Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6789
Peer-review started: January 7, 2021
First decision: February 12, 2021
Revised: March 4, 2021
Accepted: June 28, 2021
Article in press: June 28, 2021
Published online: August 16, 2021
Processing time: 210 Days and 11.6 Hours
CYP21A2 gene mutations may all cause reduction or loss of 21-hydroxylase activity, leading to development of congenital adrenal hyperplasia (CAH) with different clinical phenotypes. For families with CAH children, genetic testing of the parents and genetic counseling are recommended to assess the risk of recurrence.
We report a case of CAH with a high suspicion before delivery. The risk of the child suffering from CAH during the pregnancy had been underestimated due to the deviation of genetic counseling and genetic testing results. Our report confirmed a CYP21A2 homozygous deletion in this case, CYP21A2 heterozygous deletion in the mother, and a rare 2+0 CYP21A2 deletion in the father.
It is important to analyze the distribution of CYP21A2 gene in the two alleles of parents of children with CAH.
Core Tip: This study suggests that carriers of type 2+0 are likely to be a trap in genetic testing and genetic counseling.
- Citation: Xi N, Song X, Wang XY, Qin SF, He GN, Sun LL, Chen XM. 2+0 CYP21A2 deletion carrier — a limitation of the genetic testing and counseling: A case report. World J Clin Cases 2021; 9(23): 6789-6797
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6789.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6789
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive genetic diseases caused by adrenocortical hormone synthesis disorders[1]. Enzyme deficiencies involving adrenocortical hormone synthesis disorders include 21-hydroxylase deficiency (21-OHD), 11-β OHD, 17-OHD, 3β-hydroxy steroid dehydrogenase deficiency and 18-OHD, of which 21-OHD is the most common type and is the cause of > 90% of CAH cases[2]. Deficiency of enzymes leads to corticosteroid synthesis disorders, accumulation of precursor substances, increase in androgen bypass metabolites, adrenocorticotropic hormone secretion, and adrenal hypertrophy[3]. Depending on the extent of 21-OHD, it is divided into classic and nonclassical type, in which the former includes salt loss type and simple masculine type[4]. According to previous reports, the incidence of classic CAH is around 1:5000-1:15000 due to ethnic differences[5]. The gene encoding 21-hydroxylase is CYP21A2. Gene mutations including substitutions, deletions and point mutations may all cause the reduction or loss of enzyme activity, leading to the development of CAH with different clinical phenotypes. For families with CAH children, genetic testing of the parents and genetic counseling are recommended to assess the risk of recurrence.
We report a case of CAH with a high suspicion before delivery and confirmed diagnosis after delivery. The risk of the child suffering from CAH during the pregnancy was underestimated due to the deviation of genetic counseling on his brother (who died after birth) and the father’s genetic test results. The genetic test confirmed that the case carried a CYP21A2 homozygous deletion, the mother carried a CYP21A2 heterozygous deletion, and the father carried a rare 2+0 CYP21A2 deletion.
A 30-year-old pregnant woman came to our prenatal diagnosis center for consultation at 26 wk of pregnancy. During this pregnancy, the mother only accepted routine obstetric examination procedures. She came to our center for consultation because she had concerns and hoped to receive a detailed prenatal diagnosis through color Doppler ultrasound examination.
The patient had a free present medical history.
The patient was pregnant for the second time and gave birth to the first child, a boy, in the hospital 19 mo ago. No special circumstances occurred during delivery. More than 10 d later, the newborn was referred to a superior hospital because of severe choking on milk, crying, and restlessness. Further examination revealed that the boy had electrolyte disturbances (hyperkalemia and hyponatremia), multiple organ damage (renal insufficiency, myocardial damage and brain damage), metabolic acidosis, moderate dehydration, and undescended bilateral testicles. Diagnosis of CAH, newborn pneumonia or neonatal sepsis was then considered. Unfortunately, the child died after rescue failed.
The CYP21A2 gene of the patient’s first dead child and his parents was detected by multiplex ligation-dependent probe amplification (MLPA), polymerase chain reaction (PCR) and Sanger sequencing and found a heterozygous deletion of CYP21A2 gene exons 1-7 that was inherited from the mother. At the same time, there were multiple pathogenic point mutations in the CYP21A2 gene and these mutations did not come from the father. Based on the results of this genetic testing, the pregnant woman was told that these point mutations in the child were new. Although the mother is a carrier of CYP21A2 gene deletion, the father is not. Thus, the probability of having CAH offspring is very low.
No physical examination was done at the center in this study.
Because of the abnormal findings on color Doppler ultrasound examination, combined with the medical history of pregnant women who have had a child with CAH, we recommended amniocentesis for gender diagnosis and CYP21A2 genetic testing. After consultation with the pregnant woman and her family, the proposal for amniocentesis was rejected.
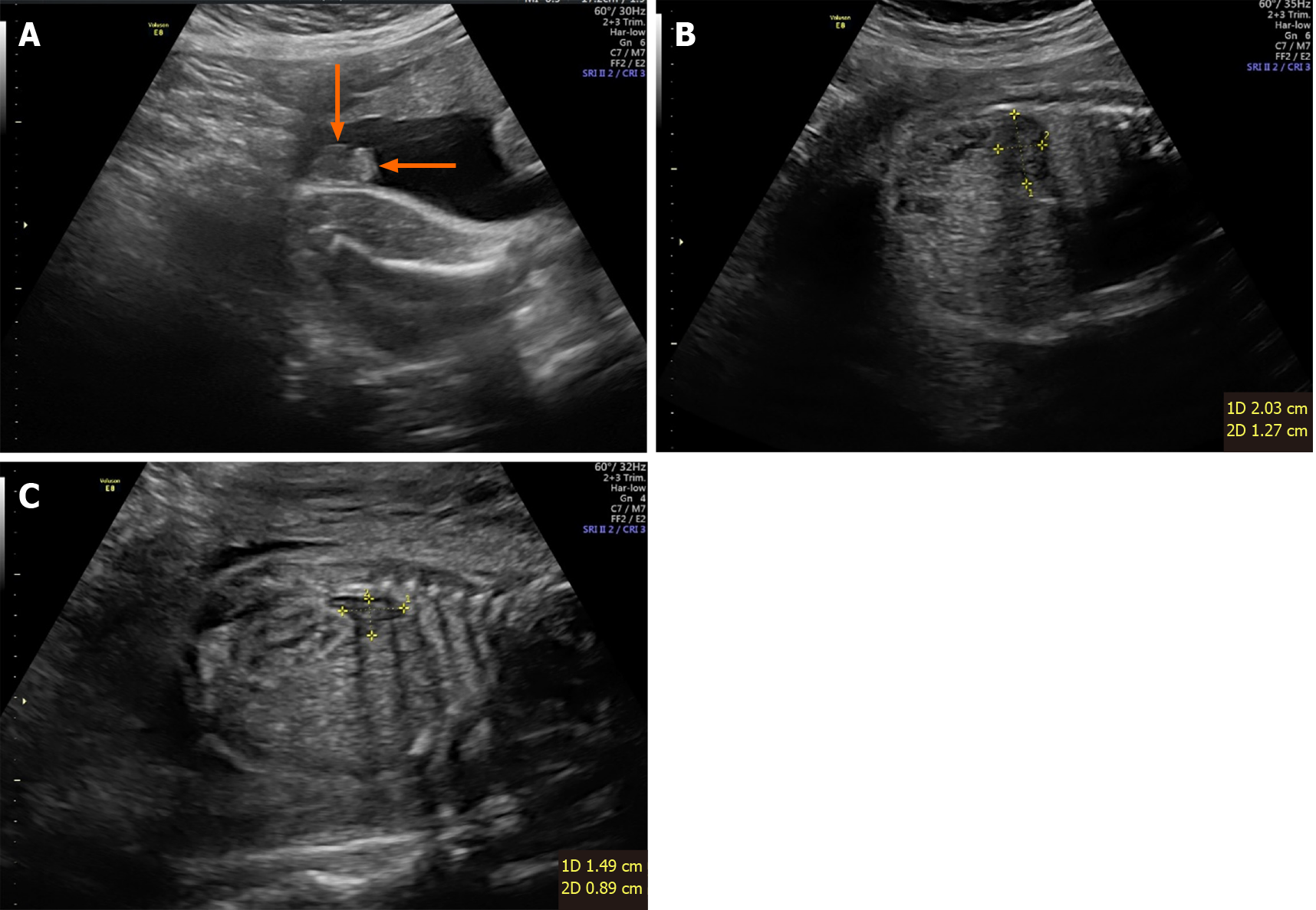
At 27+5 wk of pregnancy, the woman underwent color Doppler ultrasound exa
Subsequent diagnosis of the pregnant woman was done at other institutions and not provided to this study.
Subsequent treatment of the pregnant woman was done at other institutions and not provided to this study.
After 5 wk, the pregnant woman gave birth to a newborn with abnormal genitalia, difficult to distinguish sex, increased body hair, and thick hair on the head and face. The newborn died after rescue failure due to electrolyte disturbance and multiple organ damage. Fluorescence in situ hybridization showed that the gender of the newborn was female without aneuploidy detected by the probe.
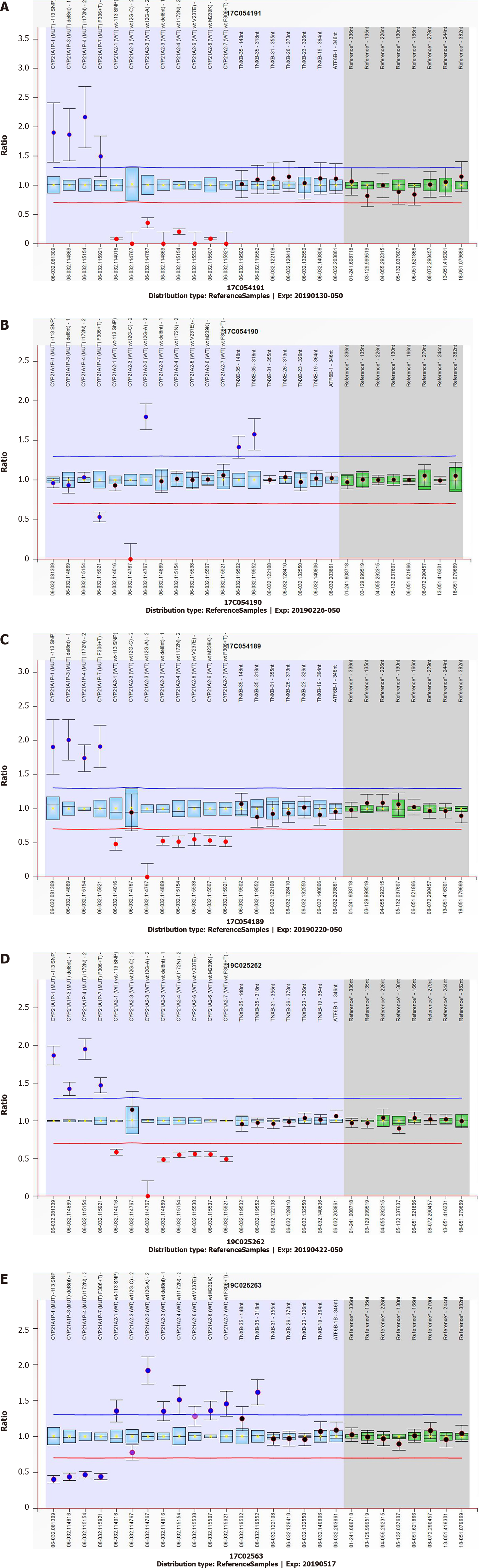
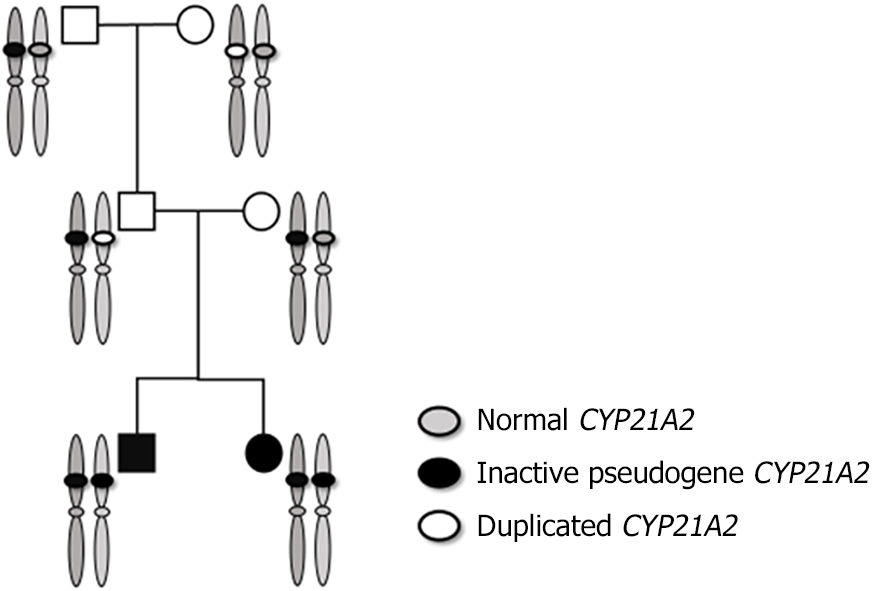
Having given birth to two CAH children, the parents required more detailed genetic testing. The CYP21A2 gene of the neonate, parents and grandparents was tested using the SALSA MLPA P050 kit from MBC-Holland of the Netherlands (Figure 2) and showed a homozygous deletion of the CYP21A2 gene in the newborn and a heterozygous deletion in the mother. The deletion and pathogenic point mutation of the CYP21A2 gene were not found in the father. However, the heterozygous deletion of the CYP21A2 gene was found in the grandfather and CYP21A2 gene duplication in the grandmother.
Deoxyribonucleic acid (DNA) high-throughput sequencing technology, Gerber’s self-developed detection method and PGXCloud system 2.7.5, PGD analysis software 2.0 were used to identify the single nucleotide polymorphism site in the family CYP21A2 gene (NM_000500.7 chr6: 32006093-32009447 forward transcription) and adjacent regions and found that the two alleles of the child were from the father and mother, and the two alleles of the father were from the grandfather and grandmother of the child.
The highly homologous pseudogene CYP21A1P is closely linked to CYP21A2. The gene CYP21A2, pseudogene CYP21A1P, three other genes RP1, C4 and TNXB, and two pseudogenes RP2 and TNXA together form a genetic unit called the RCCX module[6,7]. Both CYP21A2 functional genes and pseudogenes contain 10 exons, and the nucleotide sequence homology between exons is up to 98%. The nucleotide sequence homology between introns is as high as 96%[6,8]. Although pseudogenes do not encode functional proteins, their high sequence homology with functional genes brings major challenges to genetic testing. The MLPA and gene sequencing results of the children of this family showed a homozygous deletion of the CYP21A2 gene, and the reason for the deletion is presumed to be pseudogene recombination. The fetal and maternal MLPA test results confirmed that she was a carrier of a CYP21A2 single copy deletion, which was also speculated to be pseudogene recombination. The father’s test results showed no copy number deletion or pathogenic point mutation of the CYP21A2 gene. Based on this family, two consecutive CAH children with similar phenotypes were born, which is inconsistent with the genetic principle. We speculate the following four possibilities: (1) new mutation; (2) paternal gonad mosaicism; (3) patient was maternal uniparental disomy (UPD); and (4) father had a CYP21A2 gene duplication in one haplotype, and the other haplotype CYP21A2 gene was recombined by pseudogenes, which was 2+0 type.
For speculation on the pathogenicity of new mutations, the genetic test results of the first child in this family found a heterozygous deletion of a large fragment of CYP21A2 and multiple pathogenic point mutations. Although the technical details and original documents of the current genetic testing are no longer available, we speculate that it may be that the amplification product was interfered with by pseudogenes due to primer design or the control of the experimental system. The subsequent sequencing results revealed the multiple pathogenic point mutations, which were not new mutations of the functional gene, but a false-positive test result. Large-scale sequencing found that the average new mutation rate of the human genome is 1.20 × 10-8 per generation, and 1 or 2 are generally present in the coding region[9]. Therefore, the probability of multiple new point mutations on a single gene is extremely low. Approximately 1% of the pathogenic variants of CYP21A2 come from new mutations[10]. As a result, the possibility that CYP21 mutations in both the child and the elder brother come from de novo mutations is even below (1.20 × 10-8)2. Considering that the de novo mutations in exon regions are rarer and low-frequency pathogenic CYP21 mutations come from de novo mutations, this possibility is even much lower and can be basically ruled out.
Also, due to the low incidence of UPD, the likelihood of maternal UPD in 2 consecutive children is low. Because we failed to collect the father’s sperm samples, we cannot completely rule out the assumption of a high proportion of chimeras in the father’s gonads. However, based on the rule of embryonic development and previous studies on chimerism in monogenic diseases, there is a low probability of a high proportion of chimerism in a single organ, and chimeric signals are generally found in organs with multiple tissue sources[11]. From our blood-based MLPA and haplotype sequencing results, we did not see a clear chimeric signal and therefore we speculate that this possibility is also low.
One hypothesis we are most interested in is that the father is a 2+0 type carrier. There are reports of CYP21A2 duplication haplotypes, and the incidence of this haplotype is sometimes equal to or even higher than the population carrying rate of the classic pathogenic mutation of the CYP21A2 gene[12-14], suggesting the possibility of 2+0 pathogenicity. According to a previously published method for SMN1 gene 2+0 carriers[15], we conducted MLPA testing on the grandparents of the children and found that the grandfather was a carrier of a single deletion due to pseudogene recombination. The grandmother of the child had 3 copies of the CYP21A2 gene. To further understand the status of chromatids in pedigree inheritance and exclude the possibility of maternal UPD, we did haplotype analysis of pedigree members. The results confirmed 2 CYP21A2 deleted alleles; 1 from the mother and 1 from the father. The 2 alleles of the child’s father also came from the child’s grandfather and grandmother. Comprehensive testing found that that the grandmother was type 2+1, the grandfather was type 1+0, and the father inherited a haplotype from the grandmother’s CYP21A2 duplication and the grandfather’s haplotype with CYP21A2 gene copy number deletion to form the 2+0 type.
The 2 alleles of the brother and the child were inherited from the parents and were recombined by the pseudogene and the CYP21A2 gene deletion type allele. This confirms our hypothesis about the genetic test results of the brothers: false positives caused by the pseudogenes interference with sequencing results (Figure 3).
Genetic counseling for the family is important and necessary to help the family cope with this hard time and deciding whether or not to get pregnant in the future. Thus, we organized a group discussion with the family to let the guardian of the child know about this situation. To keep them fully informed, the group included a representative from the laboratory that performed the genetic testing, a genetic counselor, and the primary care provider. We also suggested a group discussion with the family in future research if a similar situation occurs.
To our knowledge, this is the first report of a 2+0 silent CYP21A2 deletion carrier. Previous studies have found a CYP21A2 duplication haplotype combined with another CYP21A2 deleted haplotype in patients with CAH salt loss. In the CYP21A2 duplication haplotypes, both copies of CYP21A2 carry severe pathogenic variants[13,14]. The haplotype frequency of CYP21A2 duplication is 1.6%–3.5%. Most of the haplotypes of CYP21A2 duplication have one CYP21A2 gene carrying the Q318X mutation. No 2+0 carriers were found in non-CAH populations[13,14,16,17].
This report implies that current quantitative copy number variation (CNV) detection methods such as PCR and MLPA have some limitations to detect silent carriers of pathological genetic disorders, such as gene duplication paired with gene loss on the opposite chromosome or allele (2+0) in this report. MLPA has been regarded as the gold standard for CNV determination. Furthermore, laboratories worldwide commonly rely on MLPA for the diagnosis and research of genetic disorders[18]. Nevertheless, MLPA is limited to analyzing the distribution in the two alleles and has a risk of missed detection of 2/0 carriers. For example, Alías et al[15] tested 1562 individuals to determine their SMA carrier status using MLPA, while the exclusive use of such a quantitative detection method of only independent individuals in a given family led to failure in identifying carrier status. In their study, all blood relatives characterized as 2/0 carriers were identified by studying their respective parents, but not by MLPA.
The situation is similar for the PCR test method. The protocol is based on long-range PCR amplification with allele-specific primers, followed by DNA sequencing. PCR together with the Sanger sequencing is a robust testing strategy aiming to determine whether a point mutation or indel exists[19]. However, pathological genetic disorders, such as gene duplication paired with gene loss on the opposite chromosome or allele (2+0) may be missed using PCR sequencing testing. In addition, CYP21A2 has a duplicated pseudogene called CYP21A1P and they share 98% and 96% sequence homology in exons and in noncoding regions. All the reasons above make the definition of the 2+0 carrier in this report more complicated.
This study suggests that carriers of type 2+0 are likely to be a limitation of genetic testing and counseling. The father of the child was interpreted as a non-CAH carrier in a previous genetic testing report, which caused the risk of recurrent CAH to be erroneously underestimated by genetic counseling. This pregnancy did not have opportunities for earlier corresponding genetic testing for the fetus or intrauterine intervention. These results reveal that there are some limitations of the current testing strategy to detect silent carriers of pathological genetic disorders, such as gene duplication paired with gene loss on the opposite chromosome or allele (2+0). Thus, only detecting the CNV in the CYP21A2 gene with current detection methods is not sufficient. For couples who have had children with CAH, in addition to routine detection of the copy number of the CYP21A2 gene and pathogenic point mutations, it is also important to analyze the distribution of the CYP21A2 gene in the two alleles.
Manuscript source: Unsolicited manuscript
Specialty type: Geriatrics and gerontology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jelski W, Wang R S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li X
| 1. | Krone N, Arlt W. Genetics of congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23:181-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349:776-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 541] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 3. | Wedell A. An update on the molecular genetics of congenital adrenal hyperplasia: diagnostic and therapeutic aspects. J Pediatr Endocrinol Metab. 1998;11:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21:245-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 266] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Thil'en A, Nordenström A, Hagenfeldt L, von Döbeln U, Guthenberg C, Larsson A. Benefits of neonatal screening for congenital adrenal hyperplasia (21-hydroxylase deficiency) in Sweden. Pediatrics. 1998;101:E11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Higashi Y, Yoshioka H, Yamane M, Gotoh O, Fujii-Kuriyama Y. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene. Proc Natl Acad Sci USA. 1986;83:2841-2845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 357] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Yang Z, Mendoza AR, Welch TR, Zipf WB, Yu CY. Modular variations of the human major histocompatibility complex class III genes for serine/threonine kinase RP, complement component C4, steroid 21-hydroxylase CYP21, and tenascin TNX (the RCCX module). A mechanism for gene deletions and disease associations. J Biol Chem. 1999;274:12147-12156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | White PC, New MI, Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci USA. 1986;83:5111-5115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 354] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, Gudjonsson SA, Sigurdsson A, Jonasdottir A, Wong WS, Sigurdsson G, Walters GB, Steinberg S, Helgason H, Thorleifsson G, Gudbjartsson DF, Helgason A, Magnusson OT, Thorsteinsdottir U, Stefansson K. Rate of de novo mutations and the importance of father's age to disease risk. Nature. 2012;488:471-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1456] [Cited by in RCA: 1432] [Article Influence: 110.2] [Reference Citation Analysis (0)] |
| 10. | Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. Predicting phenotype in steroid 21-hydroxylase deficiency? J Clin Endocrinol Metab. 2000;85:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 205] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Yang X, Liu A, Xu X, Yang X, Zeng Q, Ye AY, Yu Z, Wang S, Huang AY, Wu X, Wu Q, Wei L, Zhang Y. Genomic mosaicism in paternal sperm and multiple parental tissues in a Dravet syndrome cohort. Sci Rep. 2017;7:15677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Bachega TA, Billerbeck AE, Madureira G, Arnhold IJ, Medeiros MA, Marcondes JA, Longui CA, Nicolau W, Bloise W, Mendonca BB. Low frequency of CYP2B deletions in Brazilian patients with congenital adrenal hyperplasia due to 21-hydroxylas deficiency. Hum Hered. 1999;49:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Koppens PF, Hoogenboezem T, Degenhart HJ. Duplication of the CYP21A2 gene complicates mutation analysis of steroid 21-hydroxylase deficiency: characteristics of three unusual haplotypes. Hum Genet. 2002;111:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Koppens PF, Hoogenboezem T, Halley DJ, Barendse CA, Oostenbrink AJ, Degenhart HJ. Family studies of the steroid 21-hydroxylase and complement C4 genes define 11 haplotypes in classical congenital adrenal hyperplasia in The Netherlands. Eur J Pediatr. 1992;151:885-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Alías L, Barceló MJ, Bernal S, Martínez-Hernández R, Also-Rallo E, Vázquez C, Santana A, Millán JM, Baiget M, Tizzano EF. Improving detection and genetic counseling in carriers of spinal muscular atrophy with two copies of the SMN1 gene. Clin Genet. 2014;85:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Parajes S, Quinteiro C, Domínguez F, Loidi L. High frequency of copy number variations and sequence variants at CYP21A2 locus: implication for the genetic diagnosis of 21-hydroxylase deficiency. PLoS One. 2008;3:e2138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Witchel SF. Congenital Adrenal Hyperplasia. J Pediatr Adolesc Gynecol. 2017;30:520-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Bashton M, Hollis R, Ryan S, Schwab CJ, Moppett J, Harrison CJ, Moorman AV, Enshaei A. Concordance of copy number abnormality detection using SNP arrays and Multiplex Ligation-dependent Probe Amplification (MLPA) in acute lymphoblastic leukaemia. Sci Rep. 2020;10:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Maremonti E, Brede DA, Olsen AK, Eide DM, Berg ES. Ionizing radiation, genotoxic stress, and mitochondrial DNA copy-number variation in Caenorhabditis elegans: droplet digital PCR analysis. Mutat Res. 2020;858-860:503277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |