Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6775
Peer-review started: March 5, 2021
First decision: April 29, 2021
Revised: May 9, 2021
Accepted: May 19, 2021
Article in press: May 19, 2021
Published online: August 16, 2021
Processing time: 153 Days and 5.6 Hours
Although metastatic adenocarcinoma of the ileum is not uncommon, solitary metastasis to the seminal vesicle has not been reported. We report a patient with recurrent hematospermia diagnosed with metastasis to the seminal vesicle following ileal adenocarcinoma resection, his subsequent management and outcome.
A 46-year-old man presented with recurrent episodes of painless hematospermia. This was not associated with any lower urinary tract symptoms. He had a past medical history of ileal tumor at the terminal ileum with solitary mesenteric lymph node metastasis on presentation, and underwent partial ileectomy and lymphadenectomy 4 years ago. Subsequent investigations included positron-emission tomography and computed tomography imaging confirmed the very unusual diagnosis of a solitary tumor at the left seminal vesicle. Laparoscopic left-sided vesiculectomy was carried out. Histological analysis with immunohistochemistry showed that CDX-2 was positive and CK7 was negative, and the appearance was consistent with the diagnosis of recurrent metastatic adenocarcinoma of his previously treated intestine primary. The patient had an uneventful post-operative recovery. He received adjuvant chemoradiotherapy following surgery. He remained asymptomatic until he developed multiple bone and pulmonary metastases one year after surgery.
Clinicians should be aware of hematospermia as the first symptom of metastatic recurrence in patients with a history of ileal adenocarcinoma.
Core Tip: A 46-year-old man with a history of a partial ileectomy and adjuvant chemotherapy for ileal adenocarcinoma 4 years prior to presentation at our clinic with hematospermia. Subsequent investigations confirmed the very unusual diagnosis of a solitary tumor at the left seminal vesicle. Laparoscopic left-sided vesiculectomy was carried out. Histological analysis with immunohistochemistry showed that CDX-2 was positive and CK7 was negative, and the appearance was consistent with the diagnosis of recurrent metastatic adenocarcinoma of his previously treated intestine primary. He received adjuvant chemoradiotherapy following surgery. He remained asymptomatic until he developed multiple bone and pulmonary metastasis one year after surgery.
- Citation: Cheng XB, Lu ZQ, Lam W, Yiu MK, Li JS. Solitary seminal vesicle metastasis from ileal adenocarcinoma presenting with hematospermia: A case report. World J Clin Cases 2021; 9(23): 6775-6780
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6775.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6775
Small bowel adenocarcinoma (SBA) is rare, with an incidence of malignant SBA less than 1 per 100000[1]. Owing to its nonspecific clinical presentation and the lack of effective investigative tools for exploring the small bowel, SBA is usually diagnosed at an advanced stage. At diagnosis, 35% to 40% of patients have distant metastases[2,3]. The most common sites of metastases are liver, lung, and bone[4]. To our knowledge, solitary seminal vesicle (SV) metastasis from SBA has not been reported in the literature.
A 46-year-old man presented to our urology outpatient clinic with recurrent episodes of painless hematospermia.
Hematospermia was not associated with any lower urinary tract symptoms. He had initially been treated with several courses of oral antibiotics for almost three months but did not improve. The patient denied prior trauma or genitourinary tract infection.
He had a past medical history of ileal tumor at the terminal ileum with solitary mesenteric lymph node metastasis on presentation, and underwent partial ileectomy and lymphadenectomy 4 years ago. This was followed by adjuvant capecitabine chemotherapy and intraperitoneal infusion chemotherapy with 5-fluorouracil. The patient had an uneventful recovery and had been in remission since. His tumor markers including carcinoembryonic antigen (CEA), alpha-fetoprotein, human chorionic gonadotropin, and lactate dehydrogenase nadir were within the normal range following treatment.
This patient had no special personal and family history.
Physical examination of his external genitalia was unremarkable. Testicular and epididymal examinations were normal and his vasa deferens was smooth and not thickened. Digital rectal examination revealed a smooth, small, benign feeling prostate.
Urinalysis and urine culture did not identify evidence of urinary tract infection or hematuria. Semen culture, urethral swabs, mycobacterial cultures and viral serology were all negative. His prostate specific antigen level was not elevated, but interestingly his CEA level was found to be newly elevated to 21 ng/mL.
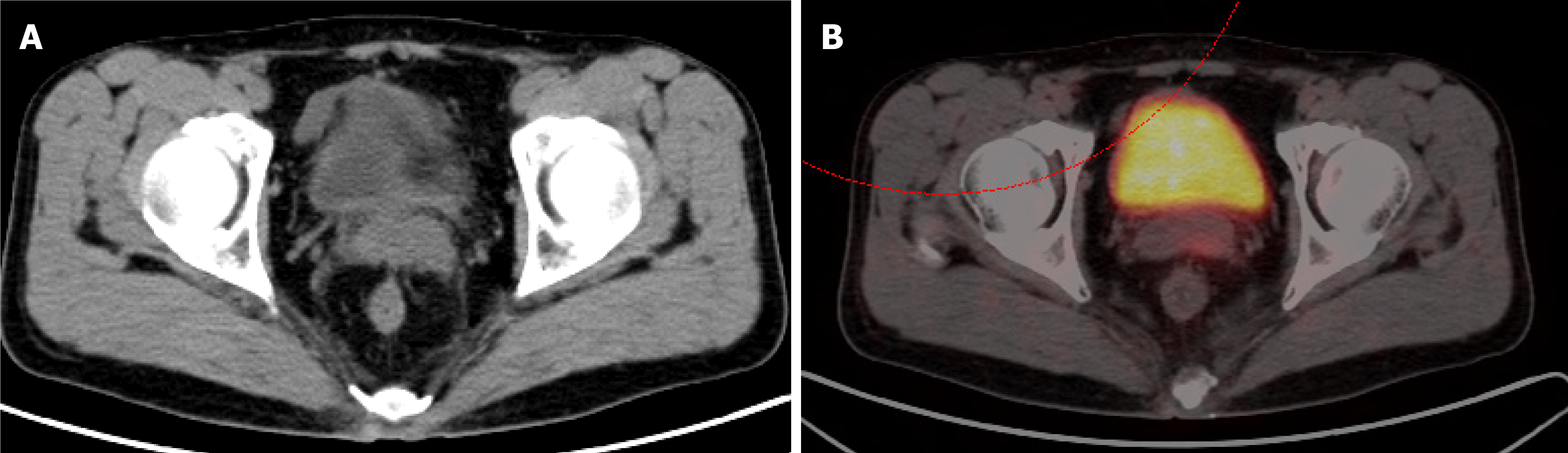
The patient subsequently underwent positron-emission tomography (PET)/computed tomography (CT) imaging. This identified a solitary 2.1 cm × 1.6 cm lesion in the left SV with a standard uptake value of 5.8, the appearance of which was consistent with metastatic recurrence (Figure 1). There was no evidence of tumor recurrence at the original surgical site, or other sites of distal metastasis.
Considering the patient’s history, laboratory examinations and imaging examinations, the doctors diagnosed the mass in the SV as a metastatic focus.
After carefully counselling the patient, he underwent a laparoscopic resection of the single left SV recurrence. Under general anesthesia, a prophylactic left-sided ureteral stent was inserted due to the tumor’s anticipated proximity to the ureter. This was followed by an uneventful laparoscopic transperitoneal complete excision of the left SV. Intraoperative frozen sections showed negative tumor margins.
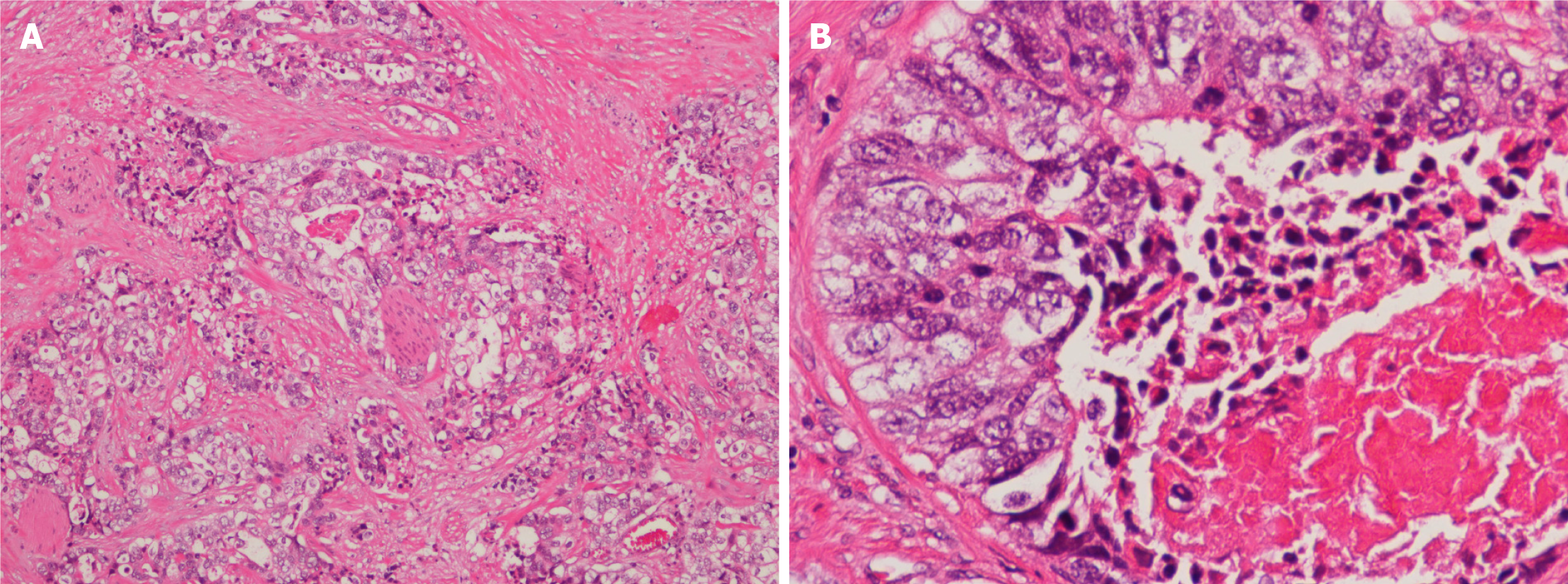
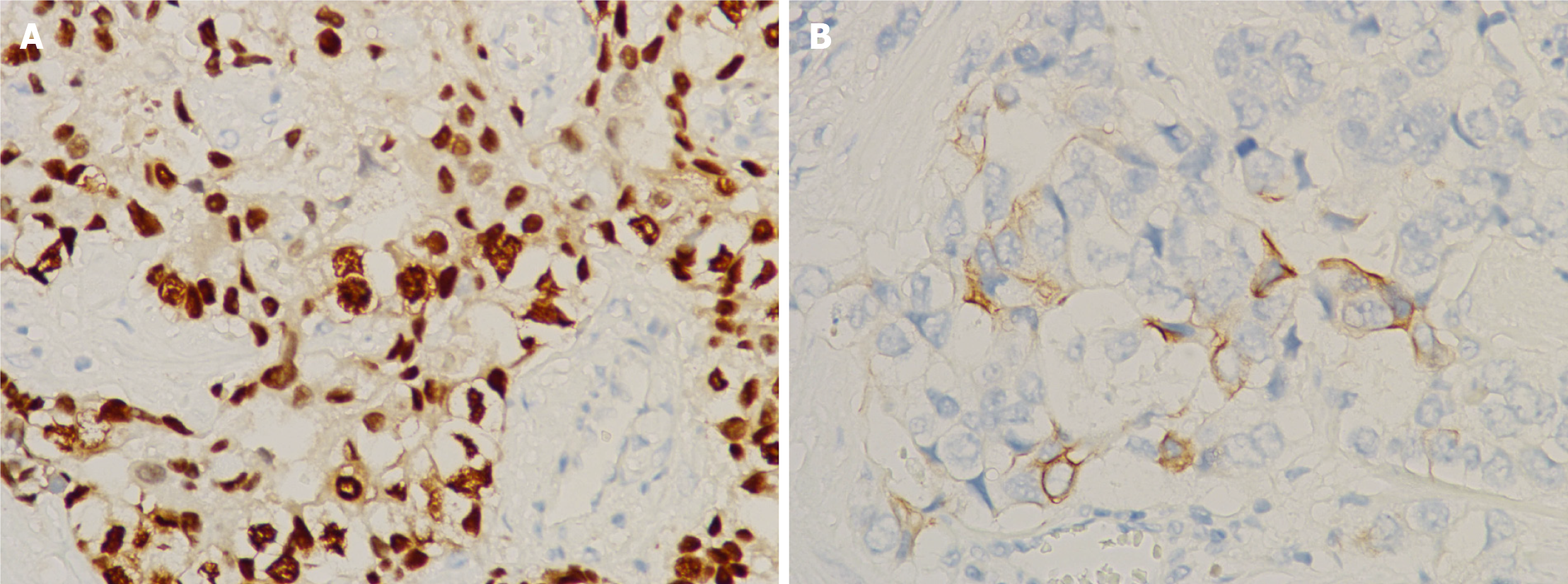
The patient had an uneventful post-operative recovery, and was discharged home on day 2 post-surgery. Pathology of the excised tumor showed solid nests and tubular patterns (Figure 2). Immunohistochemical examination demonstrated that the tumor cells were positive for CDX-2 and CK20 (Figure 3), but negative for CK7. These findings confirmed the pathological diagnosis of metastatic adenocarcinoma recurrence originating from his SBA. The patient subsequently received adjuvant radiochemotherapy (intensity-modulated radiotherapy combined with capecitabine).
The patient’s hematospermia resolved and he remained asymptomatic following completion of his treatment. Unfortunately, his CEA level, having initially dropped to 6.5 ng/mL following surgery, was found to be elevated at 43.5 ng/mL one year after treatment. A repeat PET/CT scan showed multiple lung and bone metastases. He later also developed visible hematuria and was confirmed to have metastatic recurrence of the disease in the trigone area of his bladder following a cystoscopy and bladder biopsy. The patient is alive with multiple metastases and is now receiving palliative chemotherapy with cetuximab.
Primary adenocarcinoma of the SVs is rare and approximately only 60 cases have been reported in the literature[5]. Seminal vesicle metastasis from other organs is even rarer. It has been reported only in renal cell carcinoma, hepatocellular carcinoma, and colon adenocarcinoma[6-8], and were all case reports.
Hematospermia is generally regarded as a benign condition, with inflammatory causes and infection being the commonest underlying etiology. However, hematospermia has previously been reported as a rare first sign of underlying systemic or urological malignancy[9]. Our case highlights the importance of thorough history taking in patients presenting with hematospermia, and personalized investigations should be arranged accordingly. The determination of CEA level should be considered part of the investigation of hematospermia in patients with a history of colorectal or SBA malignancy. To our knowledge, this is the first case of solitary SV metastasis from a SBA primary presenting as hematospermia.
In this case, elevated CEA prompted subsequent PET/CT imaging, which identified a solitary mass in the left SV. The role of percutaneous or transrectal biopsy of the SV mass can be considered to confirm the diagnosis. However, following careful counselling of the patient, a ‘laparoscopic resection’ approach was agreed for both diagnostic and curative purposes, due to the highly suspicious clinical, biochemical and radiological findings.
Surgical access to the SV tumor can be potentially challenging. Kavoussi et al[10] previously described the principles of the laparoscopic transperitoneal approach to the SV, with the benefits of providing a clear vision when operating deep in the pelvic region, minimal blood loss, reduced post-operative pain and shortened length of stay post-surgery when compared with the open approach. Our case confirmed the safety and feasibility of laparoscopic surgery in treating the SV tumor. Technically, it is also crucial to be aware that the ipsilateral ureter is often in close proximity to the SV tumor. The prophylactic insertion of an ipsilateral ureteric JJ stent, such as in our case, could potentially help in identifying the ureter intra-operatively and avoid ureteric injury.
A previous retrospective study reported that distant recurrence of SBA following treatment of the primary tumor was common, and accounted for 86% of patients with disease recurrence on follow-up[11]. The optimal treatment strategy for patients with SBA has yet to been determined, including the role of adjuvant chemotherapy, radiotherapy and targeted therapy. Radical surgical resection remains the mainstay of treatment in patients with SBA, which has been reported to potentially improve prognosis in patients including in those with distant metastasis or recurrence[11]. As such, our patient underwent a laparoscopic seminal vesiculectomy. However, the reported prognosis of metastatic SBA is poor, with a median overall survival of 19 mo, and the five-year survival rate in patients with metastatic disease is reported to be as low as 3%-4%[12].
In conclusion, we report a rare case of SV metastasis from a SBA primary presenting with hematospermia. Although most patients with hematospermia have a benign underlying etiology, it is essential to undertake a thorough history assessment and physical examination, and arrange for personalized investigations accordingly, especially in patients with a history of malignancy. Laparoscopic seminal vesicu
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bains L, Mehdipour P S-Editor: Gong ZM L-Editor: Webster JR P-Editor: Wang LYT
| 1. | Reynolds I, Healy P, Mcnamara DA. Malignant tumours of the small intestine. Surgeon. 2014;12:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 349] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Talamonti MS, Goetz LH, Rao S, Joehl RJ. Primary cancers of the small bowel: analysis of prognostic factors and results of surgical management. Arch Surg. 2002;137:564-70; discussion 570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 148] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Rompteaux P, Gagnière J, Gornet JM, Coriat R, Baumgaertner I, Lecomte T, Afchain P, Zaanan A, Pocard M, Bachet JB, Bonichon-Lamichhane N, Bouché O, Faucheron JL, Forestier J, Lecaille C, Manfredi S, Tougeron D, Terrebonne E, Chehimi M, Villing AL, Sarda C, Legoux JL, Benamouzig R, Aparicio T. Resection of small bowel adenocarcinoma metastases: Results of the ARCAD-NADEGE cohort study. Eur J Surg Oncol. 2019;45:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Sollini M, Silvotti M, Casali M, Giovanardi F, Zadro A, Froio A, Erba PA, Versari A. The role of imaging in the diagnosis of recurrence of primary seminal vesicle adenocarcinoma. World J Mens Health. 2014;32:61-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Matsuzaki K, Yasunaga Y, Fukuda S, Oka T. Seminal vesicle metastasis of renal cell carcinoma. Urology. 2009;74:1017-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Gong L, Zheng M, Li Y, Zhang W, Bu W, Shi L, Yan H. Seminal vesicle metastasis after partial hepatectomy for hepatocellular carcinoma. BMC Cancer. 2011;11:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Hsu YL, Lin IC, Tung CL. 18F-FDG PET/CT of Seminal Vesicle Metastasis From Ascending Colon Adenocarcinoma. Clin Nucl Med. 2017;42:138-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Meng MV, Werboff LH. Hematospermia as the presenting symptom of metastatic malignant melanoma of unknown primary origin. Urology. 2000;56:330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Kavoussi LR, Schuessler WW, Vancaillie TG, Clayman RV. Laparoscopic approach to the seminal vesicles. J Urol. 1993;150:417-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Nakanoko T, Koga T, Taketani K, Hirayama Y, Yoshiya S, Minagawa R, Kai M, Kajiyama K, Maehara Y. Characteristics and Treatment Strategies for Small Bowel Adenocarcinoma in Advanced-stage Cases. Anticancer Res. 2015;35:4135-4138. [PubMed] |
| 12. | Howe JR, Karnell LH, Menck HR, Scott-Conner C. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985-1995. Cancer. 1999;86:2693-2706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |