Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6832
Peer-review started: April 24, 2021
First decision: May 24, 2021
Revised: June 4, 2021
Accepted: June 16, 2021
Article in press: June 16, 2021
Published online: August 16, 2021
Processing time: 103 Days and 13.4 Hours
Triphalangeal thumb-polysyndactyly syndrome (TPT-PS) is a rare type of congenital limb deformity, and most studies focus on the genetics. Case reports of the sonographic characteristics of TPT-PS during pregnancy are rare.
A 30-year-old woman (G3P1) who had pregnancies with TPT-PS fetuses is presented. The possibility of TPT-PS was shown by ultrasound performed at the 19th wk of pregnancy, featuring hands with six metacarpals, an extra digit at the 5th finger side, and an abnormally widened thumb. Whole-exome sequencing was subsequently conducted. The results showed that exons 1-17 of the LMBR1 gene had a heterozygous duplication, with a length of approximately 253 kb.
We suggest prenatal ultrasound examination combined with genetic testing to diagnose TPT-PS accurately and to help clinicians and patients make decisions.
Core Tip: Triphalangeal thumb-polysyndactyly syndrome (TPT-PS) is a rare type of congenital limb deformity that has autosomal dominant inheritance. A case of TPT-PS diagnosed by ultrasound in pregnancy is presented. Features of antenatal ultrasound include hands with six metacarpals, an extra digit at the 5th finger side, an abnormally widened thumb or thumb shaped like a trident (bifida), and the absence of interpha
- Citation: Zhang SJ, Lin HB, Jiang QX, He SZ, Lyu GR. Prenatal diagnosis of triphalangeal thumb-polysyndactyly syndrome by ultrasonography combined with genetic testing: A case report. World J Clin Cases 2021; 9(23): 6832-6838
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6832.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6832
Triphalangeal thumb-polysyndactyly syndrome (TPT-PS, OMIM-174500), also known as preaxial polydactyly type II, is an autosomal dominant anomaly characterized by triphalangeal thumbs, pre- and post-axial polydactyly, complex polysyndactyly, and isolated syndactyly[1]. During limb development, sonic hedgehog (SHH), normally expressed in the zone of polarizing activity (ZPA) in the posterior limb bud, regulates the anterior-posterior axis of the limb bud[2]. The expression of SHH in the limb bud is regulated by an enhancer known as ZPA regulatory sequence (ZRS), which is a highly conserved noncoding sequence lying within intron 5 of the LMBR1 gene, approximately 1 Mb upstream of SHH[3]. Disruption of ZRS can lead to additional expression of SHH at the anterior part of the limb bud, which finally causes TPT-PS[4]. A review of the literature shows that point mutations in ZRS[3,5,6], point mutations in pre-ZRS[4], and duplications including ZRS can cause TPT-PS[1,7-12]. A collection of genetic abnormalities causing TPT-PS is presented in Table 1.
| Ref. | Position/range |
| Lettice et al[3], 2003 | Point mutation: 105C>G |
| Wang et al[6], 2007 | Point mutation: 4420C>T |
| VanderMeer et al[5], 2012 | Point mutation: 287C>A |
| Potuijt et al[4], 2018 | Point mutation: Pre-ZRS (chr7: 156585476G>C) |
| Klopocki et al[1], 2008 | Duplication: 589 kb |
| Sun et al[7], 2008 | Duplication: 131-398 kb |
| Wieczorek et al[8], 2010 | Duplication: 276 kb |
| Lohan et al[9], 2014 | Duplication: 255 kb |
| Xing et al[10], 2014 | Duplication: 442 kb |
| Liu et al[11], 2017 | Duplication: 288 kb |
| Xu et al[12], 2020 | Duplication: 180 kb |
Most of the literature reporting TPT-PS focuses on the genetics, but genetic testing is difficult to perform in prenatal screening because of its high cost. By ultrasonography, polydactyly can be detected as early as the 10th wk of gestation[13]. It is considered to be present if an extra digit containing bone is detected[14]. With improvements in ultrasound imaging technology, the detection rate of polydactyly has significantly increased. Whole-exome sequencing (WES) is an important way to detect abnormal genes associated with fetal structural malformations and can be an appropriate tool to confirm abnormal ultrasound findings during pregnancy[15]. At present, there is little literature reporting the sonographic characteristics of TPT-PS during pregnancy. Here, we report a case of TPT-PS with a summary of its prenatal sonographic features and show that heterozygous duplication encompassing exons 1-17 of the LMBR1 gene with a length of approximately 253 kb is also related to TPT-PS.
In July 2020, a 30-year-old female patient at the 19th wk of pregnancy (G3P1) was admitted to our hospital due to a history of abnormal pregnancies.
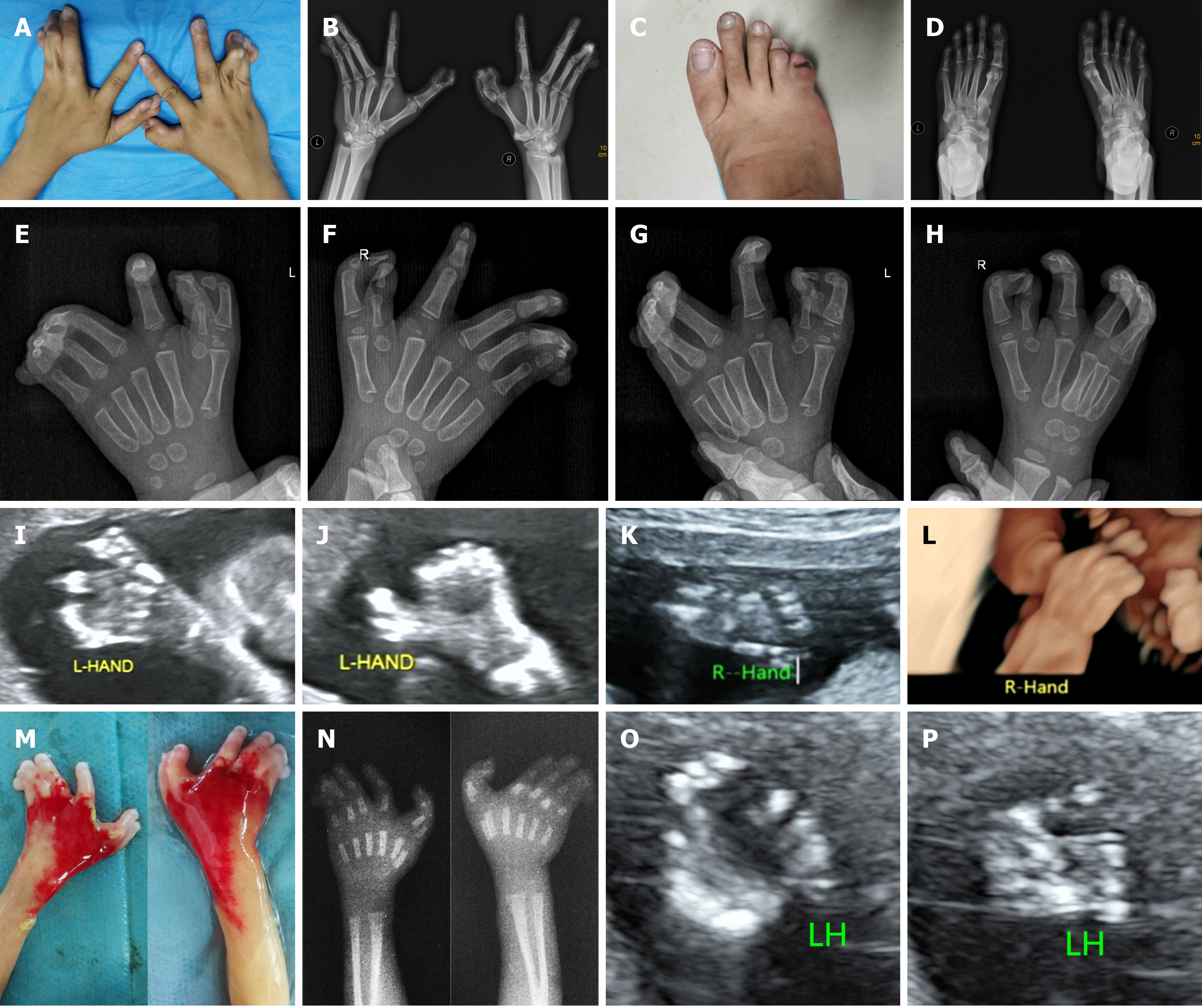
The patient suffered from malformation of both hands and the right foot (Figure 1A-D).
In 2015, a baby boy was born with triphalangeal thumbs, pre- and post-axial polysyndactyly, and cutaneous fusion of the 3rd, 4th, and 5th fingers (Figure 1E-H). In 2017, a pregnancy ended in miscarriage with a fetus demonstrating triphalangeal thumbs and complex polysyndactyly. According to the sonogram, there was thumb polydactyly shaped like a trident (bifida) and the absence of interphalangeal space between the 3rd, 4th, and 5th fingers (Figure 1I-L).
No deformity was found in the patient's parents or relatives.
Physical examination showed that the pregnant woman had triphalangeal thumbs, pre- and post-axial polysyndactyly, and cutaneous fusion of the 3rd, 4th, and 5th fingers, accompanied by deviant posture of the hands, while the index finger was unaffected. Her right foot manifested postaxial polysyndactyly (Figure 1A-D).
Laboratory examinations showed no abnormalities.
On ultrasound, hands with six metacarpals were documented. Through multiplane scanning, an echo of the extra digit at the 5th finger side was evident, and the thumb appeared be doubled, leaving the thumb abnormally widened. The morphological appearance of the 3rd, 4th, and 5th fingers was plausibly normal at the beginning of our examination. However, no separation of those digits was detected throughout the examination, leading to a diagnosis of syndactyly (Figure 1O and P).
The final diagnosis was TPT-PS.
According to the observed deformities, such as hands with six metacarpals, an extra digit at the 5th finger side, and an abnormally widened thumb, polysyndactyly was considered. Given that the patient had abnormal fetuses in three consecutive pregnancies and that the X-ray examination of herself and her son demonstrated a triphalangeal thumb, TPT-PS was diagnosed. After prenatal consultation, the patient chose WES to make a definite diagnosis and requested termination of the pregnancy.
At the 20th wk of gestation, ultrasound-guided cordocentesis was conducted with amniotic cavity injection of ethacridine lactate (Rivanol, Guangxi Hefeng Pharmaceutical Co., Ltd., China) for labor induction. Umbilical cord blood (3.5 mL) was successfully obtained. The peripheral blood of the pregnant woman, her husband, and her son was also extracted for WES analysis. However, none of the patient's parents or relatives were willing to undergo a blood draw for genetic testing.
The fetus was stillborn 1 d after Rivanol injection. Postmortem and X-ray examination of the aborted fetus showed six metacarpals, triphalangeal thumbs, fused extra preaxial fingers, and cutaneous syndactyly of the 3rd, 4th, and 5th digits with a knob-like pedicled finger, while the index finger was unaffected (Figure 1M and N). Additionally, the right foot presented postaxial polysyndactyly.
Twenty-four days after the extraction, WES showed a heterozygous duplication encompassing exons 1-17 of the LMBR1 gene (NM_022458.3) in the fetus, the pregnant woman, and her son. The size of their duplication was 253 kb. According to the inclusion criteria set by the American College of Medical Genetics and Genomics (ACMG), the Clinical Genome Resource (ClinGen), the disease gene variation database, and literature reports, the duplication was confirmed as a pathogenic variation, and TPT-PS was confirmed. The duplication was absent from the patient’s husband.
We report a case of prenatal sonographic detection of a fetus with TPT-PS. Subsequently, autopsy, X-ray examination, history investigation, and WES were used to verify the ultrasound diagnosis. In the history investigation, we found that the pregnant woman had abnormal fetuses in the last two pregnancies. WES revealed a duplication spanning exons 1-17 of the LMBR1 gene, including ZRS. This research may shed light on methods for prenatal TPT-PS diagnosis.
First, there was a diversity of phenotypes, including triphalangeal thumbs, pre- and post-axial polydactyly, and webbing of the digits. Therefore, the sonographic features include the following: (1) An extremely widened or bifurcated thumb accompanied by additional echo of phalangeals and, in some cases, metacarpals; (2) A redundant echo of soft tissue with or without a bony echo at the postaxial field; and (3) A lack of interphalangeal space and/or fingers that never separate throughout the examination. Polydactyly and/or syndactyly of the lower extremities can sometimes be detected. These features can lead to a preliminary diagnosis of TPT-PS. When suspected by ultrasound, the triphalangeal thumb and polydactyly should be confirmed in the coronal and axial planes. Three-dimensional ultrasonography might be helpful to provide more details for the evaluation. Given that polydactyly is associated with approximately 300 syndromic abnormalities and that the presence of a triphalangeal thumb can be predictive of other combined congenital anomalies, further ultrasound scans are essential for the exclusion of other anomalies[16-18]. However, it is still difficult to diagnose TPT-PS accurately by prenatal ultrasonography only. Hence, additional information is needed to confirm this diagnosis.
Second, because TPT-PS is an autosomal dominant malformation, a detailed survey of the patient’s history will be conducive to a prenatal diagnosis. It should be noted that mild and subclinical limb phenotypes without the presence of a triphalangeal thumb may occur in patients who are presumed to be unaffected. For instance, family members who do not have a triphalangeal thumb might have broadness of the thumb or a double flexion fold in the thumb. These mild anomalies might not cause evident aesthetic or functional problems, so the report will indicate that the individual is as unaffected. Consequently, the width, number of flexion folds, strength, and digital opposition of the thumb and whether the lunula of the thumb is duplicated should be evaluated when investigating the patient’s history[19].
Last, the pathogenic gene of TPT-PS was confirmed to be the LMBR1 gene, which is mapped to chromosome 7q36.3[1]. In our case, all the affected individuals with available samples for WES carried the mutation of the LMBR1 gene, while the unaffected individual did not, confirming that the mutation is cosegregated in this family. Hence, genetic testing is an ideal approach to confirm the ultrasound diagnosis. Several case studies have reported the use of genetic testing after abnormal ultrasound findings[20-22]. Chen and Huang[20] reported a case of Apert syndrome diagnosed by prenatal ultrasound combined with magnetic resonance imaging and WES using a method similar to ours. As a screening tool, ultrasound mainly provides diagnostic information regarding the phenotypes of genetic syndromes. Additional genetic testing is performed for genotypic confirmation of the ultrasound findings. With the advent of the era of precision medicine, ultrasound screening combined with genetic testing may become a trend in the diagnosis of genetic syndromes. Moreover, genetic evaluation is indicated for likely unaffected family members for complete reassurance regarding whether they harbor the disease-related gene. This will contribute to the study of genetics and to the reproduction plans of affected families.
We suggest prenatal ultrasound examination combined with genetic testing to accurately diagnose TPT-PS and help clinicians and patients make decisions.
We sincerely thank the family and all participants for their cooperation in providing the medical data necessary for publication. We also wish to thank the BGI-Guangzhou Medical Laboratory for performing the whole-exome sequencing.
Manuscript source: Unsolicited manuscript
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Watanabe A S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Klopocki E, Ott CE, Benatar N, Ullmann R, Mundlos S, Lehmann K. A microduplication of the long range SHH limb regulator (ZRS) is associated with triphalangeal thumb-polysyndactyly syndrome. J Med Genet. 2008;45:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Hill RE, Heaney SJ, Lettice LA. Sonic hedgehog: restricted expression and limb dysmorphologies. J Anat. 2003;202:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, Oostra BA, Goode D, Elgar G, Hill RE, de Graaff E. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 2003;12:1725-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 878] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 4. | Potuijt JWP, Baas M, Sukenik-Halevy R, Douben H, Nguyen P, Venter DJ, Gallagher R, Swagemakers SM, Hovius SER, van Nieuwenhoven CA, Galjaard RH, van der Spek PJ, Ahituv N, de Klein A. A point mutation in the pre-ZRS disrupts sonic hedgehog expression in the limb bud and results in triphalangeal thumb-polysyndactyly syndrome. Genet Med. 2018;20:1405-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | VanderMeer JE, Afzal M, Alyas S, Haque S, Ahituv N, Malik S. A novel ZRS mutation in a Balochi tribal family with triphalangeal thumb, pre-axial polydactyly, post-axial polydactyly, and syndactyly. Am J Med Genet A. 2012;158A:2031-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Wang ZQ, Tian SH, Shi YZ, Zhou PT, Wang ZY, Shu RZ, Hu L, Kong X. A single C to T transition in intron 5 of LMBR1 gene is associated with triphalangeal thumb-polysyndactyly syndrome in a Chinese family. Biochem Biophys Res Commun. 2007;355:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Sun M, Ma F, Zeng X, Liu Q, Zhao XL, Wu FX, Wu GP, Zhang ZF, Gu B, Zhao YF, Tian SH, Lin B, Kong XY, Zhang XL, Yang W, Lo WH, Zhang X. Triphalangeal thumb-polysyndactyly syndrome and syndactyly type IV are caused by genomic duplications involving the long range, limb-specific SHH enhancer. J Med Genet. 2008;45:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Wieczorek D, Pawlik B, Li Y, Akarsu NA, Caliebe A, May KJ, Schweiger B, Vargas FR, Balci S, Gillessen-Kaesbach G, Wollnik B. A specific mutation in the distant sonic hedgehog (SHH) cis-regulator (ZRS) causes Werner mesomelic syndrome (WMS) while complete ZRS duplications underlie Haas type polysyndactyly and preaxial polydactyly (PPD) with or without triphalangeal thumb. Hum Mutat. 2010;31:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Lohan S, Spielmann M, Doelken SC, Flöttmann R, Muhammad F, Baig SM, Wajid M, Hülsemann W, Habenicht R, Kjaer KW, Patil SJ, Girisha KM, Abarca-Barriga HH, Mundlos S, Klopocki E. Microduplications encompassing the Sonic hedgehog limb enhancer ZRS are associated with Haas-type polysyndactyly and Laurin-Sandrow syndrome. Clin Genet. 2014;86:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Xing XS, Zhu HW, Chen C, Wang SS, Luo Y, Zhang X. Mutation analysis of a Chinese pedigree with triphalangeal thumb-polysyndactyly syndrome. Genet Mol Res. 2014;13:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Liu Z, Yin N, Gong L, Tan Z, Yin B, Yang Y, Luo C. Microduplication of 7q36.3 encompassing the SHH longrange regulator (ZRS) in a patient with triphalangeal thumbpolysyndactyly syndrome and congenital heart disease. Mol Med Rep. 2017;15:793-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Xu J, Wu J, Teng X, Cai L, Yuan H, Chen X, Hu M, Wang X, Jiang N, Chen H. Large duplication in LMBR1 gene in a large Chinese pedigree with triphalangeal thumb polysyndactyly syndrome. Am J Med Genet A. 2020;182:2117-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183:755-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Ficara A, Syngelaki A, Hammami A, Akolekar R, Nicolaides KH. Value of routine ultrasound examination at 35-37 week' gestation in diagnosis of fetal abnormalities. Ultrasound Obstet Gynecol. 2020;55:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | Petrovski S, Aggarwal V, Giordano JL, Stosic M, Wou K, Bier L, Spiegel E, Brennan K, Stong N, Jobanputra V, Ren Z, Zhu X, Mebane C, Nahum O, Wang Q, Kamalakaran S, Malone C, Anyane-Yeboa K, Miller R, Levy B, Goldstein DB, Wapner RJ. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 375] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 16. | Society for Maternal-Fetal Medicine, Rac MWF, McKinney J, Gandhi M. Polydactyly. Am J Obstet Gynecol. 2019;221:B13-B15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Potuijt JWP, Galjaard RH, van der Spek PJ, van Nieuwenhoven CA, Ahituv N, Oberg KC, Hovius SER. A multidisciplinary review of triphalangeal thumb. J Hand Surg Eur Vol. 2019;44:59-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Malik S. Polydactyly: phenotypes, genetics and classification. Clin Genet. 2014;85:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Potuijt JWP, Hoogeboom J, de Graaff E, van Nieuwenhoven CA, Galjaard RJH. Variable expression of subclinical phenotypes instead of reduced penetrance in families with mild triphalangeal thumb phenotypes. J Med Genet. 2020;57:660-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Chen L, Huang FX. Apert syndrome diagnosed by prenatal ultrasound combined with magnetic resonance imaging and whole exome sequencing: A case report. World J Clin Cases. 2021;9:912-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 21. | Yates CL, Monaghan KG, Copenheaver D, Retterer K, Scuffins J, Kucera CR, Friedman B, Richard G, Juusola J. Whole-exome sequencing on deceased fetuses with ultrasound anomalies: expanding our knowledge of genetic disease during fetal development. Genet Med. 2017;19:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 22. | Vora NL, Powell B, Brandt A, Strande N, Hardisty E, Gilmore K, Foreman AKM, Wilhelmsen K, Bizon C, Reilly J, Owen P, Powell CM, Skinner D, Rini C, Lyerly AD, Boggess KA, Weck K, Berg JS, Evans JP. Prenatal exome sequencing in anomalous fetuses: new opportunities and challenges. Genet Med. 2017;19:1207-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |