Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6747
Peer-review started: January 29, 2021
First decision: April 5, 2021
Revised: April 27, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: August 16, 2021
Processing time: 188 Days and 10.3 Hours
Postpancreatectomy hemorrhage (PPH) is the most severe type of complication after pancreatic surgery, although the effect of antithrombotic therapy (ATT) on PPH is largely unknown. The safety and efficacy of chemical thromboprophylaxis for venous thromboembolism (VTE) remains controversial.
To elucidate the effect of ATT on PPH.
Published articles between 2013 and 2020 were searched from PubMed and Google Scholar, and after careful reviewing of all studies, studies concerning ATT and pancreatic surgery were included. Data such as study design, type of surgical procedures, type of antithrombotic drugs, and surgical outcome were extracted from the studies.
Nineteen published articles with a total of 37863 patients who underwent pancreatic surgery were included in the systematic review. Fourteen were cohort studies, with only three being prospective in nature. Two studies demonstrated that in patients receiving chronic ATT, which were mostly managed by heparin bridging, the risk of PPH was higher compared with those without ATT, and one study showed that patients with direct-acting oral anticoagulants managed by heparin bridging had significantly higher postoperative bleeding rates than others. The remaining six studies reported that pancreatic surgery can be safely performed in patients receiving chronic ATT, even under preoperative aspirin continuation. Concerning chemical thromboprophylaxis for VTE, most studies have shown a potentially high risk of PPH in patients undergoing chemical thromboprophylaxis; however, its effectiveness against VTE has not been statistically demonstrated, particularly among Asian patients.
Pancreatic surgery in chronically ATT-received patients can be safely performed without an increase in the occurrence of PPH, although the safety and efficacy of chemical thromboprophylaxis for VTE during pancreatic surgery is still controversial. Further investigation using reliable studies with good design is required to establish definite protocols or guidelines.
Core Tip: A total of 19 published articles on antithrombotic therapy and pancreatic surgery have been reviewed systematically. The articles showed that the risk of perioperative thromboembolic and/or bleeding complications in patients with heparin bridging or continued antiplatelets was not significantly higher than in patients with no antithrombotic or interrupted antiplatelets, although medical thromboprophylaxis for venous thromboembolism remains controversial when performing pancreatectomy for malignancy.
- Citation: Fujikawa T, Naito S. Safety of pancreatic surgery with special reference to antithrombotic therapy: A systematic review of the literature. World J Clin Cases 2021; 9(23): 6747-6758
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6747.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6747
Heart disease, cerebrovascular disease, and cancer are the three leading causes of death in the world. With the aging of society in recent years, more patients with cardiovascular and/or cerebrovascular disease can have opportunities to require non-cardiac surgery. Most patients receive antithrombotic therapy (ATT) to prevent thromboembolism. The perioperative period of these patients is at high risk of both bleeding and thromboembolism, which is very troublesome for surgeons[1,2].
ATT is classified into two types of drugs: antiplatelet drugs and anticoagulants. Antiplatelet drugs are frequently used for prevention of cerebrovascular or cardiovascular diseases, and can prevent thromboembolism by reduction of platelet aggregation. Antiplatelet agents include thienopyridine (e.g., clopidogrel, prasugrel, or ticlopidine), aspirin, and type III phosphodiesterase inhibitor (e.g., cilostazol)[3,4]. Anticoagulants, on the other hand, prevent coagulation of blood by suppressing the coagulation cascade. They are usually used for deep vein thrombosis, atrial fibrillation, and acute coronary syndrome, and cardiac endoprostheses. Anticoagulants are also used for perioperative thromboprophylaxis of venous thromboembolism (VTE). Oral anticoagulants include warfarin, factor Xa inhibitors (e.g., rivaroxaban, apixaban, edoxaban), and direct thrombin inhibitors (e.g., dabigatran)[4,5]. The latter two types are called direct-acting oral anticoagulants (DOACs) or non-vitamin K antagonist oral anticoagulants (NOACs), and now increasingly used. Table 1 summarizes the type and the duration of action of each antithrombotic agent.
| Class of agents | Type | Specific agents | Duration of action |
| Antiplatelets | |||
| Thienopyridines | Clopidogrel (Plavix), Ticlopidine (Panardine), Prasugrel (Effient), Ticagrelor (Brilinta) | 5-7 d1 | |
| Type III PDE inhibitor | Cilostazol (Pretal) | 2 d | |
| Acetylsalicylic acid | Aspirin | 7-10 d | |
| Other NSAIDs | Ibuprofen (Brufen, Advil), Loxoprofen (Loxonin), Diclofenac (Voltaren) etc. | Varies | |
| Anticoagulants | |||
| Heparin (unfractionated) | Heparin | 1-2 h | |
| Heparin (LMWH) | Dalteparin (Fragmin, iv), Enoxaparin (Clexane, s.c.), Nadroparin (s.c.) | 2-4 h, 6-12 h, 6-12 h | |
| Vitamin K antagonist | Warfarin (Coumadin) | 5 d | |
| Factor Xa inhibitor (s.c.) | Fondaparinux (Arixtra) | 1-1.5 d | |
| DOACs | |||
| Direct thrombin inhibitor | Dabigatran (Pradaxa) | 1-2 d | |
| Factor Xa inhibitors | Rivaroxaban (Xarelto), Apixaban (Eliquis), Edoxaban (Lixiana) | 1-2 d |
Pancreatic surgery for malignancy is one of the most invasive abdominal surgical procedures and may expose patients to high risks of severe postoperative complications. Although post-pancreatectomy mortality has dropped significantly to less than 5% owing to advances in perioperative management and surgical procedures[6,7], postoperative complication rates remain high at 18%-50%[6-9]. Common types of post-pancreatectomy complications are post-pancreatectomy hemorrhage (PPH), deep surgical site infection (SSI) and postoperative pancreatic fistula (POPF). PPH is related to high mortality rates of up to 60%[6-14], with an incidence of 3%-16% after overall pancreatic resection[6-9] and 8%-29% after pancreaticoduodenectomy[11-14]. When performing pancreatic surgery in ATT-received patients, both rigorous hemostasis procedures and strict perioperative antithrombotic management are required to prevent both thromboembolic and bleeding complications. To date, there is no consensus on the safety of pancreatic resection and proper perioperative antithrombotic management in patients receiving ATT, and the optimal thromboprophylaxis for venous thromboembolism (VTE) also remains unclear.
The aim of the present review study is to assess the effect of ATT and chemical thromboprophylaxis on thromboembolic complications and PPH in pancreatic surgery.
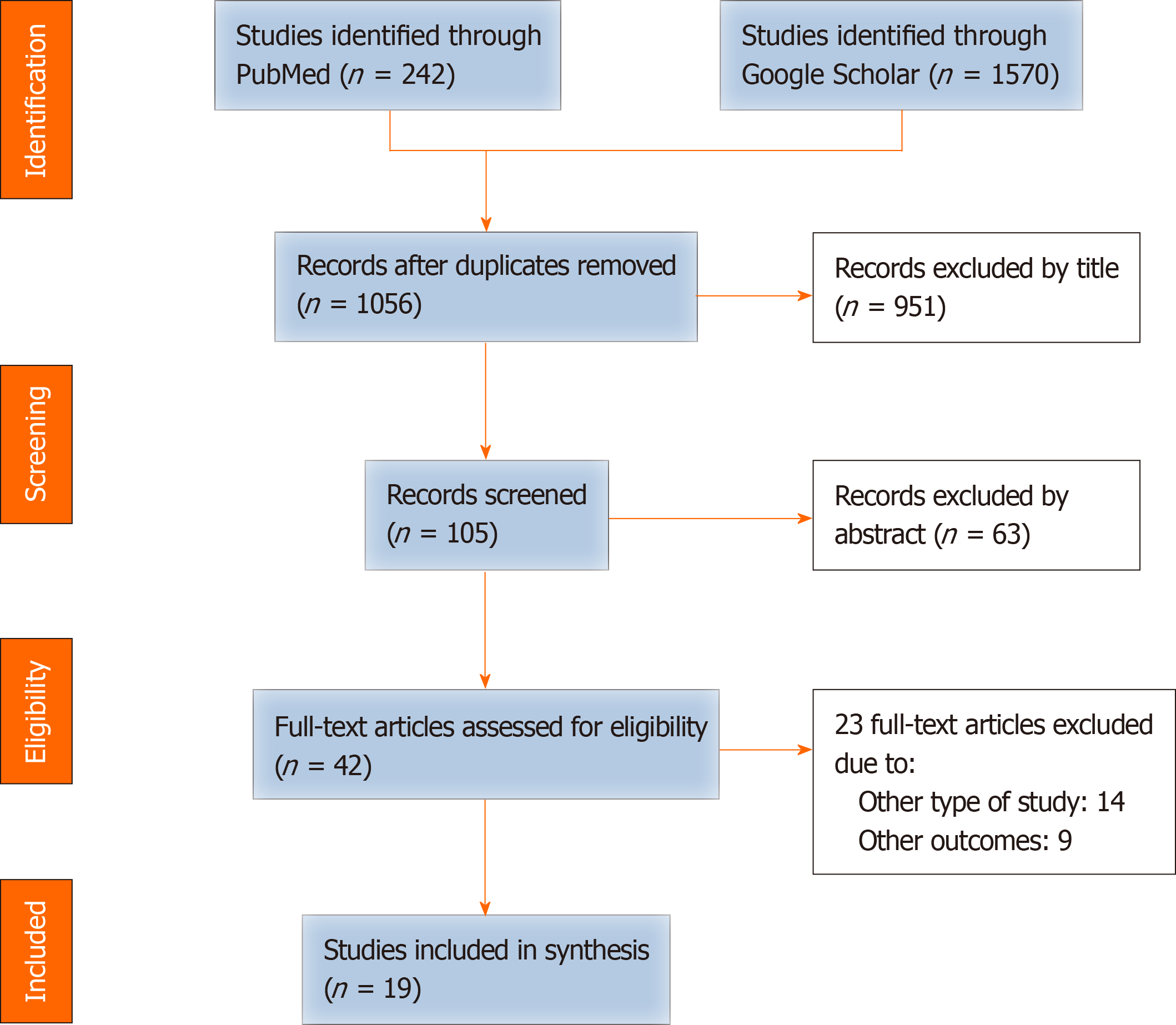
Articles written in English and published between 2013 and 2020 were collected from PubMed and Google Scholar (Figure 1). The following key words were used for the search: Antithrombotic therapy, aspirin, clopidogrel, antiplatelet therapy, anticoagulation, warfarin, DOAC, NOAC, bleeding, postpancreatectomy hemorrhage, pancreatic surgery, and pancreatectomy. The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) 2015 checklist[15]. Articles were included when published in the peer review journal. The eligible studies included randomized clinical trials, prospective or retrospective cohort studies, or case-control studies; guidelines, review articles, or case series/reports were not included.
After removing duplicates, articles were excluded systematically by careful review of each study. The quality of each study was assessed depending on the study design, and eligible articles were determined. Complete data were extracted from each study, including the design of the study, year of publication, sample size, type of surgical procedures, type of antithrombotic drugs, and surgical outcome (both bleeding and thromboembolic complications). Bleeding events included two categories: Increased surgical blood loss (SBL) and PPH. PPH was defined according to the International Study Group of Pancreatic Surgery (ISGPS)[16].
Collection and screening of research were performed from November 2020 to December 2020. In total, 19 published articles, with a total of 37863 patients undergoing pancreatic surgery, were analyzed. None was a randomized clinical trial, and all were cohort studies or case-control studies. Fourteen out of 19 were observational cohort studies, with only three being prospective in nature. The included articles consisted of 9 studies concerning the management of chronically ATT-received patients[10,17-24] (Table 2) and 10 studies regarding chemical thromboprophylaxis for VTE (Table 3)[25-34]. Among the studies on the management of chronically ATT-received patients, one was a multicenter retrospective cohort analysis[18] and another was an analysis using the propensity score matching method[21]. All 10 articles on chemical thromboprophylaxis for VTE were observational studies, including three prospective and seven retrospective cohort studies.
| Ref. | Year, Type | Surgery type | Drug use and exposure | Bleeding events | TE, mortality |
| Nakamura et al[17] | 2020 mRCS | Pancreatic surgery | Patients with ATT (n = 144); Patients without ATT (control, n = 1297) | PPH 8.3% in ATT vs 2.0% in control (P < 0.001); SBL was identical (P = 0.338) | TE 1.4% vs 0.4% (P = 0.149); Mortality 2.8% vs 2.1% (P = 0.542) |
| Fujikawa et al[18] | 2020 RCS | Major digestive surgery including pancreatic surgery | Patients with continued use of ASA (n = 421); Patients with discontinuation of APT (n = 542); Patients not on APT (control, n = 2019) | BC 3.8% in continued ASA vs 3.5% in discontinuation vs 1.3% in control (P < 0.001); BC rate comparable after adjusting | TE 0.5% in continued ASA or control vs 2.8% in discontinuation (P < 0.001); Mortality 0.7%/0.6% vs 1.1% (P = 0.340) |
| Komokata et al[19] | 2020 CCS | Pancreaticoduodenectomy | Patients with ATT (n = 30); Patients without ATT (control, n = 47) | PPH 16.7% in ATT vs 6.4% in control (P = 0.250); SBL was identical (P = 0.454) | TE 13.3% vs 0% (P = 0.020); Mortality 6.7% vs 2.1% (P = 0.557) |
| Fujikawa et al[20] | 2019 RCS | Gastroenterological surgery including pancreatic resection | Patients with DOAC w/o HEP (n = 69); Patients with DOAC w/ HEP (n = 34); Patients with WF w/ HEP (control, n = 231) | BC 1.4% in DOAC w/o HEP vs 14.7% in DOAC w/HEP vs 4.8% in control (P = 0.011); SBL was identical (P = 0.772) | TE 0% vs 0% vs 0.9% (P = 0.637); Mortality 0% vs 1% vs 1.3% (P = 0.791) |
| Ishida et al[21] | 2017 PSM | HBP surgery including pancreatic surgery | Patients with ACT (n = 39); Patients with APT (n = 77); Patients without ATT (control, n = 770) | BC 0.0% in ACT vs 1.3% in APT vs 3.4% in control (P = 0.32); SBL was identical (P = 0.99) | TE 0% vs 1.3% vs 0.8% (P = 0.75); Mortality 0% vs 0% vs 1.2% (P = 0.50) |
| Fujikawa et al[22] | 2019 CCS | HBP surgery including pancreatic surgery | Patients with DOAC (n = 35); Patients with WF (control, n = 80) | BC 2.9% in DOAC vs 0% in WF (P = 0.304); SBL was identical (P = 0.782) | No TE event in both groups; No mortality in both groups |
| Fujikawa et al[23] | 2018 RCS | Pancreaticoduodenectomy | Patients with APT (n = 31); Patients without APT (control, n = 69) | PPH 12.9% in APT vs 2.9% in control (P = 0.072); SBL was identical (P = 0.704) | Only one TE (3.2%) in APT group; No mortality in both groups |
| Mita et al[10] | 2016 CCS | Pancreatic surgery | Patients with ATT (n = 34); Patients without ATT (control, n = 124) | PPH 29.4% in ATT vs 6.5% in control (P = 0.039) | Mortality 11.8% vs 2.4% (P = 0.005) |
| Wolf et al[24] | 2014 CCS | Pancreatic surgery | Patients with continued use of ASA (n = 289); Patients without ASA (control, n = 728) | SBL 400 mL in ASA vs 400 mL in control (P = 0.661) | CV complications 10.1% vs 7.0% (P = 0.107) |
| Ref. | Year, Type | Surgery type | Drug use and exposure | Bleeding events | TE, mortality |
| Eguchi et al[25] | 2020 PCS | Major HBP surgery including pancreatic surgery | Patients with TP [LMWH (enoxaparin), n = 133, single arm] | Major BC 2.3%; Minor BC 5.2% | No TE event in whole cohort |
| Hashimoto et al[26] | 2017 PCS | Pancreatic surgery | Patients with TP [LMWH (enoxaparin), n = 103, single arm] | Major PPH 2.9% | Asymptomatic VTE 1.9%; No symptomatic VTE; No motality |
| Imamura et al[27] | 2017 PCS | Pancreatic surgery | Patients with TP [LMWH (enoxaparin), n = 151, single arm] | Major PPH 3.3%; Minor PPH 3.3% | No PE event in whole cohort |
| Hanna-Sawires et al[28] | 2019 RCS | Pancreatic surgery | Patients with single LMWH (nadroparin) (n = 80); Patients with double dose LMWH (n = 80); Patients with split dose LMWH (n = 80) | CR-PPH 16.0% in double LMWH vs 3.8% in others (P = 0.015) | VTE was identical among groups |
| Fong et al[29] | 2020 RCS | Pancreatic surgery | Patients with TP (preop heparin, n = 1062); Patients without TP (control, n = 386) | (Not mentioned) | VTE 2.6% in TP vs 1.3% in control (P = 0.079), increased AOR in TP (AOR 2.93, P = 0.031) |
| Doughtie et al[30] | 2014 RCS | Major HBP surgery including pancreatic surgery | Patients with preop TP (LMWH, n = 93); Patients without preop TP (control, n = 130) | Major BC 10.9% in preop TP vs 3.1% in control (P = 0.026); SBL was identical | VTE 1.1% vs 6.1% (P = 0.05) |
| Hayashi et al[31] | 2014 RCS | Major HBP surgery including 211 pancreatic surgery | Patients with TP (n = 207); Patients without TP (control, n = 142) | BC 26.6% in TP vs 8.5% in control (P < 0.05); Rate of major BC is identical | VTE 2.9% vs 7.7% (P < 0.05) |
| Skertich et al[32] | 2019 RCS | Surgery for NETs including pancreatic surgery | Patients with abdominal NETs (n = 7226, single arm) | (not mentioned) | VTE 2.0% in whole cohort; VTE 3.4% in malignant PNETs |
| Rashid et al[33] | 2019 RCS | Pancreatic surgery for malignant diseases | Patients with extended DOAC [dabigatran] until POD28 (n = 134, single arm) | Major PPH 2.0% and minor PPH 2.0% | Post-discharge VTE 2.0% |
| Beal et al[34] | 2018 RCS | Major HBP surgery including pancreatic surgery | Patients with hepatectomy or pancreatectomy (n = 48860, single arm) | (not mentioned) | VTE 3.2% after hepatectomy; VTE 1.1% after pancreatectomy; Post-discharge VTE 1.1% in all |
In nine studies concerning the management of patients receiving chronic ATT, three focused on the safety of perioperative heparin bridging, and three others investigated the safety of perioperative aspirin continuation during pancreatic surgery. In 10 studies regarding chemical thromboprophylaxis for VTE, patients were mostly managed perioperatively by LMWH. Two recently published studies focused on post-discharge VTE.
In all nine studies concerning the management of chronically ATT-received patients, the authors generally reported the safety and feasibility of pancreatic surgery even in ATT-received patients (Table 2). However, three articles investigated the safety of perioperative management using perioperative heparin bridging during or after pancreatectomy, and showed the significant impact of heparin bridging on the increased risk of PPH[10,17,20]. Nakamura et al[17] recently reported a retrospective multicenter study, analyzing 144 chronically ATT-received patients, the patients in the ATT group had a higher frequency of PPH compared with the control (8.3% vs 2.0%), and 75% of ATT-received patients with late-onset PPH were managed by perioperative heparin bridging. They concluded that ATT use was a significant risk factor for PPH, and the use of combined ATT or perioperative heparin bridging may be at particularly high risk for PPH.
Another retrospective cohort study reported the impact of chronic ATT on PPH after pancreatic resection[10] and concluded that rigid thromboprophylaxis including heparin bridging was significantly associated with PPH. The incidence of PPH in the study was relatively high, at 11.4% (and 4.4% was regarded as grade C), and PPH-associated mortality rate was 16.7%. Fujikawa et al[20] recently reported 334 anticoagulant-treated patients undergoing digestive surgery including pancreatectomy, showed that DOAC therapy with heparin bridging is the most significant risk factor for postoperative bleeding complications. The results of these studies suggested that perioperative heparin bridging is a potential risk factor for postoperative bleeding events including PPH and that it should be avoided as much as possible for the management of ATT-received patients during pancreatectomy.
In contrast, three papers focused on the feasibility of perioperative aspirin continuation during pancreatectomy[18,23,24]. Two retrospective cohort studies demonstrated that preoperative aspirin continuation is not associated with increased rates of SBL or PPH in chronically antiplatelet-received patients during or after pancreatic surgery[23,24]. Another recently published large-scale retrospective cohort studies[18], reviewing more than 3000 consecutive patients undergoing major digestive malignancy surgery including pancreatectomy, showed that the discontinuation of preoperative antiplatelet therapy is the most significant risk factor for thromboembolic complications and that the continuation of preoperative aspirin therapy significantly reduced the occurrence of thromboembolic complication. However, it was not related to bleeding complications or PPH. These three articles suggested that continuation of aspirin is safe and should be considered preferable even when performing pancreatic surgery, particularly in chronically antiplatelet-received patients with thromboembolic risks.
All 10 articles concerning chemical thromboprophylaxis for VTE were observational cohort studies, including three prospective and seven retrospective in nature (Table 3). All three prospective cohort studies were performed in Japan, and it was demonstrated that the rates of PPH under chemical prophylaxis during pancreatic surgery were acceptable and the rates of VTE were also relatively low; however, the effect of prophylaxis on the occurrence of VTE was not statistically shown due to the small sample size and single-arm design[25-27]. Other retrospective observational studies showed potentially elevated risks of PPH in patients receiving chemical thromboprophylaxis, although its efficacy for VTE is not statistically relevant[28-34]. Two studies conducted in the United States showed relatively high rates of postdischarge VTE after pancreatic surgery (1.1%-2.0%) and suggested the importance of extended chemical prophylaxis in this patient population[33,34], although no studies concerning postdischarge VTE were reported from Asian countries.
Analysis of these studies has shown a potentially high risk of PPH in patients undergoing chemical thromboprophylaxis, but not the effectiveness of chemical prophylaxis against VTE after pancreatic surgery, especially in the Asian patient population.
To the best of our knowledge, this is the first systematic review study to assess the effect of ATT and medical thromboprophylaxis on thromboembolic complications and PPH during and after pancreatic surgery. The current review summarized 19 published articles with a total of 37863 patients receiving pancreatic surgery with special reference to ATT. Regarding the effect of ATT on PPH in patients undergoing pancreatectomy, some studies demonstrated that chronically ATT-received patients managed by heparin bridging potentially had an elevated risk of PPH, although the majority of included studies reported that pancreatic surgery can be safely performed in patients receiving chronic ATT, even under preoperative aspirin continuation. Concerning chemical thromboprophylaxis for VTE, most studies have shown a potentially high risk of PPH in patients undergoing chemical thromboprophylaxis, although their effectiveness against VTE has not been statistically demonstrated.
For the management of patients receiving chronic ATT, guidelines regarding antithrombotic management during non-cardiac surgery were recently updated and demonstrated that the prevention of thromboembolic events is more crucial than bleeding complications, since it might cause severe sequelae or death[4,35-38]. However, major gastroenterological surgery including pancreatic surgery showed scarce evidence concerning the definite protocol or guidelines.
Our hospital is a high-volume center for referrals to patients with gastrointestinal cancer who are taking antithrombotic drugs. For this reason, we currently use a centralized protocol for antithrombotic drug management in ATT-received patients receiving elective gastroenterological surgery including pancreatic surgery, which was established and has been updated with reference to several guidelines and recently reported studies concerning perioperative antithrombotic management for endoscopic procedures or non-cardiac surgeries[4,5,35-37]. In general, the management includes three ways according to the type of ATT: antiplatelets, warfarin, and DOACs. In patients with thromboembolic risks, continued aspirin monotherapy is performed in patients receiving antiplatelet therapy, and warfarin is substituted by DOAC bridging (preferred) or heparin bridging. In the case of DOAC therapy, short-period discontinuation of DOACs (usually 1-2 d) without heparin bridging is recommended. However, if the thromboembolic risk is very high, heparin bridging might be considered. Postoperatively, every antithrombotic agent is re-instituted as soon as possible (POD1-2).
Concerning the management of patients with antiplatelet drugs, some studies such as POISE-2 study have suggested that a slight increase in bleeding risk was observed in patients with continued antiplatelets during non-cardiac surgery[39,40], but most of other studies demonstrated that the bleeding events were not significantly increased[24,41]. Moreover, one recently published large-scale retrospective cohort studies showed that the continuation of preoperative aspirin therapy significantly reduced the occurrence of thromboembolic complication but was not related to bleeding complications or PPH[18]. In the current systematic review, two recently reported studies demonstrated that in patients receiving chronic antiplatelets or anticoagulants, which were mostly managed by heparin bridging, the risk of PPH was higher compared with those without ATT[10,17]. However, three other studies reported that preoperative continuation of aspirin monotherapy is safe and feasible during hepatobiliary-pancreatic or gastrointestinal surgery[18,23,24], and the remaining studies showed no difference in the occurrence of bleeding between ATT-received group and the group without ATT. Although the proper management of patients undergoing antiplatelet therapy during pancreatic surgery remains controversial, preferred perioperative antithrombotic management such as continuation of preoperative aspirin mono
In the clinical setting, some institutions may be instructed to use heparin bridging during the perioperative cessation of antiplatelet agents when cardiologists judge the risk of thromboembolism as high in APT-recipient patients, which may be because most surgeons and cardiologists do not recognize the preferred option of continued aspirin monotherapy for perioperative antiplatelet management. Since bridging therapy with heparin is currently considered a strong risk factor for postoperative bleeding and should be avoided as much as possible[42], and because its mechanism of action is different from that of antiplatelet drugs, heparin bridging during cessation of antiplatelet therapy should not be used.
Regarding DOACs, only two reports were included in the current review[20]. These studies suggested that perioperative DOAC management without heparin bridging was safe and feasible even for patients who underwent hepatobiliary-pancreatic or gastrointestinal surgery; however, patients who were managed by heparin bridging during DOAC cessation are at high risk of postoperative bleeding. Currently, DOACs are increasingly prescribed for the prevention of arterial or venous thromboembolism. DOACs are fast-acting agents and their anticoagulant effects also quickly fade within 48 h after their withdrawal[35]. One large-scale multicenter prospective cohort study (the PAUSE study) was recently published, which examined outcomes in 3,007 adult patients with atrial fibrillation who underwent DOAC therapy and received an elective non-cardiac procedure or surgery[43]. DOAC therapy was interrupted 1–2 days prior and reinstituted 1–2 days after the procedure or surgery. The occurrence of major bleeding 30 days after the procedure or surgery was 0.90%–1.85%, and arterial thromboembolic complication was occurred at the rate of 0.16%–0.60%. The study recommended that a centralized perioperative management of DOACs without heparin bridging can be performed safely for patients with atrial fibrillation. Although the PAUSE study included a relatively small number of patients undergoing major gastroenterological surgery, the included studies in the current review also suggested that optimal DOAC management without heparin bridging is recommended even for patients who undergo major digestive surgeries such as hepatobiliary-pancreatic or gastrointestinal cancer surgery[20,22].
Regarding the thromboprophylaxis for VTE during pancreatic surgery, most studies in the current review have shown a potential risk of PPH in patients undergoing chemical thromboprophylaxis, but their effectiveness against VTE has not been statistically demonstrated, especially in Asian patients undergoing surgery. VTE is a fatal complication during the perioperative period, and prevention is of utmost importance. Although some Western guidelines for VTE treatment recommend chemical thromboprophylaxis during non-cardiac surgery[44-46], the racial differences in the incidence of VTE between Western people and Asians were observed[47]. Additionally, in one systematic review concerning chemical prophylaxis for VTE in Asian surgical patients[48], the risk of perioperative VTE in Asian patients is low even in the context of risk factors typically regarded as high risk. The most recently published two large-scale cohort studies (with more than 1000 patients) conducted in Japan also showed that the incidence of clinically relevant VTE during or after pancreatic surgery was 0%-0.3%[17,18]. Currently, the safety and efficacy of chemical thromboprophylaxis with anticoagulants for VTE during pancreatic surgery is still controversial, particularly in Asian populations. It is important to build evidence to classify risks individually according to race.
Presently, there are only limited numbers of studies regarding the management of ATT during pancreatic surgery. This patient population is expanding further, as the population ages and the prevalence of cardiovascular disease increases. Using reliable studies with good design, the definite guideline should be established. Currently, two promising studies, registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry, are ongoing; one prospective multicenter cohort study (UMIN000038280, “Study on the safety and feasibility of gastroenterological surgery in patients undergoing antithrombotic therapy (GSATT Study)”), and one retrospective multicenter cohort study (UMIN000037621, “Impact of antithrombotic agents on the clinical course after pancreatectomy”)[49]. In the future, the safety and feasibility of ATT management during pancreatic surgery will be elucidated by well-designed analyses like these two studies.
Pancreatic surgery in chronically ATT-burdened patients can be safely performed without an increase in the occurrence of PPH and thromboembolic complications. The safety and efficacy of chemical thromboprophylaxis with anticoagulants for VTE during pancreatic surgery is still controversial. Further investigation using reliable studies with good design is required to establish definite protocols or guidelines.
Postpancreatectomy hemorrhage (PPH) is the most severe type of complication after pancreatic surgery, although the effect of antithrombotic therapy (ATT) on PPH is largely unknown. The safety and efficacy of chemical thromboprophylaxis for venous thromboembolism (VTE) also remains controversial.
The goal of the current systematic review study was to elucidate the effect of ATT on PPH after pancreatic resection.
The objectives of the current systematic review study was to elucidate the effect of ATT on PPH.
Published articles between 2013 and 2020 were searched from PubMed and Google Scholar, and studies involving pancreatic surgery and ATT were included after careful review of each study. Data such as design of the study, type of surgical procedures, antithrombotic drugs used, and surgical outcome (both PPH and thromboembolic complications) were extracted from each study.
Nineteen published articles with a total of 37863 patients who underwent pancreatic surgery were included in the systematic review. Fourteen were cohort studies, with only three being prospective in nature. Two studies demonstrated that in patients receiving chronic ATT, which were mostly managed by heparin bridging, the risk of PPH was higher compared with those without ATT, and one study showed that patients with direct-acting oral anticoagulants managed by heparin bridging had significantly higher postoperative bleeding rates than others. The remaining six studies reported that pancreatic surgery can be safely performed in patients receiving chronic ATT, even under preoperative aspirin continuation. Concerning chemical thromboprophylaxis for VTE, most studies have shown a potentially high risk of PPH in patients undergoing chemical thromboprophylaxis; however, its effectiveness against VTE has not been statistically demonstrated, particularly among Asian patients.
Pancreatic surgery in chronically ATT-received patients can be safely performed without an increase in the occurrence of PPH, although the safety and efficacy of chemical thromboprophylaxis for VTE during pancreatic surgery is still controversial.
Further investigation using reliable studies with good design is required to establish definite protocols or guidelines.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Havre RF, Zorbas KA S-Editor: Ma YJ L-Editor: A P-Editor: Liu JH
| 1. | Mita K, Ito H, Murabayashi R, Sueyoshi K, Asakawa H, Nabetani M, Kamasako A, Koizumi K, Hayashi T. Postoperative bleeding complications after gastric cancer surgery in patients receiving anticoagulation and/or antiplatelet agents. Ann Surg Oncol. 2012;19:3745-3752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Thachil J, Gatt A, Martlew V. Management of surgical patients receiving anticoagulation and antiplatelet agents. Br J Surg. 2008;95:1437-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Fujikawa T, Tanaka A, Abe T, Yoshimoto Y, Tada S, Maekawa H, Shimoike N. Does antiplatelet therapy affect outcomes of patients receiving abdominal laparoscopic surgery? J Am Coll Surg. 2013;217:1044-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | ASGE Standards of Practice Committee; Acosta RD, Abraham NS, Chandrasekhara V, Chathadi KV, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fisher DA, Fonkalsrud L, Hwang JH, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Shergill AK, Wang A, Cash BD, DeWitt JM. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 450] [Article Influence: 50.0] [Reference Citation Analysis (1)] |
| 5. | Fujimoto K, Fujishiro M, Kato M, Higuchi K, Iwakiri R, Sakamoto C, Uchiyama S, Kashiwagi A, Ogawa H, Murakami K, Mine T, Yoshino J, Kinoshita Y, Ichinose M, Matsui T; Japan Gastroenterological Endoscopy Society. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 348] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 6. | Darnis B, Lebeau R, Chopin-Laly X, Adham M. Postpancreatectomy hemorrhage (PPH): predictors and management from a prospective database. Langenbecks Arch Surg. 2013;398:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Yekebas EF, Wolfram L, Cataldegirmen G, Habermann CR, Bogoevski D, Koenig AM, Kaifi J, Schurr PG, Bubenheim M, Nolte-Ernsting C, Adam G, Izbicki JR. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Correa-Gallego C, Brennan MF, D'Angelica MI, DeMatteo RP, Fong Y, Kingham TP, Jarnagin WR, Allen PJ. Contemporary experience with postpancreatectomy hemorrhage: results of 1,122 patients resected between 2006 and 2011. J Am Coll Surg. 2012;215:616-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Roulin D, Cerantola Y, Demartines N, Schäfer M. Systematic review of delayed postoperative hemorrhage after pancreatic resection. J Gastrointest Surg. 2011;15:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Mita K, Ito H, Takahashi K, Hashimoto M, Nagayasu K, Murabayashi R, Asakawa H, Koizumi K, Hayashi T, Fujino K. Postpancreatectomy Hemorrhage After Pancreatic Surgery in Patients Receiving Anticoagulation or Antiplatelet Agents. Surg Innov. 2016;23:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Ricci C, Casadei R, Buscemi S, Minni F. Late postpancreatectomy hemorrhage after pancreaticoduodenectomy: is it possible to recognize risk factors? JOP. 2012;13:193-198. [PubMed] |
| 12. | Welsch T, Eisele H, Zschäbitz S, Hinz U, Büchler MW, Wente MN. Critical appraisal of the International Study Group of Pancreatic Surgery (ISGPS) consensus definition of postoperative hemorrhage after pancreatoduodenectomy. Langenbecks Arch Surg. 2011;396:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Sanjay P, Fawzi A, Fulke JL, Kulli C, Tait IS, Zealley IA, Polignano FM. Late post pancreatectomy haemorrhage. Risk factors and modern management. JOP. 2010;11:220-225. [PubMed] |
| 14. | Rumstadt B, Schwab M, Korth P, Samman M, Trede M. Hemorrhage after pancreatoduodenectomy. Ann Surg. 1998;227:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 15832] [Article Influence: 1583.2] [Reference Citation Analysis (1)] |
| 16. | Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1941] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 17. | Nakamura K, Sho M, Satoi S, Kosaka H, Akahori T, Nagai M, Nakagawa K, Takagi T, Yamamoto T, Yamaki S. Impact of Antithrombotic Agents on Postpancreatectomy Hemorrhage: Results from a Retrospective Multicenter Study. J Am Coll Surg. 2020;231:460-469. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Fujikawa T, Kawamura Y, Takahashi R, Naito S. Risk of postoperative thromboembolic complication after major digestive surgery in patients receiving antiplatelet therapy: Lessons from more than 3,000 operations in a single tertiary referral hospital. Surgery. 2020;167:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Komokata T, Aryal B, Tada N, Kaieda M, Nuruki K. Impact of antithrombotic therapy on the outcomes with focus on bleeding and thromboembolic events in patients undergoing pancreticoduodenectomy. ANZ J Surg. 2020;90:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Fujikawa T, Takahashi R, Naito S. Perioperative antithrombotic management of patients who receive direct oral anticoagulants during gastroenterological surgery. Ann Gastroenterol Surg. 2020;4:301-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Ishida J, Fukumoto T, Kido M, Matsumoto I, Ajiki T, Kawai H, Hirata K, Ku Y. Hemorrhagic and Thromboembolic Complications after Hepato-Biliary-Pancreatic Surgery in Patients Receiving Antithrombotic Therapy. Dig Surg. 2017;34:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Fujikawa T, Fujikawa T, Tanaka A. Safety of Hepatobiliary and Pancreatic Surgery in Patients Receiving Direct oral Anticoaulants (DOACs). J Gastroenterol Hepatol Res. 2019;8:3009-3013. [DOI] [Full Text] |
| 23. | Fujikawa T, Kawamoto H, Tanaka A. Effect of Antiplatelet Therapy on Surgical Blood Loss and Post-Pancreatectomy Hemorrhage in Patients Undergoing. J Gastroenterol Hepatol Res. 2018;7:1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Wolf AM, Pucci MJ, Gabale SD, McIntyre CA, Irizarry AM, Kennedy EP, Rosato EL, Lavu H, Winter JM, Yeo CJ. Safety of perioperative aspirin therapy in pancreatic operations. Surgery. 2014;155:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Eguchi H, Kawamoto K, Tsujie M, Yukawa M, Kubota M, Asaoka T, Takeda Y, Noda T, Shimizu J, Nagano H, Doki Y, Mori M. A Prospective, Multi-Center Phase I Study of Postoperative Enoxaparin Treatment in Patients Undergoing Curative Hepatobiliary-Pancreatic Surgery for Malignancies. Dig Surg. 2020;37:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Hashimoto D, Nakagawa S, Umezaki N, Yamao T, Kitano Y, Yamamura K, Kaida T, Arima K, Imai K, Yamashita YI, Chikamoto A, Baba H. Efficacy and safety of postoperative anticoagulation prophylaxis with enoxaparin in patients undergoing pancreatic surgery: A prospective trial and literature review. Pancreatology. 2017;17:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Imamura H, Adachi T, Kitasato A, Tanaka T, Soyama A, Hidaka M, Fujita F, Takatsuki M, Kuroki T, Eguchi S. Safety and efficacy of postoperative pharmacologic thromboprophylaxis with enoxaparin after pancreatic surgery. Surg Today. 2017;47:994-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Hanna-Sawires RG, Groen JV, Klok FA, Tollenaar RAEM, Mesker WE, Swijnenburg RJ, Vahrmeijer AL, Bonsing BA, Mieog JSD. Outcomes following pancreatic surgery using three different thromboprophylaxis regimens. Br J Surg. 2019;106:765-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Fong ZV, Sell NM, Fernandez-Del Castillo C, Del Carmen G, Ferrone CR, Chang DC, Warshaw AL, Polk HC Jr, Lillemoe KD, Qadan M. Does preoperative pharmacologic prophylaxis reduce the rate of venous thromboembolism in pancreatectomy patients? HPB (Oxford). 2020;22:1020-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Doughtie CA, Priddy EE, Philips P, Martin RC, McMasters KM, Scoggins CR. Preoperative dosing of low-molecular-weight heparin in hepatopancreatobiliary surgery. Am J Surg. 2014;208:1009-15; discussion 1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Hayashi H, Morikawa T, Yoshida H, Motoi F, Okada T, Nakagawa K, Mizuma M, Naitoh T, Katayose Y, Unno M. Safety of postoperative thromboprophylaxis after major hepatobiliary-pancreatic surgery in Japanese patients. Surg Today. 2014;44:1660-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Skertich NJ, Gerard J, Poirier J, Hertl M, Pappas SG, Schadde E, Keutgen XM. Do All Abdominal Neuroendocrine Tumors Require Extended Postoperative VTE Prophylaxis? J Gastrointest Surg. 2019;23:788-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Rashid MF, Jackson TL, Morgan JA, Dwyer FA, Schrope BA, Chabot JA, Kluger MD. Dabigatran (Pradaxa) Is Safe for Extended Venous Thromboembolism Prophylaxis After Surgery for Pancreatic Cancer. J Gastrointest Surg. 2019;23:1166-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Beal EW, Tumin D, Chakedis J, Porter E, Moris D, Zhang XF, Abdel-Misih S, Dillhoff M, Manilchuk A, Cloyd J, Schmidt CR, Pawlik TM. Identification of patients at high risk for post-discharge venous thromboembolism after hepato-pancreato-biliary surgery: which patients benefit from extended thromboprophylaxis? HPB (Oxford). 2018;20:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Kato M, Uedo N, Hokimoto S, Ieko M, Higuchi K, Murakami K, Fujimoto K. Guidelines for Gastroenterological Endoscopy in Patients Undergoing Antithrombotic Treatment: 2017 Appendix on Anticoagulants Including Direct Oral Anticoagulants. Dig Endosc. 2018;30:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 36. | Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O'Gara PT, Sabatine MS, Smith PK, Smith SC Jr. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123-e155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 1027] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 37. | Polkowski M, Jenssen C, Kaye P, Carrara S, Deprez P, Gines A, Fernández-Esparrach G, Eisendrath P, Aithal GP, Arcidiacono P, Barthet M, Bastos P, Fornelli A, Napoleon B, Iglesias-Garcia J, Seicean A, Larghi A, Hassan C, van Hooft JE, Dumonceau JM. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy. 2017;49:989-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 38. | Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, Dunn AS, Kunz R. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S-50S. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1079] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 39. | Devereaux PJ, Mrkobrada M, Sessler DI, Leslie K, Alonso-Coello P, Kurz A, Villar JC, Sigamani A, Biccard BM, Meyhoff CS, Parlow JL, Guyatt G, Robinson A, Garg AX, Rodseth RN, Botto F, Lurati Buse G, Xavier D, Chan MT, Tiboni M, Cook D, Kumar PA, Forget P, Malaga G, Fleischmann E, Amir M, Eikelboom J, Mizera R, Torres D, Wang CY, VanHelder T, Paniagua P, Berwanger O, Srinathan S, Graham M, Pasin L, Le Manach Y, Gao P, Pogue J, Whitlock R, Lamy A, Kearon C, Baigent C, Chow C, Pettit S, Chrolavicius S, Yusuf S; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 588] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 40. | of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 705] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 41. | Fang X, Baillargeon JG, Jupiter DC. Continued Antiplatelet Therapy and Risk of Bleeding in Gastrointestinal Procedures: A Systematic Review. J Am Coll Surg. 2016;222:890-905.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 43. | Douketis JD, Spyropoulos AC, Duncan J, Carrier M, Le Gal G, Tafur AJ, Vanassche T, Verhamme P, Shivakumar S, Gross PL, Lee AYY, Yeo E, Solymoss S, Kassis J, Le Templier G, Kowalski S, Blostein M, Shah V, MacKay E, Wu C, Clark NP, Bates SM, Spencer FA, Arnaoutoglou E, Coppens M, Arnold DM, Caprini JA, Li N, Moffat KA, Syed S, Schulman S. Perioperative Management of Patients With Atrial Fibrillation Receiving a Direct Oral Anticoagulant. JAMA Intern Med. 2019;179:1469-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 333] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 44. | Farge D, Frere C, Connors JM, Ay C, Khorana AA, Munoz A, Brenner B, Kakkar A, Rafii H, Solymoss S, Brilhante D, Monreal M, Bounameaux H, Pabinger I, Douketis J; International Initiative on Thrombosis and Cancer (ITAC) advisory panel. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566-e581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 439] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 45. | Schünemann HJ, Cushman M, Burnett AE, Kahn SR, Beyer-Westendorf J, Spencer FA, Rezende SM, Zakai NA, Bauer KA, Dentali F, Lansing J, Balduzzi S, Darzi A, Morgano GP, Neumann I, Nieuwlaat R, Yepes-Nuñez JJ, Zhang Y, Wiercioch W. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 549] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 46. | Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e227S-e277S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1495] [Cited by in RCA: 1467] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 47. | Liew NC, Alemany GV, Angchaisuksiri P, Bang SM, Choi G, DE Silva DA, Hong JM, Lee L, Li YJ, Rajamoney GN, Suviraj J, Tan TC, Tse E, Teo LT, Visperas J, Wong RS, Lee LH. Asian venous thromboembolism guidelines: updated recommendations for the prevention of venous thromboembolism. Int Angiol. 2017;36:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 48. | Yeo DX, Junnarkar S, Balasubramaniam S, Tan YP, Low JK, Woon W, Pang TC. Incidence of venous thromboembolism and its pharmacological prophylaxis in Asian general surgery patients: a systematic review. World J Surg. 2015;39:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Sho M, Nakamura K. Impact of antithrombotic agents on the clinical course after pancreatectomy. UMIN-CTR Clinical Trial 2019. Available from: https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000042569. |