) |
| Women | 25 (42.4) |
| Differentiation grade | |
| Moderately differentiated | 44 (74.6) |
| Poorly differentiated | 15 (25.4) |
| T3 stage | |
| T3a | 30 (50.9) |
| T3b | 24 (40.7) |
| T3c | 5 (8.5) |
| N stage | |
| N0 | 27 (45.8) |
| N1 | 22 (37.3) |
| N2 | 10 (17.0) |
| M stage | |
| M0 | 55 (93.2) |
| M1 | 4 (6.8) |
The ADC first-order textural differences between pT3a and pT3b-c rectal adenocarcinomas are shown in Table 2. Skewness was significantly lower in pT3a tumors than in pT3b-c counterparts (0.24 ± 0.31 vs 0.41 ± 0.32, P = 0.033). ADC value (including mean and percentiles) and ADC histogram related features (including kurtosis and uniformity) were not significantly different between the pT3a and pT3b-c stages. All ADC first-order texture features derived from ADC maps were delineated indepen
| ADC histogram feature | pT3a (n = 30) | pT3b-c (n = 29) | P value | ICC |
| Mean ADC (× 10-6 mm2/s)1 | 879.5 ± 202.3 | 832.8 ± 166.7 | 0.339 | 0.906 |
| 5th ADC (× 10-6 mm2/s)1 | 584.4 ± 163.3 | 533.1 ± 111.9 | 0.164 | 0.898 |
| 10th ADC (× 10-6 mm2/s)1 | 638.6 ± 164.2 | 589.5 ± 111.0 | 0.183 | 0.901 |
| 25th ADC (× 10-6 mm2/s)1 | 720.2 ± 175.8 | 664.5 ± 125.7 | 0.168 | 0.921 |
| 90th ADC (× 10-6 mm2/s)1 | 1113.8 ± 275.8 | 1055.7 ± 229.3 | 0.383 | 0.850 |
| Skewness1 | 0.24 ± 0.31 | 0.41 ± 0.32 | 0.033 | 0.925 |
| Kurtosis1 | 0.66 ± 0.52 | 0.80 ± 0.70 | 0.376 | 0.909 |
| Uniformity1 | 0.78 ± 0.06 | 0.76 ± 0.05 | 0.058 | 0.921 |
The obtained ADC second-order textural differences between pT3a and pT3b-c rectal adenocarcinomas are shown in Table 3. Energy (median, 0.782 vs 0.640, P = 0.038) and entropy (median, 9.831 vs 10.805, P = 0.001) were significantly different between pT3a and pT3b-c rectal adenocarcinomas. Specifically, energy was higher for pT3a tumors than for pT3b-c ones, while entropy was lower for the pT3a stage than for pT3b-c tumors. All ADC second-order texture features derived from ADC maps were delineated independently by two radiologists, and showed excellent agreement (ICCs ranging from 0.895 to 0.921).
| ADC texture feature | pT3a (n = 30) | pT3b-c (n = 29) | P value | ICC |
| Energy (× 10-3)2 | 0.782 (0.527, 1.399) | 0.640 (0.437, 0.779) | 0.038 | 0.895 |
| Entropy2 | 9.831 (8.969, 10.652) | 10.805 (10.189, 11.592) | 0.001 | 0.920 |
| Inertia1 | 753.0 ± 417.0 | 878.9 ± 279.0 | 0.180 | 0.918 |
| Correlation (× 10-3)2 | 0.538 (0.399, 1.255) | 0.476 (0.414, 0.642) | 0.332 | 0.921 |
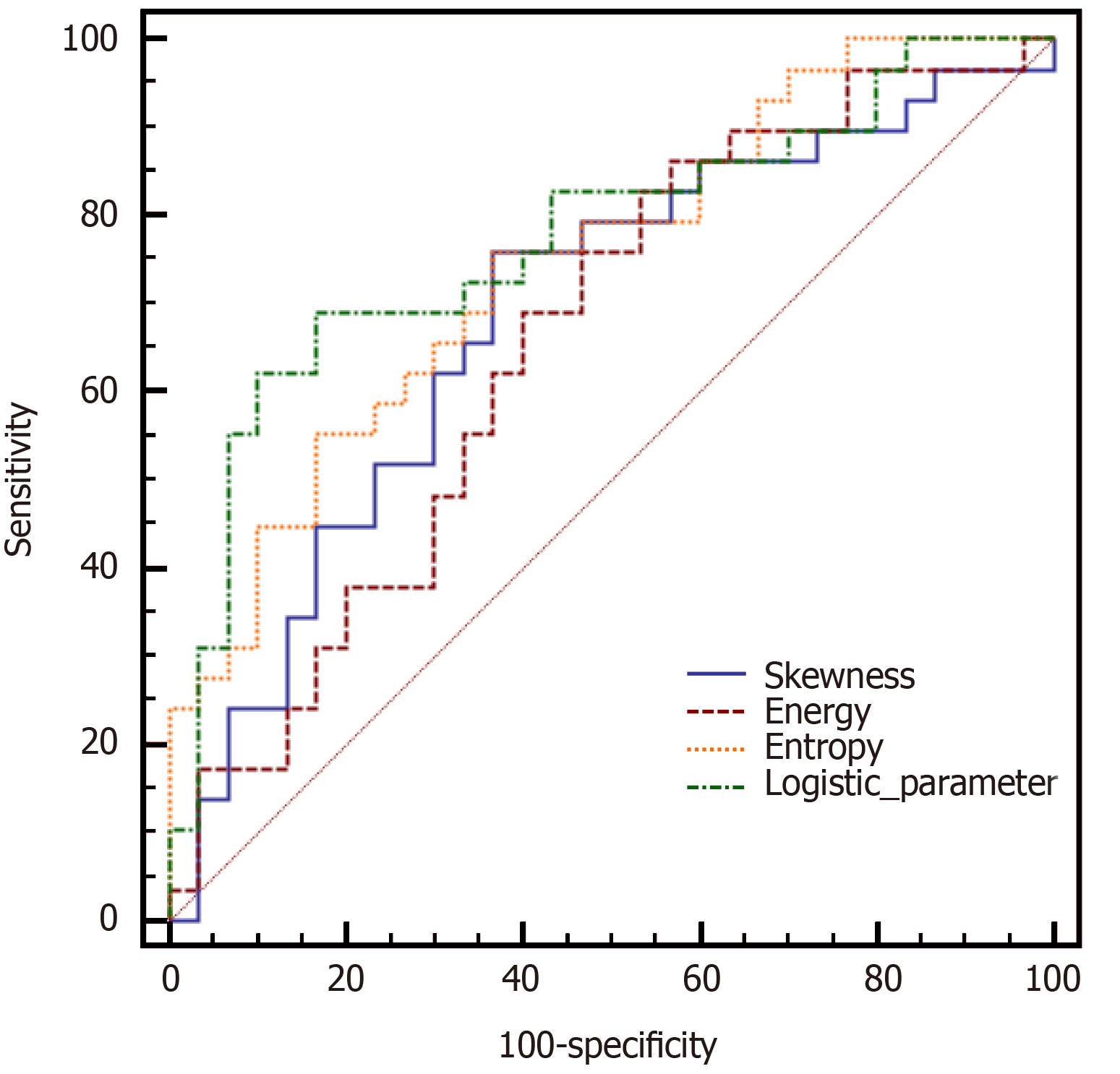
The diagnostic performances of statistically significant ADC first- and second-order texture features and logistic features using ROC analysis to determine the pT3a stage are shown in Table 4. For differentiating pT3a rectal adenocarcinomas from pT3b-c tumors, the AUCs of skewness, energy, and entropy were 0.686, 0.657, and 0.747, respectively. Using a logistic regression model that incorporated all three texture parameters, a moderate accuracy (AUC, 0.775) was achieved, with a sensitivity of 69.0% and specificity of 83.3% (Figure 3). We noted no significant AUC differences while comparing all pairs of texture parameters.
| Texture features | AUC (95%CI) | Sensitivity | Specificity | +LR | -LR | +PV | -PV | Comparison of AUC (P value) | |||
| Skewness | Energy | Entropy | Logistic feature | ||||||||
| Skewness | 0.686 (0.552-0.801) | 75.9% | 63.3% | 2.07 | 0.38 | 66.7 | 73.1 | — | 0.7469 | 0.4667 | 0.1471 |
| Energy | 0.657 (0.522-0.776) | 86.2% | 43.3% | 1.52 | 0.32 | 59.5 | 76.2 | — | 0.0956 | 0.0617 | |
| Entropy | 0.747 (0.617-0.851) | 75.9% | 63.3% | 2.07 | 0.38 | 66.7 | 73.1 | — | 0.3998 | ||
| Logistic feature | 0.775 (0.647-0.873) | 69.0% | 83.3% | 4.14 | 0.37 | 80.0 | 73.5 | — | |||
In this study, we investigated the feasibility of differentiating pT3a rectal adenocarcinomas from pT3b-c tumors using first- and second-order texture features derived from ADC maps. Skewness, energy, and entropy could significantly differentiate pT3a from pT3b-c rectal adenocarcinomas. Logistic regression analysis using all three features demonstrated a higher AUC in differentiating pT3a from pT3b-c tumors. The results indicate that texture features derived from ADC maps might potentially differentiate pT3a from pT3b-c rectal adenocarcinomas.
Statistics-based techniques are the most commonly used methods for TA[27] and contain three orders of features. First-order statistics can be obtained from the histogram of pixel intensity values, including mean intensity, maximum intensity, minimum intensity, standard deviation, uniformity, skewness, and kurtosis. Among second-order statistics, GLCM measurements calculated using spatial gray-level dependence matrices are well known, and these parameters also describe the relationship between neighboring pixels[28,29], including energy, entropy, total frequency, matrix mean, inertia, and correlation. Third-order statistics show the spatial relationships among three or more pixels[28,29]. In this study, ADC histogram features were based on first-order statistics, and GLCM measurements were based on second-order statistics.
In previous studies, the mean ADC values were significantly different among rectal cancers with different pT3 subclasses[23]. In the current study, mean ADC values were not statistically different between pT3a and pT3b-c tumors. Such a discrepancy could result from the methods used for calculating ADC. In the studies by Lu et al[11] and Tong et al[23], mean ADC values were calculated from three round ROIs that were manually delineated within solid tumor parts. On the contrary, we used whole-tumor VOI, which might better reflect the heterogeneity of the whole tumor[24]. In previous studies by our group[30,31], the 10th percentile ADC from the histogram performed better in differentiating the transition zone cancer from benign prostatic hyperplasia nodule, and was best correlated with the Gleason score of prostate cancer, because the low proportion (e.g., minimum, 10th percentile) ADC from the histogram represents the focal areas of high cellularity under the heterogeneous background[32]. Therefore, we hypothesized that the low proportion of ADC from the histogram could differentiate pT3a rectal adenocarcinomas from pT3b-c tumors. Nevertheless, the results showed that different percentile ADCs from the histogram were not statistically different between pT3a and pT3b-c tumors, suggesting that in rectal cancer, the pT3 stage is possibly more spatially homogeneous. In addition, the relatively small number of patients in the present study might have been a contributing factor.
As shown above, the pT3b-c stage was associated with higher skewness than the pT3a stage. Skewness reflects the asymmetry of pixel distribution. Positive skewness means that the distribution of the histogram has an elongated tail on the right side of the mean[24]. Higher skewness indicates elevated complexity and heterogeneity in tumors. This may be important when interpreting the finding that pT3b-c stage tumors have greater skewness than pT3a ones. The results of the present study were similar to those of Liu et al[33]. Moreover, uniformity had a near-significant difference between pT3a and pT3b-c tumors. Uniformity reflects how close the image is to a uniform distribution of gray levels, and high uniformity indicates uniformity in the image and lower heterogeneity[29]. Meng et al[34] reported a significant difference in pre-uniformity between advanced patients with rectal cancer that were responders vs non-responders in pathologic complete response. The above results revealed that uniformity was potentially advantageous in differentiating pT3a rectal adenocarcinomas from pT3b-c tumors. Hence, future studies will need larger patient cohorts to investigate the differences in uniformity between patients with pT3a and pT3b-c rectal adenocarcinomas.
As for second-order texture features, energy was significantly higher in pT3a rectal adenocarcinomas than in pT3b-c tumors, while entropy was significantly lower. Theoretically, the meaning of energy is similar to that of uniformity. Entropy represents the spatial disorder of the ADC gray-level distribution. Higher entropy and lower energy reflect greater lesional heterogeneity[35,36]. This may be important when interpreting the findings of second-order textural differences between pT3a and pT3b-c tumors. Similar findings have been reported in previous studies. For example, Caruso et al[35] observed significantly higher energy in complete responders compared with non-responders to neoadjuvant chemoradiotherapy for colorectal cancer. In studies exploring the value of ADC texture features in characterizing pathologic features of rectal cancer, pT3-4 rectal cancer was significantly higher in entropy in comparison with pT1-2[33,37].
There were several limitations in the present study. First, because of its retrospective design, selection bias was inevitable. Second, the study population was relatively small, and all patients were enrolled in the same hospital, which indicates the low generalizability of the present findings. The low proportion of ADC from the histogram could not significantly differentiate pT3a from pT3b-c tumors, which contradicted previous studies from our group. A future study would benefit from a larger study population. Third, we did not correlate TA with other MRI sequences such as T2WI. High-resolution T2WI plays a pivotal role in the preoperative staging of rectal cancer. Hence, future studies should include T2WI-based TA. Fourth, although gadolinium was used as the contrast agent for MRI, its effect was not examined in this study. Fifth, the study's outcomes only had short-term clinical applicability. Finally, first- and second-order texture features were derived from the DWI datasets, which are sensitive to the applied b-values[38]. In designing future studies, the choice of the b-value needs to be taken into account.
Texture features derived from ADC maps, especially skewness, energy, and entropy, might potentially differentiate pT3a rectal adenocarcinomas from pT3b-c tumors. This could have a practical value for the individualized management of rectal cancer patients.
The accuracy of discriminating pT3a from pT3b-c rectal cancer using high-resolution magnetic resonance imaging remains unsatisfactory. Indeed, the mean and median apparent diffusion coefficient (ADC) values are not always significantly sensitive to small changes or precise status of the tumor owing to the intrinsic chaotic environment of tumors.
Texture analysis (TA) could improve the discrimination of pT3a rectal adenocarcinomas from pT3b-c tumors, but pT3 subclasses of rectal cancer have not been previously determined by ADC maps.
To investigate the value of TA on ADC maps in differentiating pT3a rectal adenocarcinomas from pT3b-c tumors.
This case-control study assessed patients with pT3 rectal adenocarcinoma, who underwent DWI between October 2016 and December 2018. First-order (ADC values, skewness, kurtosis, and uniformity) and second-order (energy, entropy, inertia, and correlation) texture features were derived from whole-lesion ADC maps. Receiver operating characteristic (ROC) curves were used to determine the diagnostic value for pT3b-c tumors.
Totally 59 patients (34 men and 25 women) were included, with a median age of 66 years (range, 41-85 years). Thirty patients had pT3a, 24 had pT3b, and five had pT3c. Skewness was significantly lower in the pT3a stage than in pT3b-c tumors. In addition, energy and entropy were significantly different between pT3a rectal adenocarcinomas and pT3b-c tumors. For differentiating pT3a rectal adenocarcinomas from pT3b-c tumors, the areas under the curves (AUCs) of skewness, energy, and entropy were 0.686, 0.657, and 0.747, respectively. Logistic regression analysis of all three features yielded a greater AUC (0.775) in differentiating pT3a rectal adenocarcinomas from pT3b-c tumors (69.0% sensitivity and 83.3% specificity).
TA features derived from ADC maps might potentially differentiate pT3a rectal adenocarcinomas from pT3b-c tumors, especially skewness, energy, and entropy and their combination.
Future studies should include T2WI-based TA, since high-resolution T2WI plays a pivotal role in the preoperative staging of rectal cancer, and b-values should also be taken into account. In addition, features with long-term clinical applicability should be assessed. Finally, large multicenter studies are needed to confirm and increase the generalizability of the above findings.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nespoli L S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55778] [Article Influence: 7968.3] [Reference Citation Analysis (132)] |
| 2. | Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1120] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 3. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1194] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 4. | Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC, Shih YT, Walter LC, Andrews KS, Brawley OW, Brooks D, Fedewa SA, Manassaram-Baptiste D, Siegel RL, Wender RC, Smith RA. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1309] [Article Influence: 187.0] [Reference Citation Analysis (0)] |
| 5. | NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Rectal Cancer. Version 4.2020. Fort Washington: National Comprehensive Cancer Network. 2020. |
| 6. | Zinicola R, Pedrazzi G, Haboubi N, Nicholls RJ. The degree of extramural spread of T3 rectal cancer: an appeal to the American Joint Committee on Cancer. Colorectal Dis. 2017;19:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Kaur H, Choi H, You YN, Rauch GM, Jensen CT, Hou P, Chang GJ, Skibber JM, Ernst RD. MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. Radiographics. 2012;32:389-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Nomura M, Takahashi H, Fujii M, Miyoshi N, Haraguchi N, Hata T, Matsuda C, Yamamoto H, Mizushima T, Mori M, Doki Y. Clinical significance of invasion distance relative to prognosis in pathological T3 colorectal cancer. Oncol Lett. 2019;18:5614-5620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Merkel S, Mansmann U, Siassi M, Papadopoulos T, Hohenberger W, Hermanek P. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis. 2001;16:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Cho SH, Kim SH, Bae JH, Jang YJ, Kim HJ, Lee D, Park JS; Society of North America (RSNA). Prognostic stratification by extramural depth of tumor invasion of primary rectal cancer based on the Radiological Society of North America proposal. AJR Am J Roentgenol. 2014;202:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Lu ZH, Hu CH, Qian WX, Cao WH. Preoperative diffusion-weighted imaging value of rectal cancer: preoperative T staging and correlations with histological T stage. Clin Imaging. 2016;40:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Zhong G, Xiao Y, Zhou W, Pan W, Zhu Q, Zhang J, Jiang Y. Value of endorectal ultrasonography in measuring the extent of mesorectal invasion and substaging of T3 stage rectal cancer. Oncol Lett. 2017;14:5657-5663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Faletti R, Gatti M, Arezzo A, Stola S, Benedini MC, Bergamasco L, Morino M, Fonio P. Preoperative staging of rectal cancer using magnetic resonance imaging: comparison with pathological staging. Minerva Chir. 2018;73:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4541] [Cited by in RCA: 5530] [Article Influence: 614.4] [Reference Citation Analysis (3)] |
| 15. | Becker AS, Ghafoor S, Marcon M, Perucho JA, Wurnig MC, Wagner MW, Khong PL, Lee EY, Boss A. MRI texture features may predict differentiation and nodal stage of cervical cancer: a pilot study. Acta Radiol Open. 2017;6:2058460117729574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Wibmer A, Hricak H, Gondo T, Matsumoto K, Veeraraghavan H, Fehr D, Zheng J, Goldman D, Moskowitz C, Fine SW, Reuter VE, Eastham J, Sala E, Vargas HA. Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol. 2015;25:2840-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 292] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 17. | Ytre-Hauge S, Dybvik JA, Lundervold A, Salvesen ØO, Krakstad C, Fasmer KE, Werner HM, Ganeshan B, Høivik E, Bjørge L, Trovik J, Haldorsen IS. Preoperative tumor texture analysis on MRI predicts high-risk disease and reduced survival in endometrial cancer. J Magn Reson Imaging. 2018;48:1637-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | Ueno Y, Forghani B, Forghani R, Dohan A, Zeng XZ, Chamming's F, Arseneau J, Fu L, Gilbert L, Gallix B, Reinhold C. Endometrial Carcinoma: MR Imaging-based Texture Model for Preoperative Risk Stratification-A Preliminary Analysis. Radiology. 2017;284:748-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 19. | Xu Y, Xu Q, Ma Y, Duan J, Zhang H, Liu T, Li L, Sun H, Shi K, Xie S, Wang W. Characterizing MRI features of rectal cancers with different KRAS status. BMC Cancer. 2019;19:1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Oh JE, Kim MJ, Lee J, Hur BY, Kim B, Kim DY, Baek JY, Chang HJ, Park SC, Oh JH, Cho SA, Sohn DK. Magnetic Resonance-Based Texture Analysis Differentiating KRAS Mutation Status in Rectal Cancer. Cancer Res Treat. 2020;52:51-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Park H, Kim KA, Jung JH, Rhie J, Choi SY. MRI features and texture analysis for the early prediction of therapeutic response to neoadjuvant chemoradiotherapy and tumor recurrence of locally advanced rectal cancer. Eur Radiol. 2020;30:4201-4211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Crimì F, Capelli G, Spolverato G, Bao QR, Florio A, Milite Rossi S, Cecchin D, Albertoni L, Campi C, Pucciarelli S, Stramare R. MRI T2-weighted sequences-based texture analysis (TA) as a predictor of response to neoadjuvant chemo-radiotherapy (nCRT) in patients with locally advanced rectal cancer (LARC). Radiol Med. 2020;125:1216-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Tong T, Yao Z, Xu L, Cai S, Bi R, Xin C, Gu Y, Peng W. Extramural depth of tumor invasion at thin-section MR in rectal cancer: associating with prognostic factors and ADC value. J Magn Reson Imaging. 2014;40:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Just N. Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer. 2014;111:2205-2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 386] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 25. | Cortez-Conradis D, Favila R, Isaac-Olive K, Martinez-Lopez M, Rios C, Roldan-Valadez E. Diagnostic performance of regional DTI-derived tensor metrics in glioblastoma multiforme: simultaneous evaluation of p, q, L, Cl, Cp, Cs, RA, RD, AD, mean diffusivity and fractional anisotropy. Eur Radiol. 2013;23:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [PubMed] |
| 27. | Castellano G, Bonilha L, Li LM, Cendes F. Texture analysis of medical images. Clin Radiol. 2004;59:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 656] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 28. | Srinivasan G, Shobha G. Statistical texture analysis. Proc World Acad Sci Eng Technol. 2008;36:1264-1269. |
| 29. | Kim JH, Ko ES, Lim Y, Lee KS, Han BK, Ko EY, Hahn SY, Nam SJ. Breast Cancer Heterogeneity: MR Imaging Texture Analysis and Survival Outcomes. Radiology. 2017;282:665-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 30. | Lu Z, Zhao W, Ji L. The Histogram Analysis of Apparent Diffusion Coefficient Maps with Standard- or Ultrahigh-b Value Diffusion-Weighted MR Imaging for Differentiating the Gleason Grade of Prostate Cancer. J Med Imaging Health Inf. 2018;8:577-582. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Lu ZH, Ji LB, Zhao WL, Zhang YS, Wu JF, Li X, Shen JK. Differentiating Transition Zone Cancers From Benign Prostatic Hyperplasia by Histogram Analysis of Apparent Diffusion Coefficient Maps With Standard and Ultrahigh b-value Diffusion-weighted MR Imaging. J Comput Assist Tomogr. 2019;43:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Wu CJ, Wang Q, Li H, Wang XN, Liu XS, Shi HB, Zhang YD. DWI-associated entire-tumor histogram analysis for the differentiation of low-grade prostate cancer from intermediate-high-grade prostate cancer. Abdom Imaging. 2015;40:3214-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Liu L, Liu Y, Xu L, Li Z, Lv H, Dong N, Li W, Yang Z, Wang Z, Jin E. Application of texture analysis based on apparent diffusion coefficient maps in discriminating different stages of rectal cancer. J Magn Reson Imaging. 2017;45:1798-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 34. | Meng Y, Zhang C, Zou S, Zhao X, Xu K, Zhang H, Zhou C. MRI texture analysis in predicting treatment response to neoadjuvant chemoradiotherapy in rectal cancer. Oncotarget. 2018;9:11999-12008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Caruso D, Zerunian M, Ciolina M, de Santis D, Rengo M, Soomro MH, Giunta G, Conforto S, Schmid M, Neri E, Laghi A. Haralick's texture features for the prediction of response to therapy in colorectal cancer: a preliminary study. Radiol Med. 2018;123:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Duvauferrier R, Bezy J, Bertaud V, Toussaint G, Morelli J, Lasbleiz J. Texture analysis software: integration with a radiological workstation. Stud Health Technol Inform. 2012;180:1030-1034. [PubMed] |
| 37. | Li W, Jiang Z, Guan Y, Chen Y, Huang X, Liu S, He J, Zhou Z, Ge Y. Whole-lesion Apparent Diffusion Coefficient First- and Second-Order Texture Features for the Characterization of Rectal Cancer Pathological Factors. J Comput Assist Tomogr. 2018;42:642-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Becker AS, Wagner MW, Wurnig MC, Boss A. Diffusion-weighted imaging of the abdomen: Impact of b-values on texture analysis features. NMR Biomed. 2017;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |