Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6922
Peer-review started: April 25, 2021
First decision: June 6, 2021
Revised: June 8, 2021
Accepted: June 22, 2021
Article in press: June 22, 2021
Published online: August 16, 2021
Processing time: 102 Days and 6.5 Hours
Allergic bronchopulmonary aspergillosis (ABPA) is an allergic reaction to Aspergillus species that aggravates bronchial asthma. Previous studies demonstrated the glucocorticoid-sparing effect of dupilumab in patients with ABPA. There is no report of complete withdrawal of glucocorticoids after dupilumab.
The patient was a 54-year-old woman with bronchial asthma treated with inhaled corticosteroids and a long-acting beta-2 agonist. She consulted our institution for productive cough and fever in March 2017. Chest computed tomography scan revealed mucoid impaction, and the bronchial lavage fluid culture was positive for Aspergillus fumigatus. The diagnosis was ABPA. The patient was treated with oral glucocorticoids from April 2017 to November 2017. In January 2019, she had bronchial asthma exacerbation, and a chest computed tomography scan showed recurrent mucoid impaction. She was treated with oral glucocorticoids and itraconazole. In February 2020, during tapering of oral glucocorticoid, she had the third episode of bronchial asthma exacerbation and a mucoid impaction. The patient was treated with dupilumab in addition to oral glucocorticoid and itraconazole. The clinical response improved, and oral glucocorticoid was discontinued in June 2020.
This is the first case of ABPA in which complete withdrawal of glucocorticoid was possible after treatment with dupilumab.
Core Tip: Allergic bronchopulmonary aspergillosis (ABPA) is an allergic reaction to antigen from Aspergillus species that causes exacerbation of bronchial asthma, eosinophilic pneumonia, and bronchiectasis. Conventional therapy includes the administration of oral glucocorticoids and other antiasthmatic therapy. However, there are intractable cases showing repeated clinical exacerbations of bronchial asthma despite therapy with oral glucocorticoids. Previous reports have shown that the addition of monoclonal antibodies against interleukin (IL)-4/IL-13 or dupilumab to the treatment is effective and allows the reduction in the dose of glucocorticoids in ABPA patients. However, there is no report of complete withdrawal of oral glucocorticoids after therapy with dupilumab in these patients. Here, we report the first case of ABPA in which complete withdrawal of oral glucocorticoid was possible after treatment with dupilumab.
- Citation: Nishimura T, Okano T, Naito M, Tsuji C, Iwanaka S, Sakakura Y, Yasuma T, Fujimoto H, D'Alessandro-Gabazza CN, Oomoto Y, Kobayashi T, Gabazza EC, Ibata H. Complete withdrawal of glucocorticoids after dupilumab therapy in allergic bronchopulmonary aspergillosis: A case report. World J Clin Cases 2021; 9(23): 6922-6928
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6922.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6922
Allergic bronchopulmonary aspergillosis (ABPA) is an allergic reaction to Aspergillus species[1]. ABPA causes exacerbation of bronchial asthma, eosinophilic pneumonia, and bronchiectasis[1]. Treatment of ABPA is critical for the clinical control of bronchial asthma. The administration of oral glucocorticoid alone or in combination with antifungal drugs are effective therapeutic approaches[1]. However, ABPA is highly prone to relapse when the oral glucocorticoid dose is reduced or is completely discontinued. Patients with ABPA have also been reported to respond to the treatment with anti-immunoglobulin E (IgE) or anti-interleukin (IL)-5 antibody[2-4]. However, most cases of ABPA require therapy with oral glucocorticoids to control clinical exacerbations of bronchial asthma. The oral glucocorticoid-sparing effect of anti-IL-4/IL-13 antibody (dupilumab) has been previously reported[5]. However, there is no report on the complete withdrawal of glucocorticoids following dupilumab therapy. Here, we report a case of ABPA in which glucocorticoids were completely unnecessary to control bronchial asthma exacerbation after dupilumab therapy.
The patient was a 54-year-old woman that consulted the Mie Chuo Medical Center because of fever and productive cough.
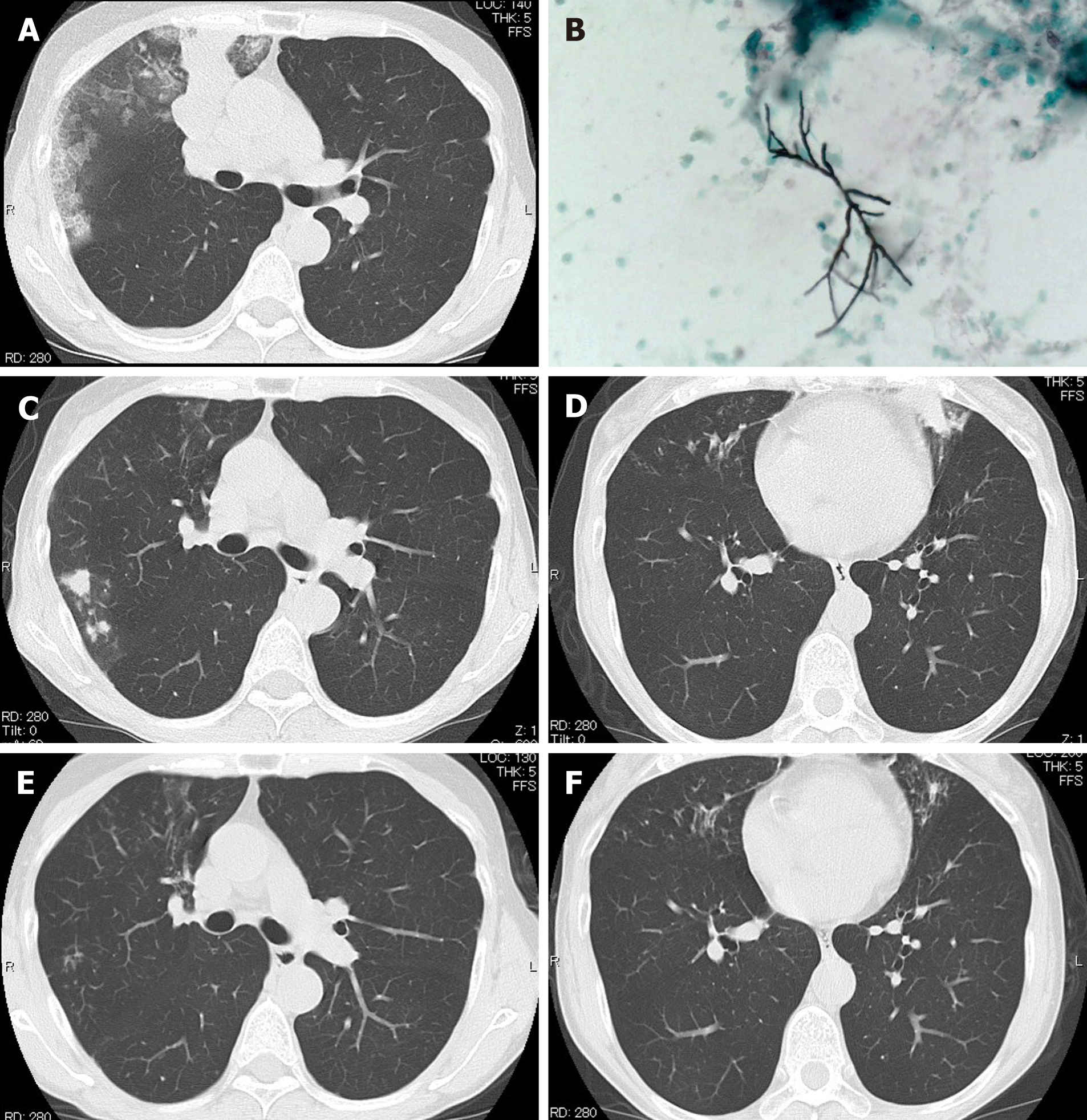
In April 2017, she presented at the outpatient clinic of Mie Chuo Medical Center for fever and productive cough. A chest computed tomography (CT) scan revealed consolidation, ground-glass opacity, and mucoid impaction of the right upper lung (Figure 1A). The culture of the bronchial lavage fluid was positive for Aspergillus fumigatus (A. fumigatus) (Figure 1B). The anti-aspergillus antibody was positive with a serum anti-aspergillus-specific IgE concentration of 28.9 UA/mL. The diagnosis was ABPA. Treatment with oral prednisolone at a dose of 0.5 mg/kg/d was started in April 2017. The clinical symptoms of the patient improved, and the oral glucocorticoid therapy was discontinued in November 2017.
However, in January 2019, she presented again with fever and exacerbation of her bronchial asthma. The chest CT scan showed a recurrence of mucoid impaction. The CT finding improved after treating the patient with oral (0.5 mg/kg/d) glucocorticoid and oral itraconazole in January 2019. The dose of oral glucocorticoid was then gradually reduced.
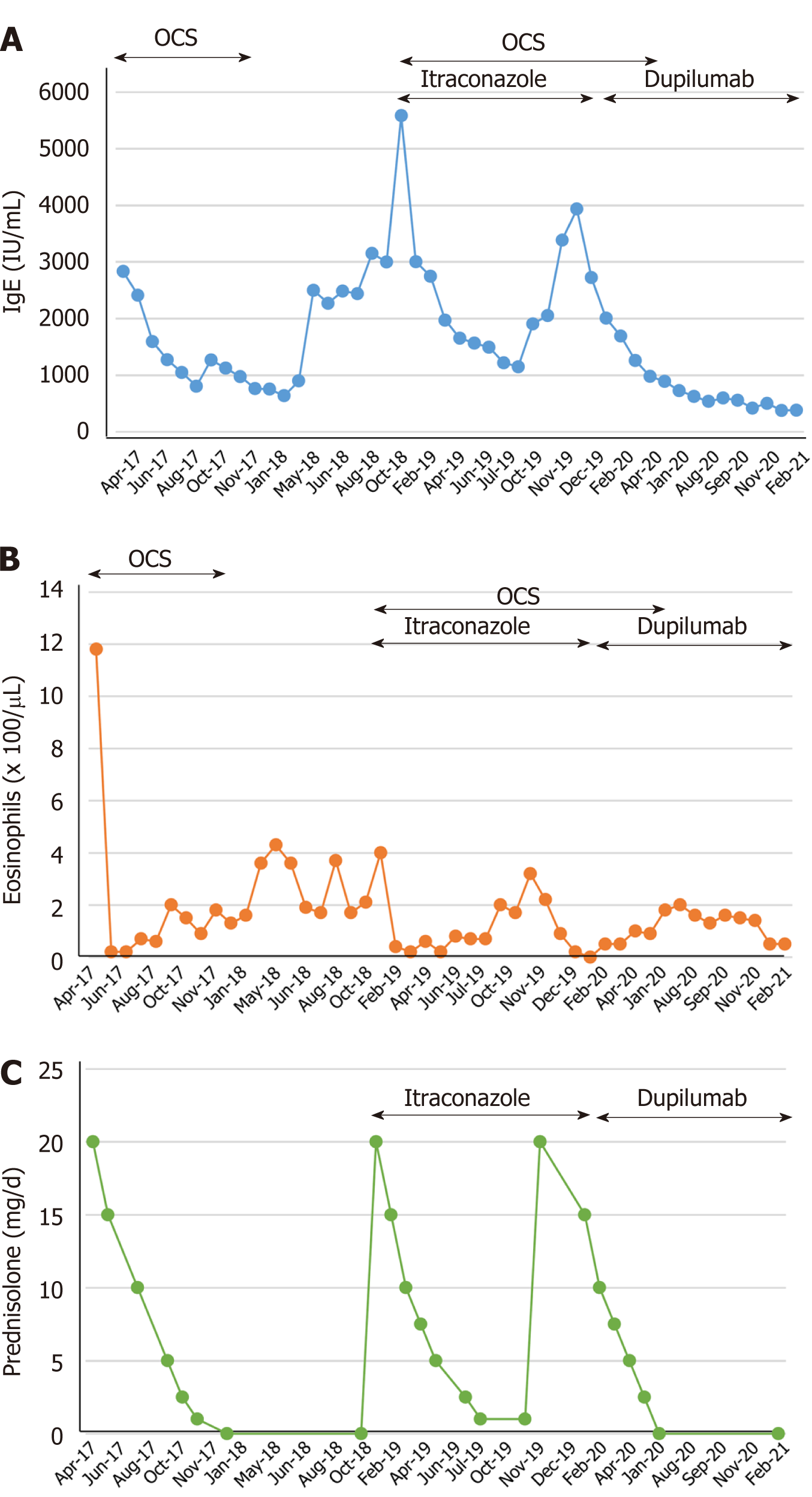
However, in November 2019, she had another episode of bronchial asthma exacerbation in association with increased serum total IgE, eosinophilia, and recurrent mucoid impaction by CT scan (Figure 1C and D). In February 2020, we started treating the patient with dupilumab (300 mg/2-wk) in addition to oral glucocorticoid (0.5 mg/kg/d) and itraconazole (100 mg/d). The mucoid impaction disappeared after the combined therapy of dupilumab and oral glucocorticoid (Figure 1E and F). We continued treating the patient with dupilumab, but discontinued the administration of oral glucocorticoid in June 2020. During follow-up, the patient showed no symptoms of bronchial asthma exacerbation or recurrence of mucoid impaction. IgE levels and the eosinophil count in peripheral blood were not increased after discontinuation of oral glucocorticoids and maintenance with dupilumab (Figure 2).
She had no medical history of any disease.
No history.
The patient's physical examination showed symptoms and signs of airway obstruction.
During her first consultation, the culture of the bronchial lavage fluid was positive for A. fumigatus (Figure 1B), and the anti-aspergillus antibody was positive with a serum anti-aspergillus-specific IgE concentration of 28.9 UA/mL. The total serum levels of IgE (3387 IU/mL) were also increased during her second consultation.
A chest CT scan revealed consolidation, ground-glass opacity, and mucoid impaction of the right upper lung (Figure 1A). During her second and third consultations for clinical exacerbation of bronchial asthma, her chest CT scan findings were compatible with a recurrence of mucoid impaction.
The final diagnosis was ABPA with repeated clinical exacerbations of bronchial asthma.
The patient was initially treated with oral prednisolone at a dose of 0.5 mg/kg/d in April 2017. The clinical symptoms of the patient improved, and the oral glucocorticoid therapy was discontinued in November 2017. The patient received treatment with oral (0.5 mg/kg/d) glucocorticoid and oral itraconazole (100 mg/d) in January 2019 during her second consultation for bronchial asthma exacerbation. The dose of oral glucocorticoid was then gradually reduced. During her third episode of bronchial asthma exacerbation, we treated the patient with dupilumab (300 mg/2-wk) in addition to oral glucocorticoid (0.5 mg/kg/d) and itraconazole (100 mg/d).
The mucoid impaction detected by the chest CT scan disappeared after the combined therapy of dupilumab and oral glucocorticoid (Figure 1E and F). We then continued treating the patient with dupilumab, but discontinued the administration of oral glucocorticoid in June 2020. Despite discontinuing her treatment with oral glucocorticoids, inhaled glucocorticoids, and a long-acting beta-2 agonist inhalation for 4 mo after starting therapy with dupilumab, she had no symptoms of bronchial asthma exacerbation or mucoid impaction by chest CT scan.
The pathogenesis of ABPA is not completely clear. Deficient fungal clearance from the airways and excessive activation of CD4+ T helper 2 (Th2) cells play important roles[1]. A predominant activation of Th2 cells in ABPA leads to increased inflammatory cytokines, including IL-4, IL-5, IL-13, CCL-17, and IL-9[6]. Failure to eradicate the fungus leads to a sustained immune response in the airways of patients with ABPA[7]. This excessive immune response induces an inflammatory response characterized by elevated serum levels of IgE, increased mast cell degranulation, enhanced activation of eosinophils and neutrophils that ultimately causes mucoid impaction, eosinophilic pneumonia, or bronchiectasis[8].
The therapeutic efficacy of monoclonal antibodies against Th2 mediators including IgE (omalizumab), IL-5 (mepolizumab), IL-5R (benralizumab), and anti-IL-4/IL-13 (dupilumab) has been reported in patients with ABPA[2-4]. The administration of dupilumab decreases the peripheral blood count of eosinophils and the serum levels of IgE in patients with severe bronchial asthma[9]. Several reports have also demon
| Ref. | Age, yr | Sex | Timing of dupilumab therapy | Treatment in combination with dupilumab | Baseline IgE (IU/mL) | Baseline eosinophil count (/µL) | Chest CT findings |
| Ramonell et al[10], 2020 | 60 | Female | Disease recurrence | OCS | 561 | 90 | Cylindrical bronchiectasis |
| Ramonell et al[10], 2020 | 51 | Female | Disease recurrence | OCS | > 2000 | 90 | Bilateral central bronchiectasis |
| Ramonell et al[10], 2020 | 33 | Male | Disease diagnosis | Voriconazole | 11290 | 1750 | Mild diffuse bronchiectasis |
| Mümmler et al[5], 2020 | 49 | Female | Disease recurrence | OCS | 7000 | 950 | Pulmonary infiltrates, diffuse pulmonary bronchiectasis and bronchial wall thickening |
| Tashiro et al[11], 2021 | 72 | Female | Disease diagnosis | None | 2525 | 5637 | Centrilobular nodules, infiltrations, and high-attenuation mucus in the bronchi with central dilatation |
| Mikura et al[9], 2021 | 45 | Male | Disease recurrence | OCS, itraconazole | 2306 | 1914 | High-attenuation mucus and ground glass opacity |
| Present case | 54 | Female | Disease recurrence | OCS, itraconazole | 2833 | 1180 | Consolidation, ground-glass opacity, and high-attenuation mucus |
This is the first report of a case of ABPA in which reduction in the dose and, sub
Manuscript source: Unsolicited manuscript
Specialty type: Allergy
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Panaitescu C, Zhuo ZQ S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Agarwal R, Sehgal IS, Dhooria S, Muthu V, Prasad KT, Bal A, Aggarwal AN, Chakrabarti A. Allergic bronchopulmonary aspergillosis. Indian J Med Res. 2020;151:529-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 2. | Altman MC, Lenington J, Bronson S, Ayars AG. Combination omalizumab and mepolizumab therapy for refractory allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2017;5:1137-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Li JX, Fan LC, Li MH, Cao WJ, Xu JF. Beneficial effects of Omalizumab therapy in allergic bronchopulmonary aspergillosis: A synthesis review of published literature. Respir Med. 2017;122:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Soeda S, Kono Y, Tsuzuki R, Yamawaki S, Katsube O, To M, To Y. Allergic bronchopulmonary aspergillosis successfully treated with benralizumab. J Allergy Clin Immunol Pract. 2019;7:1633-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Mümmler C, Kemmerich B, Behr J, Kneidinger N, Milger K. Differential response to biologics in a patient with severe asthma and ABPA: a role for dupilumab? Allergy Asthma Clin Immunol. 2020;16:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Schuyler M. The Th1/Th2 paradigm in allergic bronchopulmonary aspergillosis. J Lab Clin Med. 1998;131:194-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 875] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 8. | Knutsen AP, Slavin RG. Allergic bronchopulmonary aspergillosis in asthma and cystic fibrosis. Clin Dev Immunol. 2011;2011:843763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Mikura S, Saraya T, Yoshida Y, Oda M, Ishida M, Honda K, Nakamoto K, Tamura M, Takata S, Shimoyamada H, Fujiwara M, Ishii H. Successful Treatment of Mepolizumab- and Prednisolone-resistant Allergic Bronchopulmonary Aspergillosis with Dupilumab. Intern Med. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Ramonell RP, Lee FE, Swenson C, Kuruvilla M. Dupilumab treatment for allergic bronchopulmonary aspergillosis: A case series. J Allergy Clin Immunol Pract. 2020;8:742-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Tashiro H, Takahashi K, Kurihara Y, Sadamatsu H, Kimura S, Sueoka-Aragane N. Efficacy of dupilumab and biomarkers for systemic corticosteroid naïve allergic bronchopulmonary mycosis. Allergol Int. 2021;70:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |